Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narodowy Fundusz Zdrowia. NFZ o Zdrowiu. Nadciśnienie Tętnicze; Narodowy Fundusz Zdrowia: Warsaw, Poland, 2019. [Google Scholar]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). Blood Press. 2018, 27, 314–340. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Barricarte, A.; Ardanaz, E.; Amiano, P.; Dorronsoro, M.; Larrañaga, N.; et al. Estimation of Dietary Sources and Flavonoid Intake in a Spanish Adult Population (EPIC-Spain). J. Am. Diet. Assoc. 2010, 110, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Sampson, L.; Rimm, E.; Hollma, P.C.H.; de Vries, J.H.M.; Katan, M.B. Flavonol and Flavone Intakes in US Health Professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Fedirko, V.; Santucci de Magistris, M.; Ericson, U.; Amiano, P.; Trichopoulou, A.; et al. Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24 hour dietary recall cohort. Br. J. Nutr. 2011, 106, 1915–1925. [Google Scholar] [CrossRef]
- Cassidy, A.; O’Reilly, É.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with Quercetin Markedly Increases Plasma Quercetin Concentration without Effect on Selected Risk Factors for Heart Disease in Healthy Subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Fornal, E. The Impact of Flavonols on Cardiovascular Risk. Nutrients 2022, 14, 1973. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, M.; Moreno, L.; Vera, R.; Cogolludo, A.; Duarte, J.; Tamargo, J.; Perez-Vizcaino, F. Effects of the Flavonoid Quercetin and its Methylated Metabolite Isorhamnetin in Isolated Arteries from Spontaneously Hypertensive Rats. Planta Med. 2003, 69, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yao, R.; Liu, Y.; Wang, Z.; Huang, Z.; Du, B.; Zhang, D.; Wu, L.; Xiao, L.; Zhang, Y. Isorhamnetin protects against cardiac hypertrophy through blocking PI3K–AKT pathway. Mol. Cell. Biochem. 2017, 429, 167–177. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Torres, N.; Gutiérrez-Uribe, J.A.; Noriega, L.G.; Torre-Villalvazo, I.; Leal-Díaz, A.M.; Antunes-Ricardo, M.; Márquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.A.; et al. The effect of isorhamnetin glycosides extracted from Opuntia ficus-indica in a mouse model of diet induced obesity. Food Funct. 2015, 6, 805–815. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Guardado-Félix, D.; Rocha-Pizaña, M.R.; Garza-Martínez, J.; Acevedo-Pacheco, L.; Gutiérrez-Uribe, J.A.; Villela-Castrejón, J.; López-Pacheco, F.; Serna-Saldívar, S.O. Opuntia ficus-indica Extract and Isorhamnetin-3-O-Glucosyl-Rhamnoside Diminish Tumor Growth of Colon Cancer Cells Xenografted in Immune-Suppressed Mice through the Activation of Apoptosis Intrinsic Pathway. Plant Foods Hum. Nutr. 2021, 76, 434–441. [Google Scholar] [CrossRef]
