Select Dietary Supplement Ingredients for Preserving and Protecting the Immune System in Healthy Individuals: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scoping Review for Selection of Dietary Supplement Ingredients
2.2. Search Strategy and Selection Criteria for Systematic Review
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Echinacea
3.1.1. Outcomes
3.1.2. Adverse Events
3.1.3. Quality of the Evidence
3.2. Elderberry
3.2.1. Outcomes
3.2.2. Adverse Events
3.3. Garlic
3.3.1. Outcomes
3.3.2. Adverse Events
3.3.3. Other Studies
3.3.4. Quality of the Evidence
3.4. Vitamin A
3.4.1. Outcomes
3.4.2. Adverse Events
3.4.3. Quality of the Evidence
3.5. Vitamin C
3.5.1. Outcomes
3.5.2. Adverse Events
3.5.3. Quality of the Evidence
3.6. Vitamin D
3.6.1. Outcomes
3.6.2. Adverse Events
3.6.3. Quality of the Evidence
3.7. Vitamin E
3.7.1. Outcomes
3.7.2. Adverse Events
3.8. Zinc
3.8.1. Outcomes
3.8.2. Adverse Events
3.8.3. Quality of the Evidence
4. Discussion
Applicability to Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Brush, M. Immunity is everything: As the pandemic brings attention to the immune system, many question the attitudes and assumptions about it. Nutr. Bus. J. 2020, 6–9. [Google Scholar]
- Brush, M. Don’t talk about vaccines: Supplement brands wary to wade into the fray, for two big reasons. Nutr. Bus. J. 2021, 1–7. [Google Scholar]
- Brush, M. Amazon as crystal ball: Can natural brands and retailers use e-commerce to predict trends? Nutr. Bus. J. 2021, 13–15. [Google Scholar]
- Juntti, M. The whole pandemic package: Immunity is just the start as consumers scoop up supplements for sleep, stress and more. Nutr. Bus. J. 2020, 1–5. [Google Scholar]
- Food and Drug Administration. FDA 101: Dietary Supplements. Available online: https://www.fda.gov/consumers/consumer-updates/fda-101-dietary-supplements (accessed on 2 May 2022).
- Office of Dietary Supplements, NIH. Trans-NIH Resilience Working Group: Defining and Conceptualizing Resilience. Available online: https://ods.od.nih.gov/Research/resilience.aspx#defining (accessed on 30 September 2022).
- Nutrition Business Journal. Immune Health Special Report. 2020. Available online: https://store.newhope.com/products/immune-health-special-report (accessed on 30 September 2022).
- Rachul, C.; Marcon, A.R.; Collins, B.; Caulfield, T. COVID-19 and ‘immune boosting’ on the internet: A content analysis of Google search results. BMJ Open 2020, 10, e040989. [Google Scholar] [CrossRef] [PubMed]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary Supplements during COVID-19 Outbreak. Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients 2020, 13, 54. [Google Scholar] [CrossRef]
- Food and Drug Administration. Label Claims for Conventional Foods and Dietary Supplements. Available online: https://www.fda.gov/food/food-labeling-nutrition/label-claims-conventional-foods-and-dietary-supplements (accessed on 9 March 2021).
- Scottish Intercollegiate Guidelines Network. SIGN 50: A guideline developer’s handbook. Edinburgh 2015. Available online: https://www.sign.ac.uk/our-guidelines/sign-50-a-guideline-developers-handbook/ (accessed on 26 October 2022).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Murad, M.H.; Mustafa, R.A.; Schünemann, H.J.; Sultan, S.; Santesso, N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid.-Based Med. 2017, 22, 85–87. [Google Scholar] [CrossRef] [Green Version]
- Jawad, M.; Schoop, R.; Suter, A.; Klein, P.; Eccles, R. Safety and Efficacy Profile of Echinacea purpurea to Prevent common cold episodes: A randomized, double-blind, placebo-controlled trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 841315. [Google Scholar] [CrossRef] [Green Version]
- Ogal, M.; Johnston, S.L.; Klein, P.; Schoop, R. Echinacea reduces antibiotic usage in children through respiratory tract infection prevention: A randomized, blinded, controlled clinical trial. Eur. J. Med. Res. 2021, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.; Hughes, S.; Lourie, A.; Zweifler, J. Effects of echinacea on the frequency of upper respiratory tract symptoms: A randomized, double-blind, placebo-controlled trial. Ann. Allergy Asthma Immunol. 2008, 100, 384–388. [Google Scholar] [CrossRef]
- Sperber, S.J.; Shah, L.P.; Gilbert, R.D.; Ritchey, T.W.; Monto, A.S. Echinacea purpurea for prevention of experimental rhinovirus colds. Clin. Infect. Dis. 2004, 38, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Tiralongo, E.; Lea, R.A.; Wee, S.S.; Hanna, M.M.; Griffiths, L.R. Randomised, double blind, placebo-controlled trial of echinacea supplementation in air travelers. Evidence-Based Complement. Altern. Med. 2011, 2012, 417267. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.B.; Bauer, R.; Woelkart, K.; Hulsey, T.C.; Gangemi, J.D. An Evaluation of Echinacea angustifolia in Experimental Rhinovirus Infections. N. Engl. J. Med. 2005, 353, 341–348. [Google Scholar] [CrossRef]
- Tiralongo, E.; Wee, S.S.; Lea, R.A. Elderberry supplementation reduces cold duration and symptoms in air-travelers: A randomized, double-blind placebo-controlled clinical trial. Nutrients 2016, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Josling, P. Preventing the common cold with a garlic supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2001, 18, 189–193. [Google Scholar] [CrossRef]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef]
