The Impact of Body Weight Changes versus Exercise Capacity Changes on Health-Related Factors following a Lifestyle Intervention in Employees with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
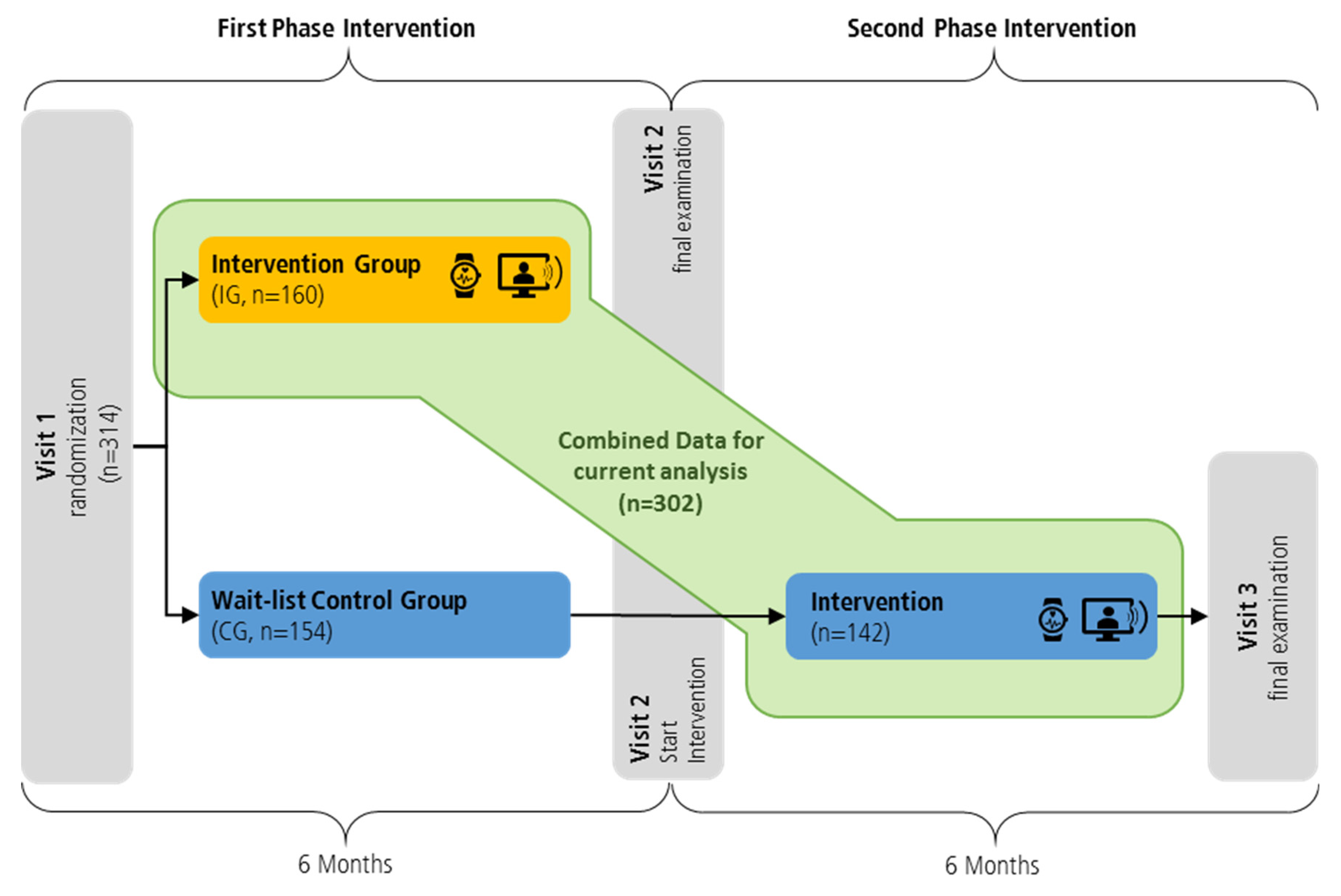
2.1. Study Design and Participants
2.2. Study Sample
2.3. Intervention
2.4. Examination Instruments
2.5. Statistical Analysis
3. Results
3.1. Physical Activity, Exercise Capacity and Nutritional Intake
3.2. Association of Health- and Work-Related Outcomes
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; SmithJr, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, e285. [Google Scholar] [CrossRef] [Green Version]
- Dekker, J.M.; Girman, C.; Rhodes, T.; Nijpels, G.; Stehouwer, C.D.A.; Bouter, L.M.; Heine, R.J. Metabolic Syndrome and 10-Year Cardiovascular Disease Risk in the Hoorn Study. Circulation 2005, 112, 666–673. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2014, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Schultz, A.B.; Edington, D.W. Analysis of the Association between Metabolic Syndrome and Disease in a Workplace Population over Time. Value Health 2010, 13, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Burton, W.N.; Chen, C.-Y.; Schultz, A.B.; Edington, D.W. The Prevalence of Metabolic Syndrome in an Employed Population and the Impact on Health and Productivity. J. Occup. Environ. Med. 2008, 50, 1139–1148. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Magkos, F.; Yannakoulia, M.; Chan, J.L.; Mantzoros, C.S. Management of the Metabolic Syndrome and Type 2 Diabetes through Lifestyle Modification. Annu. Rev. Nutr. 2009, 29, 223–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, E1077–E1086. [Google Scholar] [CrossRef]
- Kolotkin, R.L.; Andersen, J.R. A systematic review of reviews: Exploring the relationship between obesity, weight loss and health-related quality of life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; McFarlane, S.I.; Jean-Louis, G.; Myers, A.K. Energy imbalance: Obesity, associated comorbidities, prevention, management and public health implications. Adv. Obes. Weight Manag. Control 2020, 10, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pr. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Kaspy, M.S.; Semnani-Azad, Z.; Malik, V.S.; Jenkins, D.J.A.; Hanley, A.J. Metabolomic profile of combined healthy lifestyle behaviours in humans: A systematic review. Proteomics 2022, 22, e2100388. [Google Scholar] [CrossRef]
- Cabrera, A.G.; Caballero, P.; Wanden-Berghe, C.; Sanz-Lorente, M.; López-Pintor, E. Effectiveness of Workplace-Based Diet and Lifestyle Interventions on Risk Factors in Workers with Metabolic Syndrome: A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2021, 13, 4560. [Google Scholar] [CrossRef]
- Garber, C. The Health Benefits of Exercise in Overweight and Obese Patients. Curr. Sports Med. Rep. 2019, 18, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the Treatment of Depression and Anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Obesity treatment: Weight loss versus increasing fitness and physical activity for reducing health risks. iScience 2021, 24, 102995. [Google Scholar] [CrossRef]
- Haufe, S.; Kerling, A.; Protte, G.; Bayerle, P.; Stenner, H.T.; Rolff, S.; Sundermeier, T.; Kück, M.; Ensslen, R.; Nachbar, L.; et al. Telemonitoring-supported exercise training, metabolic syndrome severity, and work ability in company employees: A randomised controlled trial. Lancet Public Health 2019, 4, e343–e352. [Google Scholar] [CrossRef] [Green Version]
- Bayerle, P.; Kerling, A.; Kück, M.; Rolff, S.; Boeck, H.T.; Sundermeier, T.; Ensslen, R.; Tegtbur, U.; Lauenstein, D.; Böthig, D.; et al. Effectiveness of wearable devices as a support strategy for maintaining physical activity after a structured exercise intervention for employees with metabolic syndrome: A randomized controlled trial. BMC Sport. Sci. Med. Rehabil. 2022, 14, 24. [Google Scholar] [CrossRef]
- WHO Guidelines on Physical Activity and Sedentary Behavior. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 7 March 2022).
- Deutsche Gesellschaft fuer Ernaehrung e.V. 10 Regeln der DGE. Available online: https://www.dge.de/ernaehrungspraxis/vollwertige-ernaehrung/10-regeln-der-dge/ (accessed on 25 July 2022).
