IgE-Binding and Immunostimulating Properties of Enzymatic Crosslinked Milk Proteins as Influenced by Food Matrix and Digestibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crosslinking of Milk Proteins
2.2. Electrophoresis SDS-PAGE
2.3. Human IgE Binding by Inhibition ELISA
2.4. Bone Marrow Derived Mast Cells
2.5. Cell Viability of Mice Splenocytes
2.6. Cytokine Secretion of Mice Splenocytes
2.7. In Vitro Gastrointestinal Digestion
2.8. RP-HPLC
2.9. Statistical Analysis
3. Results and Discussion
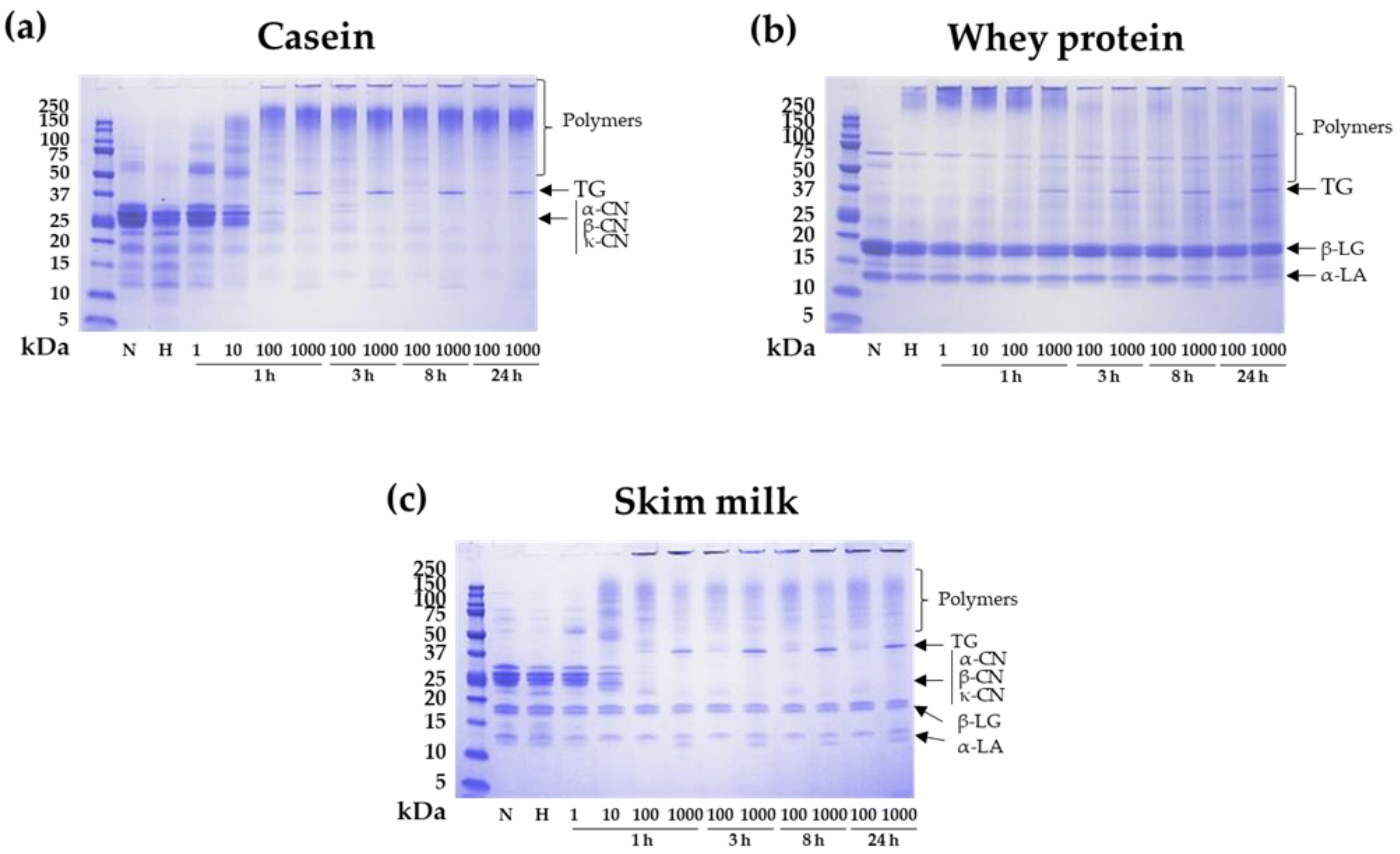
3.1. Protein Polymerization
3.2. Influence of Crosslinking on IgE-Binding Capacity
3.3. Immunostimulating Properties of Crosslinked Milk Proteins
3.4. Impact of Crosslinking on Digestibility of Milk Proteins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- McGregor, R.; Poppitt, S.D. Milk proteins and human health. In Milk Proteins: From Expression to Food; Singh, H., Boland, M., Thompson, A., Eds.; Academic Press: London, UK; Waltham, MA, USA; San Diego, CA, USA, 2014; pp. 651–669. [Google Scholar]
- Vassilopoulou, E.; Christoforou, C.; Andreou, E.; Heraclides, A. Effects of food allergy on the dietary habits and intake of primary schools’ Cypriot children. Eur. Ann Allergy Clin. Immnunol. 2017, 49, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Koubaa, M.; Barba, F.J.; Greiner, R.; George, S.; Roohinejad, S. Recent advances in the application of microbial transglutaminase crosslinking in cheese and ice cream products: A review. Int. J. Biol. Macromol. 2017, 107, 2364–2374. [Google Scholar] [CrossRef]
- Sabadin, I.S.; Villas-Boas, M.B.; de Lima Zollner, R.; Netto, F.M. Effect of combined treatment of hydrolysis and polymerization with transglutaminase on β-lactoglobulin antigenicity. Eur. Food Res. Technol. 2012, 235, 801–809. [Google Scholar] [CrossRef]
- Damodaran, S.; Li, Y. A two-step enzymatic modification method to reduce immuno-reactivity of milk proteins. Food Chem. 2017, 237, 724–732. [Google Scholar] [CrossRef]
- Olivier, C.E.; Lima, R.P.; Pinto, D.G.; Santos, R.A.; Silva, G.K.; Lorena, S.L.; Villas-Boas, M.B.; Netto, F.M.; de Lima Zollner, R. In search of a tolerance-induction strategy for cow’s milk allergies: Significant reduction of beta-lactoglobulin allergenicity via transglutaminase/cysteine polymerization. Clinics 2012, 67, 1171–1179. [Google Scholar] [CrossRef]
- Van Esch, B.C.; Gros-van Hest, M.; Westerbeek, H.; Garssen, J. Sensitizing capacity and allergenicity of enzymatically cross-linked sodium caseinate in comparison to sodium caseinate in a mouse model for CMA. Toxicol. Lett. 2013, 218, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, M.; Pieters, R.; Smit, J.; Cirkovic Velickovic, T. Cross-linking of β-lactoglobulin enhances allergic sensitization through changes in cellular uptake and processing. Toxicol. Sci. 2014, 140, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosulski, F.W.; Imafidon, G.I. Amino-acid-composition and nitrogen-to-protein conversion factors for animal and plant foods. J. Agric. Food Chem. 1990, 38, 1351–1356. [Google Scholar] [CrossRef]