- Percival, S.S. Aged Garlic Extract modifies human immunity. J. Nutr. 2016, 146, 433S–436S. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Chen, X.-R.; Zhang, L.; Luo, H.-Y.; Gao, N.; Wang, J.; Fu, G.-Y.; Mao, M. Effect of simultaneous supplementation of vitamin A and iron on diarrheal and respiratory tract infection in preschool children in Chengdu City, China. Nutrition 2013, 29, 1197–1203. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kumar, R.; Ganguly, N.K.; Kumar, L.; Walia, B.N.S. Effect of vitamin A supplementation on childhood morbidity and mortality. Indian J. Med. Sci. 2002, 56, 259–264. [Google Scholar] [PubMed]
- Constantini, N.W.; Dubnov-Raz, G.; Eyal, B.-B.; Berry, E.M.; Cohen, A.H.; Hemilä, H. The effect of vitamin C on upper respiratory infections in adolescent swimmers: A randomized trial. Eur. J. Pediatr. 2010, 170, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Barkyoumb, G.M.; Schumacher, S.S.; Johnston, C.S.; Barkyoumb, G.M.; Schumacher, S.S. Vitamin C supplementation slightly improves physical activity levels and reduces cold incidence in men with marginal vitamin c status: A randomized controlled trial. Nutrients 2014, 6, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, M.; Josling, P. Preventing the common cold with a vitamin C supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2002, 19, 151–159. [Google Scholar] [CrossRef]
- Camargo, C.A.; Sluyter, J.; Stewart, A.W.; Khaw, K.-T.; Lawes, C.M.M.; Toop, L.; Waayer, D.; Scragg, R. Effect of monthly high-dose vitamin d supplementation on acute respiratory infections in older adults: A randomized controlled trial. Clin. Infect. Dis. 2020, 71, 311–317. [Google Scholar] [CrossRef]
- De Gruijl, F.R.; Pavel, S. The effects of a mid-winter 8-week course of sub-sunburn sunbed exposures on tanning, vitamin D status and colds. Photochem. Photobiol. Sci. 2012, 11, 1848–1854. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Hemilä, H.; Cohen, A.H.; Rinat, B.; Choleva, L.; Constantini, N.W.; Hemilae, H. Vitamin D supplementation and upper respiratory tract infections in adolescent swimmers: A randomized controlled trial. Pediatr. Exerc. Sci. 2015, 27, 113–119. [Google Scholar] [CrossRef]
- Ganmaa, D.; Uyanga, B.; Zhou, X.; Gantsetseg, G.; Delgerekh, B.; Enkhmaa, D.; Khulan, D.; Ariunzaya, S.; Sumiya, E.; Bolortuya, B.; et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N. Engl. J. Med. 2020, 383, 359–368. [Google Scholar] [CrossRef]
- Goodall, E.C.; Granados, A.C.; Luinstra, K.; Pullenayegum, E.; Coleman, B.L.; Loeb, M.; Smieja, M. Vitamin D3 and gargling for the prevention of upper respiratory tract infections: A randomized controlled trial. BMC Infect. Dis. 2014, 14, 273. [Google Scholar] [CrossRef] [Green Version]
- Harrison, S.E.; Oliver, S.J.; Kashi, D.S.; Carswell, A.T.; Edwards, J.P.; Wentz, L.M.; Roberts, R.; Tang, J.C.Y.; Izard, R.M.; Jackson, S.; et al. Influence of Vitamin D supplementation by simulated sunlight or oral D3 on respiratory infection during military training. Med. Sci. Sports Exerc. 2021, 53, 1505–1516. [Google Scholar] [CrossRef]
- Hauger, H.; Ritz, C.; Mortensen, C.; Mølgaard, C.; Metzdorff, S.B.; Frøkiær, H.; Damsgaard, C.T. Winter cholecalciferol supplementation at 55°N has little effect on markers of innate immune defense in healthy children aged 4–8 years: A secondary analysis from a randomized controlled trial. Eur. J. Nutr. 2019, 58, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Sollid, S.T.; Svartberg, J.; Joakimsen, R.M.; Grimnes, G.; Hutchinson, M.Y.S. Prevention of urinary tract infections with vitamin D supplementation 20,000 IU per week for five years. Results from an RCT including 511 subjects. Infect. Dis. 2016, 48, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.C.; Seo, M.-W.; Lee, S.; Kim, S.W.; Song, J.K. Vitamin D3 supplementation reduces the symptoms of upper respiratory tract infection during winter training in vitamin d-insufficient taekwondo athletes: A randomized controlled trial. Int. J. Environ. Res. Public Health 2018, 15, 2003. [Google Scholar] [CrossRef] [Green Version]
- Laaksi, I.; Ruohola, J.; Mattila, V.; Auvinen, A.; Ylikomi, T.; Pihlajamäki, H. Vitamin D supplementation for the prevention of acute respiratory tract infection: A randomized, double-blinded trial among young Finnish men. J. Infect. Dis. 2010, 202, 809–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li-Ng, M.; Aloia, J.F.; Pollack, S.; Cunha, B.A.; Mikhail, M.; Yeh, J.; Berbari, N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009, 137, 1396–1404. [Google Scholar] [CrossRef]
- Loeb, M.; Dang, A.D.; Thiem, V.D.; Thanabalan, V.; Wang, B.; Nguyen, N.B.; Tran, H.T.M.; Luong, T.M.; Singh, P.; Smieja, M.; et al. Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influ. Other Respir. Viruses 2019, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, C.; Damsgaard, C.T.; Hauger, H.; Ritz, C.; Lanham-New, S.A.; Smith, T.J.; Hennessy, Á.; Dowling, K.; Cashman, K.D.; Kiely, M.; et al. Estimation of the dietary requirement for vitamin D in white children aged 4–8 y: A randomized, controlled, dose-response trial. Am. J. Clin. Nutr. 2016, 104, 1310–1317. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, D.R.; Slow, S.; Chambers, S.T.; Jennings, L.C.; Stewart, A.W.; Priest, P.C.; Florkowski, C.M.; Livesey, J.H.; Camargo, C.A.; Scragg, R. Effect of Vitamin D3 supplementation on upper respiratory tract infections in healthy adults. JAMA 2012, 308, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Rees, J.R.; Hendricks, K.; Barry, E.L.; Peacock, J.L.; Mott, L.A.; Sandler, R.S.; Bresalier, R.; Goodman, M.; Bostick, R.M.; Baron, J.A. