Effect of Tetragonia tetragonoides (Pall.) Kuntze Extract on Andropause Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation from NZS Leaves
2.2. Animal Study
2.3. Measure of Muscular Endurance
2.3.1. Grip Force Test
2.3.2. Forced Swim Test
2.4. Biochemical Blood Testing
2.5. Measurement of Andropause Symptoms-Related Indicators Using ELISA
2.6. PTAH Staining of Muscle Tissues
2.7. Statistical Analysis
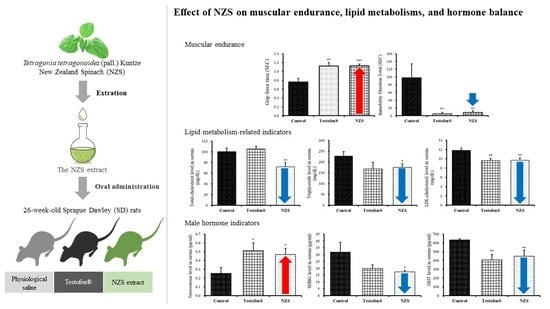
3. Results
3.1. Measure of Muscular Endurance
3.2. Effect of NZS Extract on Lipid Metabolism-Related Indicators
3.3. Effect of NZS Extract on Male Hormone Indicators
3.4. Effect of NZS Extract on LH and FSH
3.5. Effect of NZS Extract on ALT, AST, and PSA as Stability Indicators
3.6. PTAH Staining of Muscle Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matsumoto, A.M. Clinical implications of the decline in serum testosterone level with aging in men. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, 76–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midzak, A.S.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 2009, 299, 23–31. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Ekstrand, B. Regulation of 3β-hydroxysteroid dehydrogenase and sulphotransferase 2A1 gene expression in primary porcine hepatocytes by selected sex-steroids and plant secondary metabolites from chicory (Cichorium intybus L.) and wormwood (Artemisia sp.). Gene 2014, 536, 53–58. [Google Scholar] [CrossRef]
- Behre, M.; Tammela, T.; Arver, S.; Tolrá, J.R.; Bonifacio, V.; Lamche, M.; Kelly, J.; Hiemeyer, F. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male 2012, 15, 198–207. [Google Scholar] [CrossRef]
- Rosen, R.C.; Araujo, A.B.; Connor, M.K.; Gerstenberger, E.P.; Morgentaler, A.; Seftel, A.D.; Miner, M.M.; Shabsigh, R. The NERI Hypogonadism Screener: Psychometric validation in male patients and controls. Clin. Endocrinol. 2011, 74, 248–256. [Google Scholar] [CrossRef]
- Osterber, E.C.; Bernie, A.M.; Ramasamy, R. Risks of testosterone replacement therapy in men. Indian J. Urol. 2014, 30, 2–7. [Google Scholar] [CrossRef]
- Jarvis, T.R.; Chughtai, B.; Kaplan, S.A. Testosteron and benign prostatic hyperplasia. Asian J. Androl. 2015, 17, 212–216. [Google Scholar]
- Taylor, C.M. Revision of Tetragonia (Aizoaceae) in South America. Syst. Bot. 1994, 19, 575–589. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Ion relations of plants under drought and salinity. Funct. Plant Biol. 1986, 13, 75–91. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Atzori, G.; Nissim, W.; Macchiavelli, T.; Vita, F.; Azzarello, E.; Pandolfi, C.; Masi, E.; Mancuso, S. Tetragonia tetragonioides (Pallas) Kuntz. as promising salt-tolerant crop in a saline agricultural context. Agric. Water Manag. 2020, 240, 106261. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Caparrotta, S.; Masi, E.; Atzori, G.; Diamanti, I.; Azzarello, E.; Mancuso, S.; Pandolfi, C. Growing spinach (Spinacia oleracea) with different seawater concentrations: Effects on fresh, boiled and steamed leaves. Sci. Hortic. 2019, 256, 108540. [Google Scholar] [CrossRef]
- Jaworska, G. Content of nitrates, nitrites, and oxalates in New Zealand spinach. Food Chem. 2005, 89, 235–242. [Google Scholar] [CrossRef]