- Snaith, R.P. The hospital anxiety and depression scale. J. Psychosom. Res. 2002, 52, 401. [Google Scholar]
- Bullinger, M. [Assessment of health related quality of life with the SF-36 Health Survey]. Rehabilitation 1996, 35, XVII–XXIX. [Google Scholar]
- Frey, I.; Berg, A.; Grathwohl, D.; Keul, J. Freiburger Fragebogen zur körperlichen Aktivität-Entwicklung, Prüfung und Anwendung. Soz Präventivmed 1999, 44, 55–64. [Google Scholar] [CrossRef]
- van den Berg, T.I.J.; Elders, L.A.M.; de Zwart, B.C.H.; Burdorf, A. The effects of work-related and individual factors on the Work Ability Index: A systematic review. Occup. Environ. Med. 2009, 66, 211–220. [Google Scholar] [CrossRef] [Green Version]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 July 2022).
- National Heart, Lung, and Blood Institute. Metabolic Syndrome. Causes and Risk Factors. Available online: https://www.nhlbi.nih.gov/health/metabolic-syndrome/causes (accessed on 20 July 2022).
- Lavie, C.J.; Schutter, A.D.; Archer, E.; McAuley, P.A.; Blair, S.N. Obesity and Prognosis in Chronic Diseases—Impact of Cardiorespiratory Fitness in the Obesity Paradox. Curr. Sports Med. Rep. 2014, 13, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Varkevisser, R.D.M.; van Stralen, M.M.; Kroeze, W.; Ket, J.C.F.; Steenhuis, I.H.M. Determinants of weight loss maintenance: A systematic review. Obes. Rev. 2018, 20, 171–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biccirè, F.G.; Bucci, T.; Menichelli, D.; Cammisotto, V.; Pignatelli, P.; Carnevale, R.; Pastori, D. Mediterranean Diet: A Tool to Break the Relationship of Atrial Fibrillation with the Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 1260. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Namen, M.; Prendergast, L.; Peiris, C. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: A systematic review and meta-analysis. Metabolism 2019, 101, 153988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Chekroud, S.R.; Gueorguieva, R.; Zheutlin, A.B.; Paulus, M.; Krumholz, H.M.; Krystal, J.H.; Chekroud, A.M. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry 2018, 5, 739–746. [Google Scholar] [CrossRef]
- Marcos-Delgado, A.; Fernández-Villa, T.; Martínez-González, M.; Salas-Salvadó, J.; Corella, D.; Castañer, O.; Martínez, J.A.; Alonso-Gómez, M.; Wärnberg, J.; Vioque, J.; et al. The Effect of Physical Activity and High Body Mass Index on Health-Related Quality of Life in Individuals with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2020, 17, 3728. [Google Scholar] [CrossRef] [PubMed]
- Puciato, D.; Borysiuk, Z.; Rozpara, M. Quality of life and physical activity in an older working-age population. Clin. Interv. Aging 2017, 12, 1627–1634. [Google Scholar] [CrossRef] [PubMed]


| Total Group (n = 302) | Intervention Group (n = 160) | Delayed Intervention Group (Former Wait-List Control) (n = 142) | |||||
|---|---|---|---|---|---|---|---|
| n | n | n | p-Value | ||||