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin. Infect. Dis. 2013, 57, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R.; Waayer, D.; Stewart, A.W.; Lawes, C.M.M.; Toop, L.; Murphy, J.; Khaw, K.T.; Camargo, C.A., Jr. The Vitamin D Assessment (ViDA) Study: Design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J. Steroid Biochem. Mol. Biol. 2016, 164, 318–325. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ito, Y.; Yui, K.; Egawa, K.; Orimo, H. Intake of 25-hydroxyvitamin D3 reduces duration and severity of upper respiratory tract infection: A randomized, double-blind, placebo-controlled, parallel group comparison study. J. Nutr. Health Aging 2017, 22, 491–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.; Van Der Mei, I.; Stewart, N.; Blizzard, L.; Tettey, P.; Taylor, B. Weekly cholecalciferol supplementation results in significant reductions in infection risk among the vitamin D deficient: Results from the CIPRIS pilot RCT. BMC Nutr. 2015, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Urashima, M.; Mezawa, H.; Noya, M.; Camargo, C.A., Jr. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014, 5, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Graat, J.M.; Schouten, E.G.; Kok, F.J. Effect of Daily Vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons. JAMA 2002, 288, 715–721. [Google Scholar] [CrossRef]
- Van Amsterdam, J.; van der Horst-Graat, J.; Bischoff, E.; Steerenberg, P.; Opperhuizen, A.; Schouten, E. The Effect of Vitamin E supplementation on serum DHEA and neopterin levels in elderly subjects. Int. J. Vitam. Nutr. Res. 2005, 75, 327–331. [Google Scholar] [CrossRef]
- Kurugöl, Z.; Akilli, M.; Bayram, N.; Koturoglu, G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr. 2006, 95, 1175–1181. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Rerksuppaphol, S.; Rerksuppaphol, L. A randomized controlled trial of chelated zinc for prevention of the common cold in Thai school children. Paediatr. Int. Child Health 2013, 33, 145–150. [Google Scholar] [CrossRef]
- Richard, S.A.; Witzig, R.S.; Black, R.E.; Caulfield, L.E.; Zavaleta, N.; Shankar, A. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 75, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Vakili, R.; Vahedian, M.; Khodaei, G.; Mahmoudi, M. Effects of zinc supplementation in occurrence and duration of common cold in school aged children during cold season: A double-blind and placebo-controlled trial. Iran J. Pediatr. 2009, 19, 376–380. [Google Scholar]
- Veverka, D.V.; Wilson, C.; Martinez, M.A.; Wenger, R.; Tamosuinas, A. Use of zinc supplements to reduce upper respiratory infections in United States Air Force Academy Cadets. Complement. Ther. Clin. Pract. 2009, 15, 91–95. [Google Scholar] [CrossRef]
- Grebow, J. Will FDA Ever Provide a Practical Regulatory Path for Probiotics? Panel Discusses at NPA’s the Big Natural Conference. Nutritional Outlook 2020. Available online: https://www.nutritionaloutlook.com/view/will-fda-ever-provide-a-practical-regulatory-path-for-probiotics-panel-discusses-at-npa-s-the-big-natural-conference- (accessed on 25 October 2022).
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Sanislo, L.; Kuniakova, R.; Rennerova, Z.; Buchanec, J.; Banovcin, P. Imunoglukan P4H® in the prevention of recurrent respiratory infections in childhood. Cesk Pediatr. 2010, 65, 639–647. [Google Scholar]
- Laue, C.; Stevens, Y.; van Erp, M.; Papazova, E.; Soeth, E.; Pannenbeckers, A.; Stolte, E.; Böhm, R.; Gall, S.; Falourd, X.; et al. Adjuvant effect of orally applied preparations containing non-digestible polysaccharides on influenza vaccination in healthy seniors: A Double-blind, randomised, controlled pilot trial. Nutrients 2021, 13, 2683. [Google Scholar] [CrossRef]
- Karg, C.A.; Wang, P.; Vollmar, A.M.; Moser, S. Re-opening the stage for Echinacea research-Characterization of phylloxanthobilins as a novel anti-oxidative compound class in Echinacea purpurea. Phytomedicine 2019, 60, 152969. [Google Scholar] [CrossRef]
- Andrianova, I.V.; Sobenin, I.A.; Sereda, E.V.; Borodina, L.I.; Studenikin, M.I. Effect of long-acting garlic tablets “allicor” on the incidence of acute respiratory viral infections in children. Ter. Arkhiv 2003, 75, 53–56. [Google Scholar]
- Office of Dietary Supplements, NIH. Vitamin A Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 2 May 2022).
- Hemilä, H. Vitamin C supplementation and common cold symptoms: Factors affecting the magnitude of the benefit. Med. Hypotheses 1999, 52, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Abioye, A.I.; Bromage, S.; Fawzi, W. Effect of micronutrient supplements on influenza and other respiratory tract infections among adults: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e003176. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 2013, CD000980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D; National Academy Press: Washington, DC, USA, 2010. [Google Scholar]
- Brown, L.L.; Cohen, B.; Tabor, D.; Zappalà, G.; Maruvada, P.; Coates, P.M. The vitamin D paradox in Black Americans: A systems-based approach to investigating clinical practice, research, and public health-expert panel meeting report. BMC Proc. 2018, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Office of Dietary Supplements, NIH. Vitamin E Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 2 May 2022).