- Jiménez-Sáiz, R.; Martos, G.; Carrillo, W.; López-Fandiño, R.; Molina, E. Susceptibility of lysozyme to in-vitro digestion and immunoreactivity of its digests. Food Chem. 2011, 127, 1719–1726. [Google Scholar] [CrossRef] [Green Version]
- Benedé, S.; Cody, E.; Agashe, C.; Berin, M.C. Immune characterization of bone marrow-derived models of mucosal and connective tissue mast cells. Allergy Asthma Clin. Immnunol. Res. 2018, 10, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pablos-Tanarro, A.; Lopez-Expósito, I.; Lozano-Ojalvo, D.; López-Fandiño, R.; Molina, E. Antibody production, anaphylactic signs, and T-cell responses induced by oral sensitization with ovalbumin in BALB/c and C3H/HeOuJ mice. Allergy Asthma Immnunol. Res. 2015, 8, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedé, S.; Berin, M.C. Mast cell heterogeneity underlies different manifestations of food allergy in mice. PLoS ONE 2018, 13, e0190453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedé, S.; Lopez-Exposito, I.; Gimenez, G.; Grishina, G.; Bardina, L.; Sampson, H.A.; Lopez-Fandiño, R.; Molina, E. Mapping of IgE epitopes in in vitro gastroduodenal digests of beta-lactoglobulin produced with human and simulated fluids. Food Res. Int. 2014, 62, 1127–1133. [Google Scholar] [CrossRef]
- Özrenk, E. The use of transglutaminase in dairy products. Int. J. Dairy Technol. 2006, 59, 1–7. [Google Scholar] [CrossRef]
- Hsieh, J.F.; Pan, P.H. Proteomic profiling of microbial transglutaminase-induced polymerization of milk proteins. J. Dairy Sci. 2012, 95, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Villas-Boas, M.B.; Vieira, K.P.; Trevizan, G.; Zollner, R.D.; Netto, F.M. The effect of transglutaminase-induced polymerization in the presence of cysteine on b-lactoglobulin antigenicity. Int. Dairy J. 2010, 20, 386–392. [Google Scholar] [CrossRef]
- Fotschki, J.; Wróblewska, B.; Fotschki, B.; Kalicki, B.; Rigby, N.; Mackie, A. Microbial transglutaminase alters the immunogenic potential and cross-reactivity of horse and cow milk proteins. J. Dairy Sci. 2019, 103, 2153–2166. [Google Scholar] [CrossRef]
- Duarte, N.M.; Cruz, A.L.; Silva, D.C.; Cruz, G.M. Intake of whey isolate supplement and muscle mass gains in young healthy adults when combined with resistance training: A blinded randomized clinical trial (pilot study). J. Sport. Med. Phys. Fit. 2020, 60, 75–84. [Google Scholar] [CrossRef]