| Age (years) | 50 [24; 63] | 302 | 50 [25; 62] | 160 | 50 [24; 63] | 142 | 0.911 |
| Body weight (kg) | 105.0 [62.2; 165.1] | 302 | 106.5 [67.6; 158.0] | 160 | 102.1 [62.2; 165.1] | 142 | 0.140 |
| Waist circumference (cm) | 113.8 [89.0; 153.5] | 299 | 115.0 [92.0; 153.5] | 158 | 112.0 [89.0; 151.0] | 141 | 0.145 |
| Body mass index [BMI(kg/m2)] | 32.5 [22.9; 49.5] | 302 | 32.9 [22.9; 49.3] | 160 | 31.9 [23.5; 49.5] | 142 | 0.075 |
| Body fat (%) | 32.3 [14.4; 56.5] | 297 | 32.5 [14.4; 53.4] | 158 | 32.2 [16.1; 56.5] | 139 | 0.331 |
| Fat Free Mass (kg) | 71.3 ± 11.7 | 297 | 71.2 ± 11.1 | 158 | 71.4 ± 12.5 | 139 | 0.875 |
| Systolic BP (mmHg) | 135.8 [106.0; 181.0] | 298 | 136.0 [108.0; 181.0] | 157 | 133.0 [106.0; 170.0] | 141 | 0.010 |
| Diastolic BP (mmHg) | 88.8 [67.0; 120.0] | 298 | 88.5 [67.5; 113.0] | 157 | 89.0 [67.0; 120.0] | 141 | 0.186 |
| HbA1c (%) | 5.4 [4.6; 12.0] | 295 | 5.4 [4.7; 12.0] | 154 | 5.3 [4.6; 9.0] | 141 | 0.016 |
| Total cholesterol (mg/dL) | 214.3 ± 46.2 | 302 | 215.3 ± 45.9 | 160 | 213.0 ± 46.7 | 142 | 0.670 |
| HDL cholesterol (mg/dL) | 42.9 [21.5; 78.5] | 301 | 43.7 [25.6; 78.5] | 159 | 42.4 [21.5; 71.2] | 142 | 0.287 |
| LDL cholesterol (mg/dL) | 136.2 ± 38.9 | 302 | 137.8 ± 38.8 | 160 | 134.5 ± 39.2 | 142 | 0.462 |
| MetS-z-Score | 0.89 [−0.45; 4.13] | 287 | 0.91 [−0.45; 4.13] | 157 | 0.87 [−0.34; 3.16] | 130 | 0.544 |
| Every day activity (MET-h/wk) | 16.4 [0.0; 140.1] | 267 | 14.3 [0.0; 129.7] | 150 | 24.4 [0.4; 140.1] | 117 | <0.001 |
| Sports related activity (MET-h/wk) | 1.5 [0.0; 84.0] | 267 | 0.0 [0.0; 84.0] | 150 | 4.5 [0.0; 56.0] | 117 | 0.001 |
| Total physical activity (MET-h/wk) | 23.2 [0.0; 140.1] | 267 | 18.6 [0.0; 129.7] | 150 | 30.8 [1.2; 140.1] | 117 | <0.001 |
| Relative exercise capacity (watt/kg) | 1.71 ± 0.42 | 301 | 1.66 ± 0.40 | 160 | 1.78 ± 0.44 | 141 | 0.018 |
| Exercise capacity (wattmax) | 180.0 [80.0; 320.0] | 301 | 180.0 [80.0; 320.0] | 160 | 190.0 [80.0; 280.0] | 141 | 0.064 |
| Work ability (WAI) | 38.0 [16.0; 49.0] | 301 | 38.0 [21.0; 48.0] | 159 | 39.0 [16.0; 49.0] | 142 | 0.026 |
| HADS_subscale anxiety | 5.0 [0.0; 16.0] | 289 | 5.0 [0.0; 15.0] | 159 | 4.0 [0.0; 16.0] | 130 | 0.001 |
| HADS_subscale depression | 3.0 [0.0; 15.0] | 289 | 3.0 [0.0; 15.0] | 159 | 3.0 [0.0; 15.0] | 130 | 0.465 |
| SF-36_physical score | 50.8 [23.3; 64.5] | 283 | 49.8 [24.5; 64.5] | 155 | 51.9 [23.3; 61.3] | 128 | 0.047 |
| SF-36_mental score | 52.9 [16.5; 66.1] | 283 | 51.9 [16.5; 66.1] | 155 | 54.0 [20.9; 63.7] | 128 | 0.022 |
| Delta Waist Circumference | Delta Triglycerides | Delta HDL chol. | Delta Glucose conc. | Delta Systolic BP | |
|---|---|---|---|---|---|
| delta BMI (kg/m2) | 0.73 * | 0.32 * | −0.17 * | 0.27 * | 0.25 * |
| delta exercice capacity (wattmax) | −0.32* | −0.24 * | 0.07 | −0.20 * | −0.24 * |
| Dependent Variable | ||||||||||
| Delta Waist Circumference | Delta Triglycerides | HDL Cholesterol | Delta Blood Glucose | Delta Systolic BP | ||||||