- David, S.; Cunningham, R. Echinacea for the prevention and treatment of upper respiratory tract infections: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 44, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Karsch-Völk, M.; Barrett, B.; Kiefer, D.; Bauer, R.; Ardjomand-Woelkart, K.; Linde, K. Echinacea for preventing and treating the common cold. Cochrane Database Syst. Rev. 2014, 2, CD000530. [Google Scholar] [CrossRef] [Green Version]
- Harnett, J.; Oakes, K.; Carè, J.; Leach, M.; Brown, D.; Cramer, H.; Pinder, T.-A.; Steel, A.; Anheyer, D. The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Adv. Integr. Med. 2020, 7, 240–246. [Google Scholar] [CrossRef]
- Wieland, L.S.; Piechotta, V.; Feinberg, T.; Ludeman, E.; Hutton, B.; Kanji, S.; Seely, D.; Garritty, C. Elderberry for prevention and treatment of viral respiratory illnesses: A systematic review. BMC Complement. Med. Ther. 2021, 21, 112. [Google Scholar] [CrossRef]
- Lissiman, E.; Bhasale, A.L.; Cohen, M. Garlic for the common cold. Cochrane Database Syst. Rev. 2014, 2020, CD006206. [Google Scholar] [CrossRef]
- Chen, H.; Zhuo, Q.; Yuan, W.; Wang, J.; Wu, T. Vitamin A for preventing acute lower respiratory tract infections in children up to seven years of age. Cochrane Database Syst. Rev. 2008, 23, CD006090. [Google Scholar] [CrossRef]
- Boerstra, B.V.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Elberink, H.O.; Schöll, I.P.; et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy 2021, 77, 1373–1388. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Wang, S.; Yang, L.; Xia, H.; Sun, G. Excessive Vitamin A supplementation increased the incidence of acute respiratory tract infections: A systematic review and meta-analysis. Nutrients 2022, 13, 4251. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-E.; Myung, S.-K.; Cho, H. Efficacy of Vitamin D supplements in prevention of acute respiratory infection: A meta-analysis for randomized controlled trials. Nutrients 2022, 14, 818. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, X.; Gu, L.; Zhan, Y.; Chen, L.; Li, X. Association between Vitamin D and influenza: Meta-analysis and systematic review of randomized controlled trials. Front. Nutr. 2021, 8, 799709. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Miller, E.R., III; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage Vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.; Arentz, S.; Goldenberg, J.; Yang, G.; Beardsley, J.; Myers, S.P.; Mertz, D.; Leeder, S. Zinc for the prevention or treatment of acute viral respiratory tract infections in adults: A rapid systematic review and meta-analysis of randomised controlled trials. BMJ Open 2021, 11, e047474. [Google Scholar] [CrossRef]
- Wang, M.X.; Win, S.S.; Pang, J. Zinc supplementation reduces common cold duration among healthy adults: A systematic review of randomized controlled trials with micronutrients supplementation. Am. J. Trop. Med. Hyg. 2020, 103, 86–99. [Google Scholar] [CrossRef]
- Crawford, C.; Avula, B.; Lindsey, A.T.; Walter, A.; Katragunta, K.; Khan, I.A.; Deuster, P.A. Analysis of select dietary supplement products marketed to support or boost the immune system. JAMA Netw. Open 2022, 5, e2226040. [Google Scholar] [CrossRef]