- Kato, M.; Takeda, T.; Ogihara, Y.; Shimizu, M.; Nomura, T.; Tomita, Y. Studies on the structure of polysaccharide from Tetragonia tetragonoides. I. Chem. Pharm. Bull. 1985, 33, 3675–3680. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.A.; Choi, H.J.; Kang, J.S.; Choi, Y.W.; Joo, W.H. Antioxidant activities of the solvent extracts from Tetragonia tetragonioides. J. Life Sci. 2008, 18, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Kang, J.S.; Choi, Y.W.; Jeong, Y.K.; Joo, W.H. Inhibitory activity on the diabetes related enzymes of Tetragonia tetragonioides. KSBB J. 2008, 23, 419–424. [Google Scholar]
- Lee, Y.G.; Lee, H.; Ryuk, J.A.; Hwang, J.T.; Kim, H.G.; Lee, D.S.; Kim, Y.J.; Yang, D.C.; Ko, B.S.; Baek, N.I. 6-Methoxyflavonols from the aerial parts of Tetragonia tetragonoides (Pall.) Kuntze and their anti-inflammatory activity. Bioorganic. Chem. 2019, 88, 102922. [Google Scholar] [CrossRef]
- Choi, H.S.; Cho, J.Y.; Kim, S.J.; Ham, K.S.; Moon, J.H. New lignan tyramide, phenolics, megastigmanes, and their glucosides from aerial parts of New Zealand spinach, Tetragonia tetragonoides. Food Sci. Biotechnol. 2020, 29, 599–608. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.H.; Yuk, H.J.; Lee, G.J.; Kim, D.S. Tetragonia tetragonoides (Pall.) Kuntze (New Zealand Spinach) prevents obesity and hyperuricemia in high-fat diet-induced obese mice. Nutrients 2018, 10, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryuk, J.A.; Ko, B.S.; Lee, H.W.; Kim, D.S.; Kang, S.; Lee, Y.H.; Park, S.M. Tetragonia tetragonioides (Pall.) Kuntze protects estrogen-deficient rats against disturbances of energy and glucose metabolism and decreases proinflammatory cytokines. Exp. Biol. Med. 2017, 242, 593–605. [Google Scholar] [CrossRef]
- Shin, Y.S.; You, J.H.; Cha, J.S.; Park, J.K. The relationship between serum total testosterone and free testosterone levels with serum hemoglobin and hematocrit levels: A study in 1221 men. Aging Male 2016, 19, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Cha, K.M.; Kim, D.H.; Kim, H.J.; Koo, N.Y.; Oh, S.H. Ameliorating effect of Trigonella foenum-graecum seed extract on andropause symptoms via increased testosterone. J. Korean Soc. Food Sci. Nutr. 2019, 48, 161–169. [Google Scholar] [CrossRef]
- Fujiwara, M.; Iwata, M.; Inoue, T.; Aizawa, Y.; Yoshito, N.; Hayashi, K.; Suzuki, S. Decreased grip strength, muscle pain, and atrophy occur in rats following long-term exposure to excessive repetitive motion. FEBS Open Bio 2017, 7, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, P.P.; Aquino, R.T.R.; Paschoalotti, M.A.; Ribeiro, M.C.S.A.; Nasello, A.G. Muscular strength decrease in Rattus norvegicus experimentally infected by Toxocara canis. Rev. Inst. Med. Trop. São Paulo 2009, 51, 73–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slattery, D.; Cryan, J. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Edston, E.; Grontoft, L.; Johnsson, J. TUNEL: A useful screening method in sudden cardiac death. Int. J. Legal Med. 2002, 116, 22–26. [Google Scholar] [CrossRef]
- Fernández-Miró, M.; Chillarón, J.J.; Pedro-Botet, J. Testosterone deficiency, metabolic syndrome and diabetes mellitus. Med. Clin. 2016, 146, 69–73. [Google Scholar] [CrossRef]
- McLachlan, R.I.; O’Donnell, L.; Meachem, S.J.; Stanton, P.G.; De Kretser, D.M.; Pratis, K.; Robertson, D.M. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog. Horm. Res. 2002, 57, 149–179. [Google Scholar] [CrossRef]
- JéGOU, B.; Pineau, C. Paracrine regulation of spermatogenesis: The virtue of dialogue. Treb. Soc. Catalana Biol. 2007, 56, 33–46. [Google Scholar] [CrossRef]
- Gowali, F.M. Picro-hibiscin stain for degenerated muscle fibers. Lab. Med. 1995, 26, 470–473. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Jorge, A.P.; Horst, H.; de Sousa, E.; Pizzolatti, M.G.; Silva, F.R. Insulinomimetic effects of kaempferitrin on glycaemia and on 14c-glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2004, 149, 89–96. [Google Scholar] [CrossRef]