- Hansen, L.; Dragsted, L.O.; Olsen, A.; Christensen, J.; Tjønneland, A.; Schmidt, E.B.; Overvad, K. Fruit and vegetable intake and risk of acute coronary syndrome. Br. J. Nutr. 2010, 104, 248–255. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; Van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Fornal, E. Dietary Isorhamnetin Intake Is Inversely Associated with Coronary Artery Disease Occurrence in Polish Adults. Int. J. Environ. Res. Public Health 2022, 19, 12546. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Kardiol. Pol. 2019, 77, 71–159. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Galindo, P.; Rodriguez-Gómez, I.; González-Manzano, S.; Dueñas, M.; Jiménez, R.; Menéndez, C.; Vargas, F.; Tamargo, J.; Santos-Buelga, C.; Pérez-Vizcaíno, F.; et al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS ONE 2012, 7, e32673. [Google Scholar] [CrossRef]
- Cogolludo, A.; Frazziano, G.; Briones, A.M.; Cobeño, L.; Moreno, L.; Lodi, F.; Salaices, M.; Tamargo, J.; Perez-Vizcaino, F. The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc. Res. 2007, 73, 424–431. [Google Scholar] [CrossRef]
- Hussain, F.; Jahan, N.; Rahman, K.-U.; Sultana, B.; Jamil, S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their Angiotensin-Converting Enzyme (ACE) inhibition potential. Oxid. Med. Cell. Longev. 2018, 2018, 4643736. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar] [CrossRef]
- Kondratiuk, V.E.; Synytsia, Y.P. Effect of quercetin on the echocardiographic parameters of left ventricular diastolic function in patients with gout and essential hypertension. Wiad. Lek. 2018, 71, 1554–1559. [Google Scholar]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hertog, M.G.; Hollman, P.C.; Hertog, M.G.; Katan, M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Kalus, U.; Pindur, G.; Jung, F.; Mayer, B.; Radtke, H.; Bachmann, K.; Mrowietz, C.; Koscielny, J.; Kiesewetter, H. Influence of the onion as an essential ingredient of the mediterranean diet on arterial blood pressure and blood fluidity. Arzneimittelforschung 2000, 50, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.I.; Sapinho, D.; De Bree, A.; Arnault, N.; Bertrais, S.; Galan, P.; Hercberg, S. Consumption of Foods Rich in Flavonoids Is Related to A Decreased Cardiovascular Risk in Apparently Healthy French Women. J. Nutr. 2004, 134, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.; Sakai, Y.; Okamura, Y.; Yamamoto, Y. Effects of boiling on the antihypertensive and antioxidant activities of onion. J. Nutr. Sci. Vitaminol. 2004, 50, 171–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
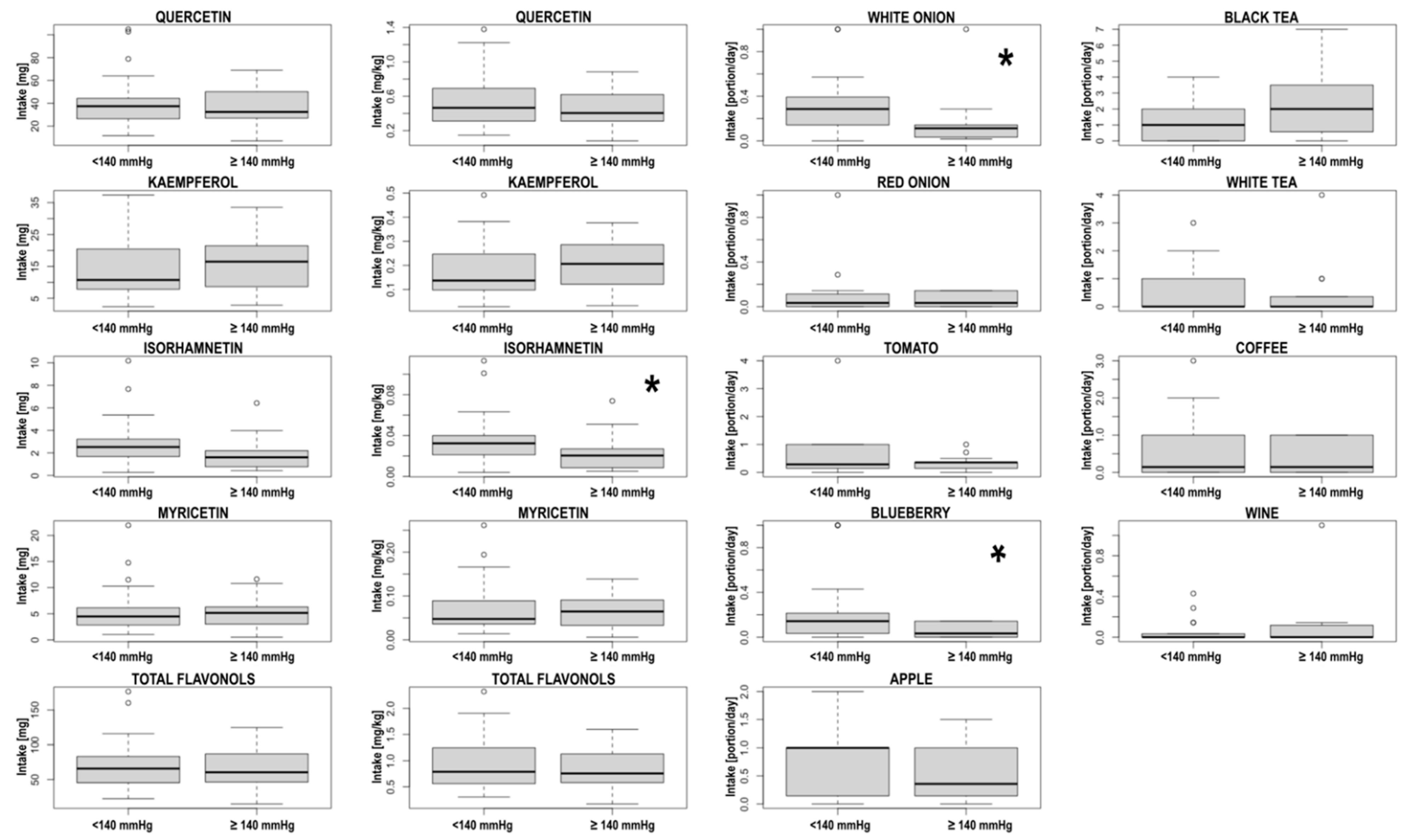
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| R | 95% CI | p | R | 95% CI | p | |
| Quercetin daily intake | −0.23 | −0.506; 0.087 | 0.15 | 0.05 | −0.264; 0.357 | 0.75 |
| Kaempferol daily intake | 0.02 | −0.292; 0.331 | 0.89 | 0.19 | −0.125; 0.477 | 0.23 |
| Isorhamnetin daily intake | −0.36 | −0.602; −0.052 | 0.02 | 0.05 | −0.263; 0.359 | 0.74 |