- Operation Supplement Safety Scorecard. Available online: https://www.opss.org/screen-your-supplement-safety-read-label-your-supplement-and-answer-these-questions (accessed on 4 March 2021).

| PICOTS | Eligibility Criteria |
|---|---|
| Populations | Otherwise healthy individuals who may or may not be experiencing a stressor, such as intense exercise, psychological stress, fatigue, air travel, sub-optimal environmental conditions, seasonal stressors such as winter time, school environments, a history of recurrent infections but not sick at the time, and exposure to a vaccination. Stressors were not pre-defined, but captured as they emerged naturally throughout the screen phase and tagged as discovered. To be consistent with the dietary intake recommendations, the authors chose to include children four years or older, or studies that included children with at least a mean age of four years, adults 18 years and older, and seniors, defined as 70 years and older but only including free living, non-institutionalized persons. Individuals with chronic conditions or taking medications were not considered eligible. |
| Dietary supplement ingredients | Dietary supplement ingredients selected from the market driven approach and delivered as a single ingredient dietary supplement product. For eligibility, the authors followed the Food and Drug Administration definition for dietary supplements. Nasal sprays and lozenges were not considered dietary supplements under this review. Studies including dietary supplements as a treatment were not eligible, but taken prophylactically, to preserve, protect or recover from, taken prior to any sign of infection or symptom occurring. If the participants included in a study were sick at the time of enrollment, those studies were not eligible. In addition, if the participants were taking other medications concurrently with the dietary supplement, then those studies were not eligible (e.g., asthma medications, other prescription drugs). Dose/amount of ingredient had to be reported in the publication to be included. |
| Control/ comparators | Placebo, waitlist, usual care. |
| Outcomes | Primary: Incidence of infection, severity, duration of symptoms, adverse events. Secondary: performance, general well-being, mood, quality of life, fatigue, sleep quality, psychological stress, dietary habits/changes, as well as biomarkers reported in conjunction with primary outcomes (e.g., C-reactive protein [CRP], Interleukins [ILs], Interferon [IFN], Tumor necrosis factor [TNF], etc.). |
| Timing | Not restricted. |
| Study designs | Efficacy: Randomized controlled trials; Additional information on safety: case reports, adverse event reporting databases and Natural Medicines adverse effects monographs. |
| No. Studies (Participants, Stressors) | Risk of Getting an Infection | Severity of Symptoms | Duration of Symptoms | Adverse Events | Research Considerations and Research Gaps |
|---|---|---|---|---|---|
| Various Echinacea products in varying amounts and duration of use, up to 4 months taken prophylactically | |||||
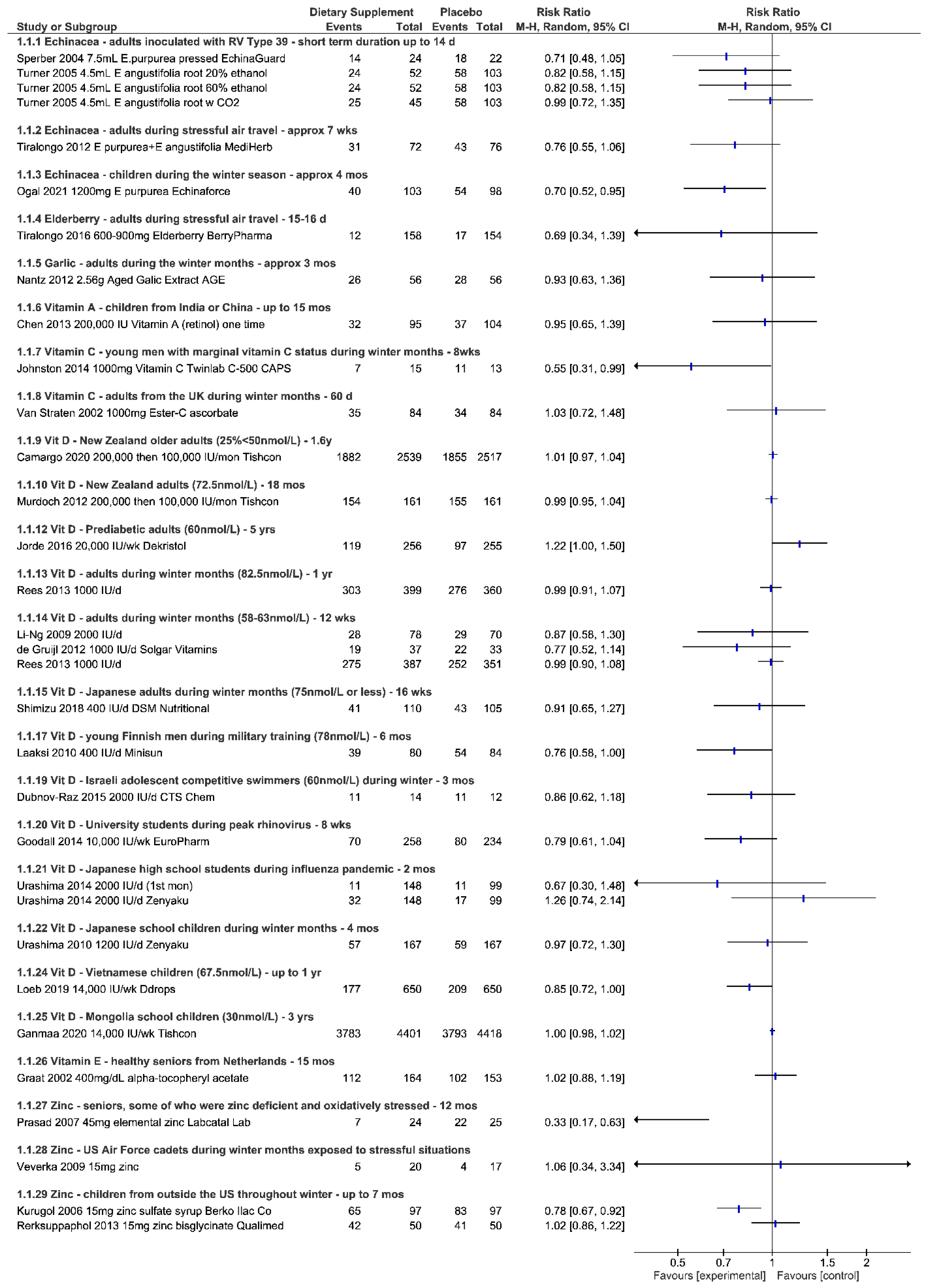