- Varshney, R.; Mishra, R.; Das, N.; Sircar, D.; Roy, P. A comparative analysis of various flavonoids in the regulation of obesity and diabetes: An in vitro and in vivo study. J. Funct. Foods 2019, 59, 194–205. [Google Scholar] [CrossRef]
- Kovac, J.R.; Pan, M.M.; Lipshultz, L.I.; Lamb, D.J. Current state of practice regarding testosterone supplementation therapy in men with prostate cancer. Steroids 2014, 89, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the lipid-modifying and antiinflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef]
- Rabiei, Z.; Rafieian-Kopaei, M.; Mokhtari, S.; Shahrani, M. Effect of dietary ethanolic extract of Lavandula officinalis on serum lipids profile in rats. Iran. J. Pharm. Res. 2014, 13, 1295. [Google Scholar]
- Mifsud, A.; Choon, A.T.; Fang, D.; Yong, E. Prostate-specific antigen, testosterone, sexhormone binding globulin and androgen receptor CAG repeat polymorphisms in subfertile and normal men. Mol. Hum. Reprod. 2001, 7, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Kim, T.S.; Park, C.K.; Lee, S.H.; Kim, J.M.; Lee, K.S.; Lee, I.K.; Park, J.W.; Lawson, M.A.; Lee, D.S. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J. Mol. Endocrinol. 2013, 50, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Bratt, O.; Lilja, H. Serum markers in prostate cancer detection. Curr. Opin. Urol. 2015, 25, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutkowski, D.M.; Goode, R.L.; Baniel, J.; Teater, C.; Cohen, P.; McNulty, A.M.; Hsiung, H.M.; Becker, G.W.; Neubauer, B.L. Growth regulation of prostatic stromal cells by prostate-specific antigen. J. Natl. Cancer Inst. 1999, 91, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M.; Maes, M.; Vanhaechke, T.; Rogiers, V. Drug-induced liver injury: Mechanisms, types and biomarkers. Curr. Med. Chem. 2013, 20, 3011–3021. [Google Scholar] [CrossRef]
- Dufau, M.L.; Winters, C.A.; Hattori, M.; Aquilano, D.; Baranao, J.L.S.; Nozu, K.; Baukal, A.; Catt, K.J. Hormonal regulation of androgen production by the Leydig cell. J. Steroid Biochem. Mol. Biol. 1984, 20, 161–173. [Google Scholar] [CrossRef]
- Payne, A.H.; Youngblood, G.L. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol. Reprod. 1995, 52, 217–225. [Google Scholar] [CrossRef]
- Wayne, C.M.; Fan, H.Y.; Cheng, X.; Richards, J.S. Follicle-stimulating hormone induces multiple signaling cascades: Evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol. Endocrinol. 2007, 21, 1940–1957. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.Y.; Kim, S.-H.; Yang, W.-K.; Lee, G.-J. Effect of Tetragonia tetragonoides (Pall.) Kuntze Extract on Andropause Symptoms. Nutrients 2022, 14, 4572. https://doi.org/10.3390/nu14214572
Lee KY, Kim S-H, Yang W-K, Lee G-J. Effect of Tetragonia tetragonoides (Pall.) Kuntze Extract on Andropause Symptoms. Nutrients. 2022; 14(21):4572. https://doi.org/10.3390/nu14214572
Chicago/Turabian StyleLee, Ka Youn, Seung-Hyung Kim, Won-Kyung Yang, and Geung-Joo Lee. 2022. "Effect of Tetragonia tetragonoides (Pall.) Kuntze Extract on Andropause Symptoms" Nutrients 14, no. 21: 4572. https://doi.org/10.3390/nu14214572
APA StyleLee, K. Y., Kim, S. -H., Yang, W. -K., & Lee, G. -J. (2022). Effect of Tetragonia tetragonoides (Pall.) Kuntze Extract on Andropause Symptoms. Nutrients, 14(21), 4572. https://doi.org/10.3390/nu14214572