- Ma, X.; Lozano-Ojalvo, D.; Chen, H.; Lopez-Fandiño, R.; Molina, E. Effect of high pressure-assisted crosslinking of ovalbumin and egg white by transglutaminase on their potential allergenicity. Innov. Food Sci. Emerg. Technol. 2015, 29, 143–150. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, C.; Xue, W.; Wang, Z. Crosslinked recombinant-Ara h 1 catalyzed by microbial transglutaminase: Preparation, structural characterization and allergic assessment. Foods 2020, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ni, S.; Wang, C.; Wang, Y. Transglutaminase-catalysed cross-linking eliminates Penaeus chinensis tropomyosin allergenicity by altering protein structure. Food Agric. Immnunol. 2019, 30, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Leszczyńska, J.; Łącka, A.; Bryszewska, M. The use of transglutaminase in the reduction of immunoreactivity of wheat flour. Food Agric. Immnunol. 2006, 17, 105–113. [Google Scholar] [CrossRef]
- Meng, S.; Tan, Y.; Chang, S.; Li, J.; Maleki, S.; Puppala, N. Peanut allergen reduction and functional property improvement by means of enzymatic hydrolysis and transglutaminase crosslinking. Food Chem. 2020, 302, 125186. [Google Scholar] [CrossRef]
- Palosuo, K.; Varjonen, E.; Nurkkala, J.; Kalkkinen, N.; Harvima, R.; Reunala, T.; Alenius, H. Transglutaminase-mediated cross-linking of a peptic fraction of ω-5 gliadin enhances IgE reactivity in wheatdependent, exercise-induced anaphylaxis. J. Allergy Clin. Immnunol. 2003, 111, 1386–1392. [Google Scholar] [CrossRef]
- Nakamura, R.; Nakamura, R.; Sakai, S.; Urisu, A.; Fukutomi, Y.; Teshima, R. Tissue transglutaminase generates deamidated epitopes on gluten, increasing reactivity with hydrolyzed wheat protein-sensitized IgE. J. Allergy Clin. Immnunol. 2013, 132, 1436–1438. [Google Scholar] [CrossRef]
- Benedé, S.; Blázquez, A.B.; Chiang, D.; Tordesillas, L.; Berin, M.C. The rise of food allergy: Environmental factors and emerging treatments. EBioMedicine 2016, 7, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Stanic, D.; Monogioudi, E.; Ercili, D.; Radosavljevic, J.; Atanaskovic-Markovic, M.; Vuckovic, O.; Raija, L.; Mattinen, M.; Buchert, J.; Cirkovic Velickovic, T. Digestibility and allergenicity assessment of enzymatically crosslinked β-casein. Mol. Nutr. Food Res. 2010, 54, 1273–1284. [Google Scholar] [CrossRef]
- Olivier, C.E.; Villas-Boas, M.B.; Netto, F.M.; Zollner, R.D.L. Allergenicity of Bos d 5 in children with cow’s milk allergyis reduced by transglutaminase polymerization. Pediatr. Allergy Immunol. Pulmonol. 2012, 25, 30–33. [Google Scholar] [CrossRef]
- Villas-Boas, M.B.; Benedé, S.; Zollner, R.D.; Netto, F.M.; Molina, E. Epitope resistance to the simulated gastrointestinal digestion of beta-lactoglobulin submitted to two-step enzymatic modification. Food Res. Int. 2015, 72, 191–197. [Google Scholar] [CrossRef]