| Myricetin daily intake | −0.08 | −0.379; 0.241 | 0.64 | 0.09 | −0.230; 0.388 | 0.59 |
| Total flavonol daily intake | −0.18 | −0.462; 0.143 | 0.28 | 0.10 | −0.222; 0.396 | 0.55 |
| Quercetin daily intake/body mass | −0.28 | −0.542; 0.037 | 0.08 | 0.04 | −0.272; 0.350 | 0.79 |
| Kaempferol daily intake/body mass | −0.05 | −0.354; 0.268 | 0.77 | 0.18 | −0.138; 0.466 | 0.26 |
| Isorhamnetin daily intake/body mass | −0.38 | −0.617; −0.076 | 0.02 | 0.07 | −0.250; 0.370 | 0.68 |
| Myricetin daily intake/body mass | −0.13 | −0.426; 0.187 | 0.41 | 0.07 | −0.247; 0.374 | 0.67 |
| Total flavonol daily intake/body mass | −0.23 | −0.503; 0.091 | 0.16 | 0.09 | −0.232; 0.387 | 0.60 |
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| R | 95% CI | p | R | 95% CI | p | |
| Quercetin daily intake | −0.32 | −0.661; 0.128 | 0.16 | −0.14 | −0.542; 0.307 | 0.53 |
| Kaempferol daily intake | 0.11 | −0.340; 0.515 | 0.64 | 0.01 | −0.422; 0.441 | 0.96 |
| Isorhamnetin daily intake | −0.65 | −0.844; −0.302 | 0.001 | −0.12 | −0.515; 0.340 | 0.64 |
| Myricetin daily intake | −0.06 | −0.478; 0.383 | 0.80 | −0.11 | −0.518; 0.336 | 0.63 |
| Total flavonol daily intake | −0.27 | −0.626; 0.187 | 0.24 | −0.13 | −0.530; 0.323 | 0.58 |
| Quercetin daily intake/body mass | −0.27 | −0.628; 0.183 | 0.24 | −0.08 | −0.497; 0.361 | 0.72 |
| Kaempferol daily intake/body mass | 0.16 | −0.292; 0.554 | 0.49 | 0.11 | −0.338; 0.517 | 0.63 |
| Isorhamnetin daily intake/body mass | −0.63 | −0.837; −0.280 | 0.002 | −0.07 | −0.491; 0.369 | 0.75 |
| Myricetin daily intake/body mass | −0.03 | −0.457; 0.405 | 0.89 | −0.08 | −0.491; 0.369 | 0.75 |
| Total flavonol daily intake/body mass | −0.21 | −0.585; 0.248 | 0.37 | −0.05 | −0.470; 0.392 | 0.84 |
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| R | 95% CI | p | R | 95% CI | p | |
| Quercetin daily intake | −0.17 | −0.580; 0.308 | 0.49 | 0.30 | −0.180; 0.663 | 0.21 |
| Kaempferol daily intake | −0.09 | −0.521; 0.381 | 0.72 | 0.34 | −0.136; 0.687 | 0.16 |
| Isorhamnetin daily intake | 0.01 | −0.447; 0.461 | 0.97 | 0.34 | −0.130; 0.691 | 0.15 |
| Myricetin daily intake | −0.14 | −0.556; 0.339 | 0.58 | 0.37 | −0.101; 0.706 | 0.12 |
| Total flavonol daily intake | −0.14 | −0.556; 0.339 | 0.58 | 0.32 | −0.157; 0676 | 0.18 |
| Quercetin daily intake / body mass | −0.27 | −0.643; 0.213 | 0.27 | 0.27 | −0.206; 0.647 | 0.26 |
| Kaempferol daily intake / body mass | −0.19 | −0.591; 0.292 | 0.44 | 0.30 | −0.179; 0.664 | 0.21 |
| Isorhamnetin daily intake / body mass | −0.06 | −0.502; −0.404 | 0.80 | 0.37 | −0.105; 0.703 | 0.12 |
| Myricetin daily intake / body mass | −0.23 | −0.618; 0.252 | 0.35 | 0.32 | −0.161; 0.674 | 0.19 |
| Total flavonol daily intake / body mass | −0.24 | −0.625; 0.242 | 0.33 | 0.29 | −0.192; 0.656 | 0.23 |
| Systolic Blood Pressure | Diastolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| p | 95% CI | R | p | 95% CI | R | |