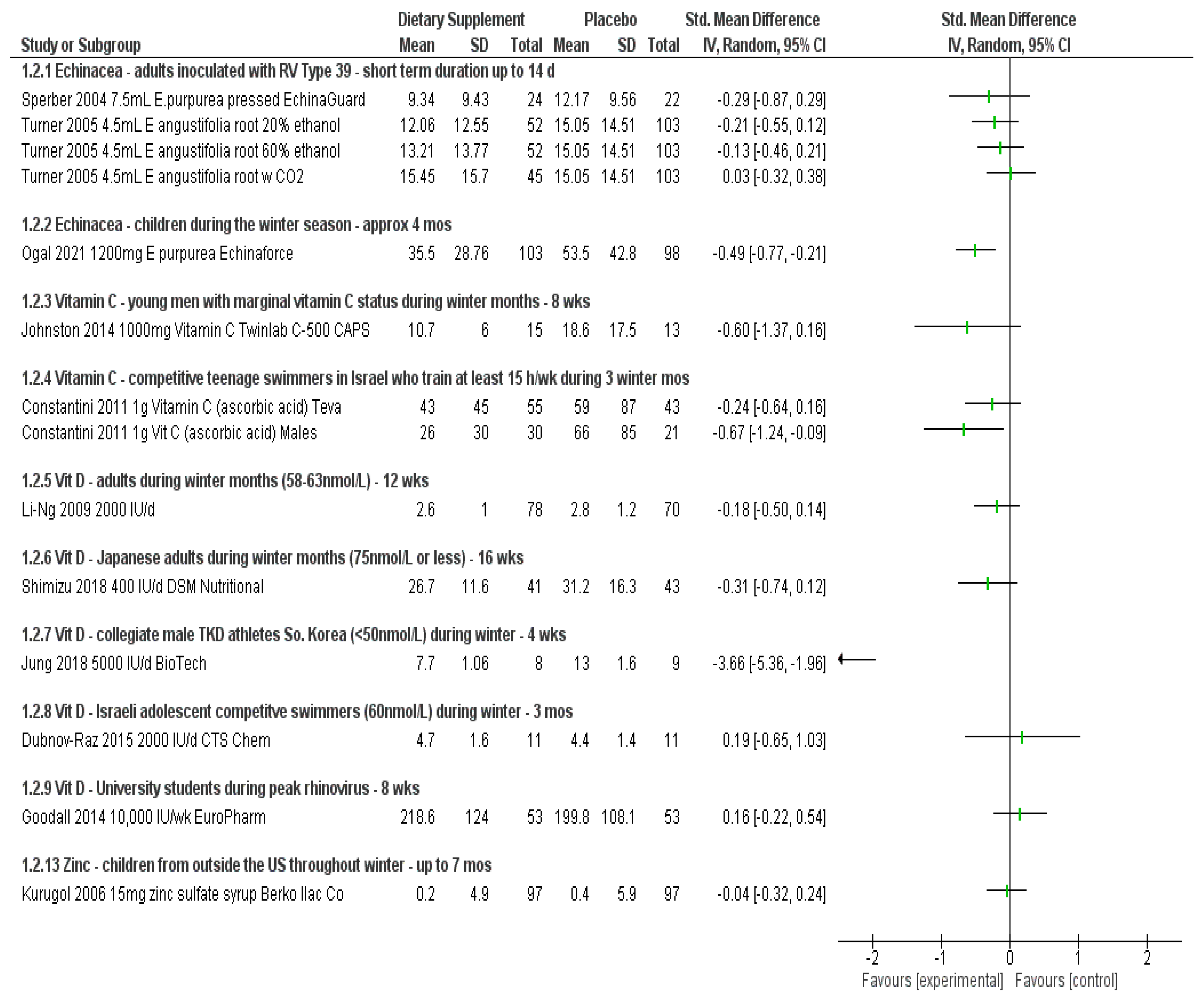
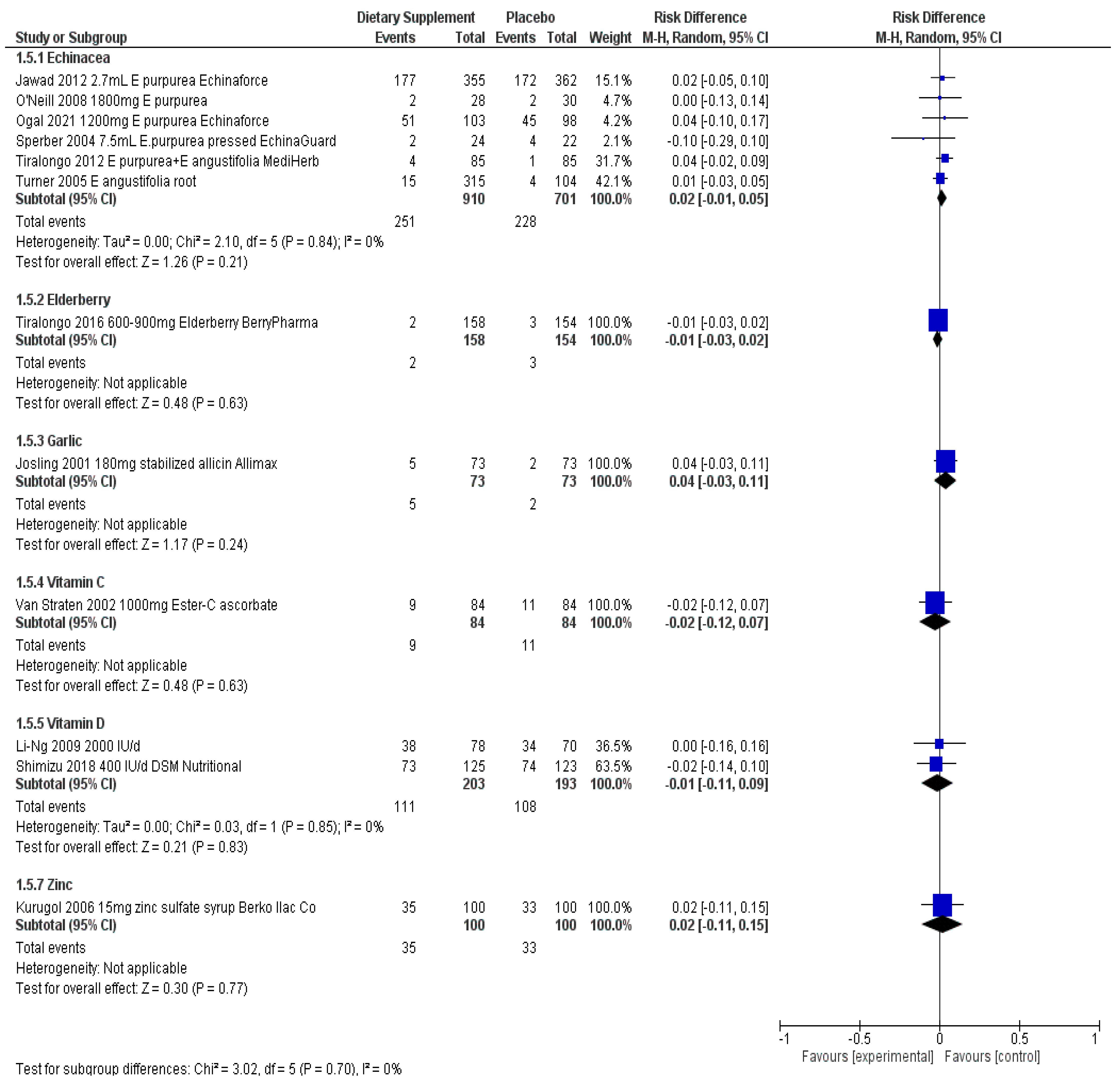
| 5 adult studies (n = 1505) Inoculation with virus (N = 2) [17,19], exposure to winter months (N = 2) [14,16], stressful air travel (N = 1) [18] | 3 studies consistently show less risk (RR 0.71 to RR 0.82; up to 29%) when taking Echinacea—none reach statistical significance on their own 2 studies did not report per person statistics | 2 studies show very small to no effect on the severity of symptoms—none reached statistical significance (SMD −0.29, 0.03) 2 studies describe less symptom severity for Echinacea group—borderline or no statistical significance | 2 studies describe reduction in duration of symptoms but not statistically significant | RD 0.02 (95% CI, −0.01, 0.05; p = 0.24, N = 5) | Studies show consistency in results for outcomes reported across studies and involving healthy adults exposed to various stressors. Echinacea products “appear” safe for short-term use. Two of the largest trials showed benefit for both adults and children taking EchinaForce over a four month period throughout winter months.
|
| 1 child study (n = 203) [15] During winter time season | RR 0.70 (95% CI, 0.52, 0.95; p = 0.023); up to 30% less risk when taking Echinacea | SMD −0.49 (95% CI, −0.77, −0.21; p = 0.001) | MD −1.4 days (95% CI, −2.39, −0.41; p = 0.008) | RD 0.04 (95% CI, −0.10, 0.17; p = 0.61, N = 1) | |
| Elderberry (Sambucus nigra L., Haschberg variety; BerryPharma Brand); 600 mg/day for 10 days before travel and 900 mg/day for 5 days (e.g., 1 day before leaving home until 4–5 days after arriving at the destination); 15–16 days total | |||||
| 1 adult study (n = 312) [20] During stressful air travel | RR 0.69 (95% CI, 0.34, 1.39; p = 0.298); up to 31% less risk when taking elderberry | the symptom score in the placebo group over these days was 583, whereas in the elderberry group it was 247 (p = 0.02) decreased the symptom load (mean 21 vs. 34) SD not provided | Number of episode days: T = 57; C = 117 (p = 0.05) On average, a 2 day shorter duration of the cold (4.75 vs. 6.88 d) | RD −0.01 (95% CI, −0.03, 0.02; p = 0.632, N = 1) | One study on the prophylactic use of Elderberry, showing decrease in severity and duration of symptoms. Elderberry “appears” safe if properly prepared.
|
| Various Garlic products in varying amounts taken throughout the cold and flu season, up to 90 days | |||||
| 2 adult studies (n = 266) [21,22] Throughout cold and flu season | 1 study showed the incidence of cold and flu was not statistically significant (RR 0.93 (95% CI, 0.63, 1.36; p = 0.71) Second study did not report per person statistics | Total number of episodes and total number of symptoms statistically significant in trials (p < 0.001) | Duration of days shown to be significant in one study (1.52 days in garlic vs. 5.01 days placebo (p < 0.001) Number of days in other trial not significant but the number of work days missed was (8 days vs. 19 placebo days (p < 0.035) | RD 0.04 (95% CI, −0.03, 0.11; p = 0.243, N = 1) | Two studies show that adult participants taking garlic supplements throughout the cold and flu season may experience less episodes and symptoms overall.