- Bai, J.; Hui, J.; Lu, Q.; Yang, A.; Yuan, J.; Gao, J.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Effect of transglutaminase cross-linking on the allergenicity of tofu based on a BALB/c mouse model. Food Func. 2020, 11, 404–413. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.F.V.; Venzke, J.G.; Rosa, R.M.; Henriques, J.A.P.; Dallegrave, E.; Flôres, S.H.; Ayub, M.A.Z. Toxicological evaluation for food applications of transglutaminase from a newly isolated Bacillus circulans BL32. Am. J. Food Technol. 2011, 6, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.; Bøgh, K.L.; Dupont, D.; Egger, L.; Gadermaier, G.; Larré, C.; Mackie, A.; Menard, O.; Adel-Patient, K.; Picariello, G.; et al. The relevance of a digestibility evaluation in the allergenicity risk assessment of novel proteins. Opinion of a joint initiative of COST action ImpARAS and COST action INFOGEST. Food Chem. Toxicol. 2019, 129, 405–423. [Google Scholar] [CrossRef]
- Havenaar, R.; de Jong, A.; Koenen, M.E.; van Bilsen, J.; Janssen, A.M.; Labij, E.; Westerbeek, H.J. Digestibility of transglutaminase cross-linked caseinate versus native caseinate in an in vitro multicompartmental model simulating young child and adult gastrointestinal conditions. J. Agric. Food Chem. 2013, 61, 7636–7644. [Google Scholar] [CrossRef]
- Macierzanka, A.; Böttger, F.; Lansonneur, L.; Groizard, R.; Jean, A.S.; Rigby, N.M.; Cross, K.; Wellner, N.; Mackie, A.R. The effect of gel structure on the kinetics of simulated gastrointestinal digestion of bovine β-lg. Food Chem. 2012, 134, 2156–2163. [Google Scholar] [CrossRef]





| Serum | IgE (kU/L) | |||
|---|---|---|---|---|
| CN | α-LA | β-LG | Cow’s Milk | |
| 1 | 40.0 | 12.1 | 8.3 | 48.2 |
| 2 | 16.1 | 5.3 | 7.8 | 23.2 |
| 3 | <100 | <100 | <100 | <100 |
| 4 | 73.2 | 29.8 | 5.0 | 54.1 |
| 5 | 20.5 | 19.2 | 7.6 | 20.1 |
| 6 | >100 | >100 | >100 | >100 |
| 7 | 62.7 | >100 | >100 | >100 |
| Undigested | Gastric | Duodenal | ||||
|---|---|---|---|---|---|---|
| −TG | +TG | −TG | +TG | −TG | +TG | |
| Casein | 0.11 | 0.79 | 35.50 | 4.96 | 289.23 | 21.19 |
| (+/−0.01) | (+/−0.15) | (+/−5.81) | (+/−0.42) | (+/−14.48) | (+/−3.92) | |
| Whey protein | 0.74 | 0.41 | 1042.59 | 87.33 | 1236.17 | 49.45 |
| (+/−0.03) | (+/−0.01) | (+/−107.98) | (+/−10.17) | (+/−112.38) | (+/−7.05) | |
| Skim milk | 0.39 | 0.72 | 99.26 | 23.81 | 31,470.27 | 9188.28 |
| (+/−0.01) | (+/−0.06) | (+/−12.32) | (+/−3.48) | (+/−3567.51) | (+/−442.36) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedé, S.; Martínez-Blanco, M.; López-Fandiño, R.; Molina, E. IgE-Binding and Immunostimulating Properties of Enzymatic Crosslinked Milk Proteins as Influenced by Food Matrix and Digestibility. Nutrients 2022, 14, 4584. https://doi.org/10.3390/nu14214584
Benedé S, Martínez-Blanco M, López-Fandiño R, Molina E. IgE-Binding and Immunostimulating Properties of Enzymatic Crosslinked Milk Proteins as Influenced by Food Matrix and Digestibility. Nutrients. 2022; 14(21):4584. https://doi.org/10.3390/nu14214584
Chicago/Turabian StyleBenedé, Sara, Mónica Martínez-Blanco, Rosina López-Fandiño, and Elena Molina. 2022. "IgE-Binding and Immunostimulating Properties of Enzymatic Crosslinked Milk Proteins as Influenced by Food Matrix and Digestibility" Nutrients 14, no. 21: 4584. https://doi.org/10.3390/nu14214584