| Independent Variables | Coefficient β | p-Value | Coefficient β | p-Value | Coefficient β | p-Value | Coefficient β | p-Value | Coefficient β | p-Value |
| delta BMI | 0.71 | <0.001 | 0.27 | <0.001 | −0.15 | 0.061 | 0.23 | 0.003 | 0.10 | 0.085 |
| delta exercise capacity | −0.03 | 0.543 | −0.07 | 0.281 | 0.01 | 0.772 | −0.05 | 0.386 | −0.14 | 0.038 |
| age | −0.02 | 0.721 | 0.02 | 0.668 | 0.09 | 0.088 | 0.04 | 0.409 | −0.05 | 0.282 |
| sex | −0.01 | 0.738 | 0.02 | 0.754 | −0.19 | 0.009 | 0.05 | 0.351 | 0.10 | 0.074 |
| baseline value of the respective | 0.01 | 0.881 | −0.51 | <0.001 | −0.40 | <0.001 | −0.48 | <0.001 | −0.46 | <0.001 |
| Dependent Variable | ||||||||||
| Delta HADS Anxiety | Delta HADS Depression | Delta Physical Score SF–36 | Delta Mental Score SF–36 | Delta WAI Total Score | ||||||
| Independent Variables | Coefficient β | p-Value | Coefficient β | p-Value | Coefficient β | p-Value | Coefficient β | p-Value | Coefficient β | p-Value |
| delta BMI | 0.06 | 0.312 | 0.05 | 0.264 | −0.19 | 0.009 | 0.04 | 0.488 | −0.07 | 0.218 |
| delta exercise capacity | −0.15 | 0.042 | −0.12 | 0.051 | 0.10 | 0.218 | 0.17 | 0.010 | 0.19 | 0.002 |
| age | −0.02 | 0.718 | 0.01 | 0.823 | −0.01 | 0.941 | 0.04 | 0.416 | −0.05 | 0.345 |
| sex | 0.07 | 0.201 | −0.01 | 0.839 | 0.06 | 0.309 | 0.01 | 0.802 | 0.06 | 0.271 |
| baseline value of the respective | −0.38 | <0.001 | −0.54 | <0.001 | −0.44 | <0.001 | −0.44 | <0.001 | −0.41 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayerle, P.; Haufe, S.; Kück, M.; Protte, G.; Kerling, A.; Ewers, S.; Boeck, H.T.; Sundermeier, T.; Ensslen, R.; Kahl, K.G.; et al. The Impact of Body Weight Changes versus Exercise Capacity Changes on Health-Related Factors following a Lifestyle Intervention in Employees with Metabolic Syndrome. Nutrients 2022, 14, 4560. https://doi.org/10.3390/nu14214560
Bayerle P, Haufe S, Kück M, Protte G, Kerling A, Ewers S, Boeck HT, Sundermeier T, Ensslen R, Kahl KG, et al. The Impact of Body Weight Changes versus Exercise Capacity Changes on Health-Related Factors following a Lifestyle Intervention in Employees with Metabolic Syndrome. Nutrients. 2022; 14(21):4560. https://doi.org/10.3390/nu14214560
Chicago/Turabian StyleBayerle, Pauline, Sven Haufe, Momme Kück, Gudrun Protte, Arno Kerling, Simone Ewers, Hedwig Theda Boeck, Thorben Sundermeier, Ralf Ensslen, Kai G. Kahl, and et al. 2022. "The Impact of Body Weight Changes versus Exercise Capacity Changes on Health-Related Factors following a Lifestyle Intervention in Employees with Metabolic Syndrome" Nutrients 14, no. 21: 4560. https://doi.org/10.3390/nu14214560
APA StyleBayerle, P., Haufe, S., Kück, M., Protte, G., Kerling, A., Ewers, S., Boeck, H. T., Sundermeier, T., Ensslen, R., Kahl, K. G., Haverich, A., Tegtbur, U., & Nachbar, L. (2022). The Impact of Body Weight Changes versus Exercise Capacity Changes on Health-Related Factors following a Lifestyle Intervention in Employees with Metabolic Syndrome. Nutrients, 14(21), 4560. https://doi.org/10.3390/nu14214560