| White onion | 0.01 | −0.624; −0.088 | −0.39 | 0.87 | −0.335; 0.288 | −0.03 |
| Red onion | 0.15 | 0.508; 0.084 | −0.23 | 0.34 | −0.166; 0.444 | 0.15 |
| Onion (total) | 0.02 | −0.616; −0.073 | −0.38 | 0.76 | −0.265; 0.357 | 0.05 |
| Tomatoes | 0.31 | −0.454; 0.153 | −0.17 | 0.04 | −0.581; −0.020 | −0.33 |
| Blueberry | 0.21 | −0.483; 0.116 | −0.20 | 0.79 | −0.349; 0.273 | −0.04 |
| Apples | 0.39 | −0.431; 0.181 | −0.14 | 0.68 | −0.371; 0.249 | −0.07 |
| Black tea | 0.58 | −0.228; 0.391 | 0.09 | 0.22 | −0.121; 0.480 | 0.20 |
| Green tea | 0.57 | −0.393; 0.225 | −0.09 | 0.25 | −0.132; 0.472 | 0.19 |
| Coffee | 0.97 | −0.306; 0.317 | 0.01 | 0.96 | −0.318; 0.305 | −0.01 |
| Wine | 0.89 | −0.337; 0.294 | −0.02 | 0.52 | −0.215; 0.409 | 0.11 |
| Systolic Blood Pressure | |||||
|---|---|---|---|---|---|
| <140 mmHg | ≥140 mmHg | ||||
| Mean | SD | Mean | SD | p | |
| Quercetin [mg/day] | 42.02 | ±24.81 | 36.73 | ±17.24 | 0.61 |
| Kaempferol [mg/day] | 13.91 | ±8.66 | 16.14 | ±8.24 | 0.39 |
| Isorhamnetin [mg/day] | 2.88 | ±2.20 | 1.90 | ±1.53 | 0.08 |
| Myricetin [mg/day] | 5.66 | ±4.80 | 5.39 | ±3.02 | 0.59 |
| Total flavonols [mg/day] | 72.09 | ±39.77 | 67.57 | ±30.94 | 0.94 |
| Quercetin [mg/kg*day] | 0.55 | ±0.33 | 0.45 | ±0.22 | 0.55 |
| Kaempferol [mg/kg*day] | 0.18 | ±0.12 | 0.20 | ±0.10 | 0.52 |
| Isorhamnetin [mg/kg*day] | 0.04 | ±0.03 | 0.02 | ±0.02 | 0.048 |
| Myricetin [mg/kg*day] | 0.07 | ±0.06 | 0.07 | ±0.04 | 0.94 |
| Total flavonols [mg/kg*day] | 0.94 | ± 0.54 | 0.83 | ±0.40 | 0.68 |
| White onion [portion/day] | 0.31 | ±0.25 | 0.17 | ±0.25 | 0.01 |
| Red onion [portion/day] | 0.09 | ±0.21 | 0.07 | ±0.06 | 0.51 |
| Tomatoes [portion/day] | 0.58 | ±0.82 | 0.34 | ±0.27 | 0.64 |
| Blueberries [portion/day] | 0.24 | ±0.31 | 0.06 | ±0.06 | 0.04 |
| Apples [portion/day] | 0.68 | ±0.51 | 0.56 | ±0.47 | 0.65 |
| Black tea [portion/day] | 1.26 | ±1.22 | 2.27 | ±2.03 | 0.10 |
| Green tea [portion/day] | 0.49 | ±0.77 | 0.45 | ±1.08 | 0.44 |
| Coffee [portion/day] | 0.76 | ±0.88 | 0.39 | ±0.47 | 0.37 |
| Wine [portion/day] | 0.05 | ±0.10 | 0.11 | ±0.29 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiolek-Kalisz, J.; Blaszczak, P.; Fornal, E. Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients. Nutrients 2022, 14, 4586. https://doi.org/10.3390/nu14214586
Popiolek-Kalisz J, Blaszczak P, Fornal E. Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients. Nutrients. 2022; 14(21):4586. https://doi.org/10.3390/nu14214586
Chicago/Turabian StylePopiolek-Kalisz, Joanna, Piotr Blaszczak, and Emilia Fornal. 2022. "Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients" Nutrients 14, no. 21: 4586. https://doi.org/10.3390/nu14214586
APA StylePopiolek-Kalisz, J., Blaszczak, P., & Fornal, E. (2022). Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients. Nutrients, 14(21), 4586. https://doi.org/10.3390/nu14214586