|
| Vitamin A (200,000 IU) taken once every four to six months, for a duration up to 15 months | |||||
| 2 child studies (n = 1719) Children from India [25] and China [24] that are less than 10 years old | Respiratory related illnesses RR 0.95 (95% CI 0.65, 1.39; p = 0.78), N = 1 study Incidence child/year for ARI T = 0.288; C = 0.361, N = 1 study | Number of respiratory related events from one study VAS 262 vs. 284 in placebo group Number of episodes in other study 88 VAS vs. 147 in placebo group | Average days experiencing respiratory related events, VAS 5.5 vs. 8.0 placebo group; SD not provided, N = 1 study | Not reported | Two studies conducted in countries with high prevalence of vitamin A deficiency, in children less than 10 years of age.
|
| Vitamin C 1000 mg/day taken prophylactically up to 90 days in duration throughout the winter months | |||||
| 3 studies (n = 237) adolescent competitive swimmers (N = 1) [26], adults in the UK (N = 1) [28], males with adequate-low plasma C levels (N = 1) [27] | 1 study in males showed up to 45% less risk of developing symptoms RR 0.55 (95% CI, 0.31, 0.99; p = 0.048) 1 study involving mostly females showed no difference RR 1.03 (95% CI, 0.72, 1.48; p = 0.88) in risk but less number of cold episodes overall. 1 study involving competitive adolescent swimmers no difference detected | Severity of symptoms reduced in two studies but not statistically significant In males alone the effect size was SMD > 0.60 (considered medium effect) in two studies, one reaching statistical significance for competitive swimmers | Duration of illness reduced in all three trials (MD > 1 day), not all statistically significant In males alone, up to 4.9 days less duration, reaching statistical significance in two trials | RD −0.02 (95% CI, −0.12, 0.07); p = 0.63, N = 1) | Three studies show some benefit of taking 1000 mg vitamin C per day throughout the winter months. Study involving males with adequate to low vitamin C levels show less risk of getting sick if taking vitamin C prophylactically during the winter months. Males and perhaps those exposed to intense exercise may benefit from less severe symptoms and reduced duration of illness. Vitamin C appears safe below the tolerable upper limit of 2 g/day.
|
| Various Vitamin D products in varying amounts, timing and duration of use, up to five years taken prophylactically | |||||
| 2 studies in adults [42] and seniors [29] (n = 5432, some with inadequate levels) 200,000 tapering to 100,000/month (3300 IU/d) Tishcon, up to 1.6 years | Two studies showed no significant reduction in risk of infection (RR 0.99, 1.01) and result remained unchanged when the analysis included winter season or baseline 25-OHD levels | One study reported No. episodes: T = 593; C = 611 and Severity per episode, median (IQR); T = 171 (86–295); C = 183 (97–316); p = 0.48 | One study reported Median (25th percentile, d) duration of symptoms per episode T = 12 (8), n = 366; C = 12 (7); n = 365; p = 0.76 and No. days missed work per episode T = 0.76 (1.25); C = 0.76 (1.26) p = 0.82 | Both reported vitamin D did not affect any reported adverse events | Two studies in adults and seniors regardless of vitamin D status taking monthly doses of vitamin D up to 1.6 years did not reduce the risk of infection. Weekly doses of vitamin D for university students during the winter showed some reduced risk of infection. Other 2 studies in adults with adequate levels did not. Children taking weekly doses in Vietnam showed reduced risk of infections but not for influenza alone while children in Mongolia with inadequate levels showed no effect for risk of infection over a longer time period. 4 studies in children taking daily vitamin D during the winter months did not show significantly reduced risk, severity or duration of illness. 3 studies involving either male during military training or taekwondo training show either less risk of infection or reduced symptom severity when taking daily vitamin D. Four studies in adults with mean adequate levels, taking daily vitamin D during the winter months showed no significant benefit in risk, severity or duration. Vitamin D appears safe when taken below the UL
|
| 3 adult studies (one involving university students) [33] (n = 1145) 10,000–20,000 IU/week (8 weeks, 16 weeks, 5 years) to include winter seasons [36,46] | Two studies showed no reduced risk Infection hazard (HR) for all URTI; T = 1.11 (0.75, 1.65); C = 1.00 (reference) p = 0.62, N = 1; RR 1.22 (95% CI, 1.00, 1.5; p = 0.055, N = 1 1 study with university students trended toward self-reported reduced risk (RR 0.79 (95% CI, 0.61, 1.04; p = 0.09) | One study reported No. episodes of RTI T = 36; C = 32 and Average daily total infection symptom severity: T = −1.08 (−3.00, 0.85); C = 5.28 (3.73, 6.82) p = 0.27 University students Complete case URTI episodes; T = 70; C = 80; p = 0.09; but no effect on symptom severity SMD 0.16 (95% CI, −0.22, 0.54; p = 0.406) | One study reported Infection duration (HR) for URTI T = 0.51 (−1.80, 2.81); C = 4.87 (3.29, 6.45); p = 0.67 University students MD −0.20 (95% CI, −0.76, 0.36; p = 0.480) | Similar in both groups, not reported or none occurred | |
| 2 child studies (n = 20,252) 14,000 IU/week up to 3 years [32,40] | Children in Vietnam (RR 0.85 (95% CI, 0.72, 1.00; p = 0.053, N = 1) Children in Mongolia with mean inadequate levels (RR 1.00 (95% CI, 0.98, 1.02; p = 0.888, N = 1) | None related to vitamin D | |||
| 4 child studies (n = 862) 400–2000 IU/day up to 5 months During winter months (one study involving adolescent competitive swimmers) [31,35,47,48] | Two studies show no reduction in risk RR 0.96, 1.26 One study reported increased risk in both groups but no difference bt groups (all p > 0.57) Competitive swimmers no significant less risk (RR 0.86 (95% CI, 0.62, 1.18; p = 0.349) | Competitive swimmers no difference in severity of symptoms SMD 0.19 (95% CI, −0.65, 1.03; p = 0.641) | One study reported Number of students absent T = 68/148; C = 38/99 and Mean absences, d; T = 1.7 (2.5); C = 1.1 (1.9); p = 0.14 Competitive swimmers no significant difference in days with illness MD −0.80 (95% CI, −2.41, 0.81; p = 0.329) | Studies either did not report on AE or reported none occurred | |
| 1 adult study in Finnish men during military training (400 IU/day; mean adequate levels), [38] 1 study in British army recruits (25% sufficient 1090 (4 weeks), 460 IU (8 weeks)) [34] and one study in So. Korean men during taekwondo training (5000 IU/day; mean inadequate levels) [37] up to 6 months (n = 440) | Finnish men during military training show less risk of infection (RR 0.76 (95% CI, 0.58, 1.00; p = 0.049) | Male TKD athletes Less symptom severity SMD −3.66 (95% CI, −5.36, −1.96; p = 0.000) British recruits: vitamin D supplementation reduced the severity of peak URTI symptoms by 15%; p < 0.05; N = 1 | Mean days absent from duty; T = 2.2 (3.2); C = 3.0 (4.0); p = 0.096, N = 1 British recruits: vitamin D supplementation reduced the days with URTI by 36% (p < 0.05); N = 1 | Not reported | |
| 4 adult studies (n = 1279) 400–2000 IU/day up to 1 year with mean adequate levels [30,39,43,45] Throughout winter months | No significant difference in four studies RR ranging from 0.77 to 0.99 | Severity of symptoms ranged from SMD −0.31 to −0.18, N = 2 considered small effect, not significant Mean episodes per person-month reported in another study not significant | Three studies reported duration in different ways, none significant days of illness per person-month T = 1.52 (2.98); C = 1.56 (2.87); adjusted RR 1.03 (0.81, 1.31), N = 1 MD 0.10 (95% CI, −1.51, 1.71; P = 0.902), N = 1 Median 10 days vs. 13 days p = 0.061, N = 1 | RD −0.01 (95% CI, −0.11, 0.09; p = 0.83) N = 2 | |
| Vitamin E 2 X 200 mg/dL of alpha-tocopheryl acetate per day for up to 15 months to include the winter months | |||||
| 1 study among seniors [49] (n = 652) noninstitutionalized individuals aged 60 years or older from Netherlands with overall healthy micronutrient status | A greater risk of experiencing an acute respiratory tract infection RR 1.02 (95% CI, 0.88, 1.19; p = 0.76, N = 1 study) | Number of symptoms, median (IQR) T = 5 (3–8); C = 4 (3–8) Number of episodes: T = 280; C = 230 | Total illness duration, days, median (IQR) T = 19 (10–30); C = 14 (6–30) | Not reported | One study showing a greater risk of experiencing an infection, greater episodes and illness duration overall.
|
| Various zinc formulations up to 30 mg/day in children and adults; in seniors up to 45 mg/day for up to 12 months duration to include the winter months | |||||
| 1 study involving US cadets during winter months exposed to stressors (n = 40) [56] | No difference in the risk of physician diagnosed infection RR 1.06 (95% CI, 0.34, 3.34; p = 0.917) | Number of symptom events: T = 135/238; C = 163/240 when comparing the subjects that had no self-report symptoms during the study to those with symptoms, subjects in the Zinc group appreciated more weeks without any symptoms (p = 0.01) | Not reported | One study in cadets exposed to winter months and physical/academic stressors taking 15 mg zinc experienced less symptom events overall.One study in seniors showed significantly less risk of infections in those taking 45 mg zinc throughout 12 month period. Three of the four studies report benefit for children taking zinc prophylactically throughout the winter months in countries outside the US, up to 20 mg/day. Zinc appears safe in amounts below the upper tolerable limit.
| |
| 4 studies involving children living outside the US throughout the winter months (n = 1355) [51,53,54,55] | One study showed less risk of developing symptoms when taking zinc RR 0.78; (95% CI, 0.67, 0.92; p = 0.003) One study did not show benefit for experiencing at least one symptom RR 1.02; (95% CI, 0.86, 1.22; p = 0.790) Two studies did not report per person but the average cold occurrence was statistically significant in favor of zinc supplement for one study | One study reported on the mean number of colds per study child: T: 1.2 + −1.4; C: 1.7 + −1.2; p = 0.003 but the severity of symptoms at day 5 was not significant SMD −0.04 (95% CI, −0.32, 0.24; p = 0.79) | Duration of symptoms reduced significantly in two studies: Duration of cold symptoms: T: 4.7 + −0.8; C: 5.3 + −0.7; MD −0.60 (95% CI, −0.78, −0.42; p = 0.0001); N = 1 Duration of at least 2 symptoms: median (IQR) T = 0.0 (0–1.0); C = 1.0 (0–5.3) p < 0.01; N = 1 Another study showed Days missing school: T = 0.55 +−1.09; C = 1.35 + −1.79; MD −0.80 (95% CI, −1.21, −0.39; p < 0.0001 | RD 0.02 (95% CI, −0.11, 0.15; p = 0.77, N = 1) | |
| One study in seniors some of which were zinc-deficient and oxidatively stressed (n = 50) [52] | Significantly less risk of experiencing an infection when taking zinc RR 0.33 (95% CI, 0.17, 0.63; p = 0.001) | Not reported | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, C.; Brown, L.L.; Costello, R.B.; Deuster, P.A. Select Dietary Supplement Ingredients for Preserving and Protecting the Immune System in Healthy Individuals: A Systematic Review. Nutrients 2022, 14, 4604. https://doi.org/10.3390/nu14214604
Crawford C, Brown LL, Costello RB, Deuster PA. Select Dietary Supplement Ingredients for Preserving and Protecting the Immune System in Healthy Individuals: A Systematic Review. Nutrients. 2022; 14(21):4604. https://doi.org/10.3390/nu14214604
Chicago/Turabian StyleCrawford, Cindy, LaVerne L. Brown, Rebecca B. Costello, and Patricia A. Deuster. 2022. "Select Dietary Supplement Ingredients for Preserving and Protecting the Immune System in Healthy Individuals: A Systematic Review" Nutrients 14, no. 21: 4604. https://doi.org/10.3390/nu14214604
APA StyleCrawford, C., Brown, L. L., Costello, R. B., & Deuster, P. A. (2022). Select Dietary Supplement Ingredients for Preserving and Protecting the Immune System in Healthy Individuals: A Systematic Review. Nutrients, 14(21), 4604. https://doi.org/10.3390/nu14214604






