Association of Zn and Cu Levels in Cord Blood and Maternal Milk with Pregnancy Outcomes among the Slovenian Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birth Cohort
2.2. Study Design
2.3. Questionnaire
2.4. Definition of Size for Gestational Age, Gestational Age
2.5. Collection of Cord Blood and Maternal Milk Samples
2.6. Measurement of Zn and Cu in Cord Blood
2.7. Measurement of Zn and Cu in Maternal Milk
2.8. Statistical Analysis
3. Results
3.1. Normality of Data Distribution
3.2. Association of Maternal Characteristics with Cu and Zn Levels in Cord Blood and Maternal Milk
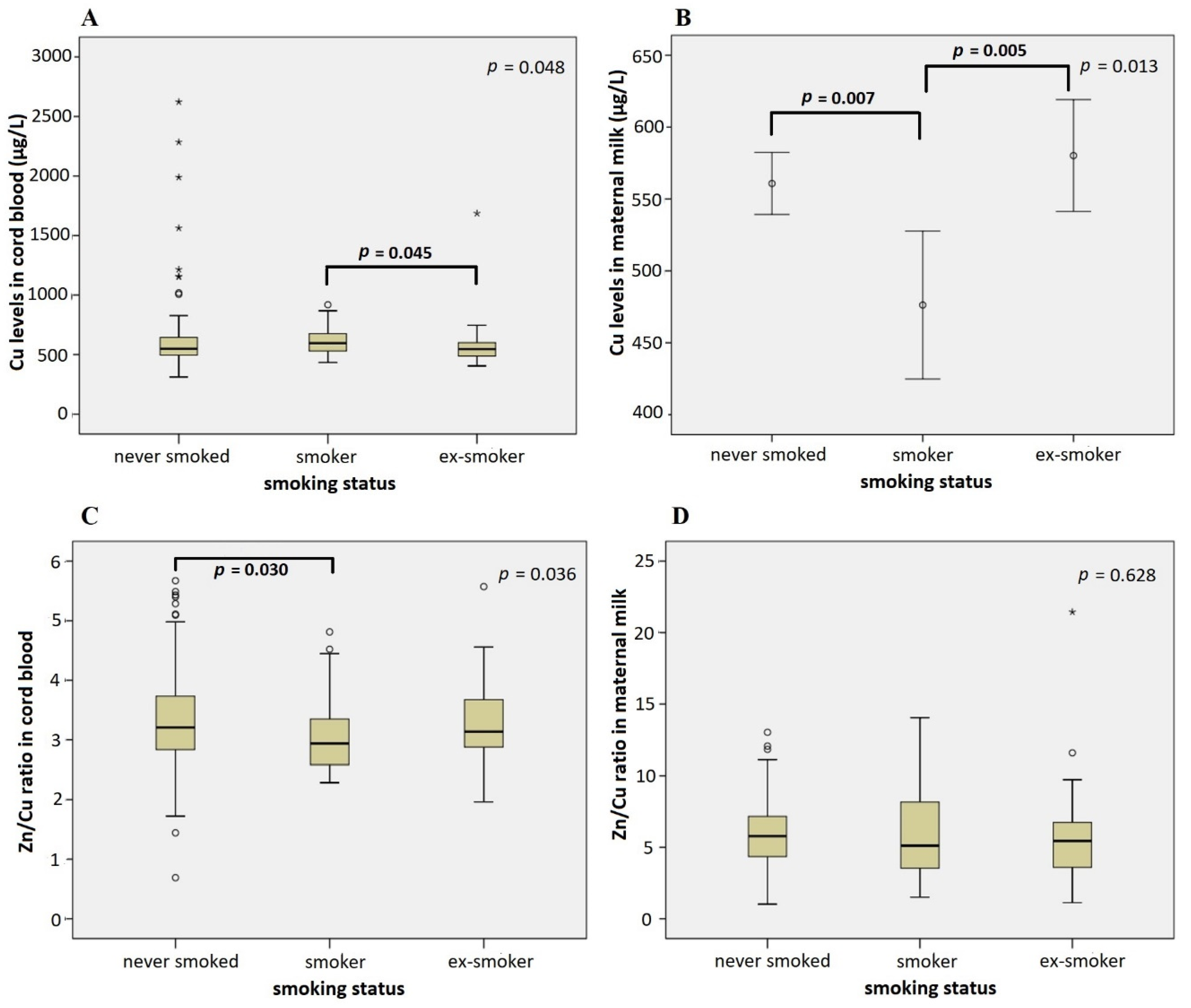
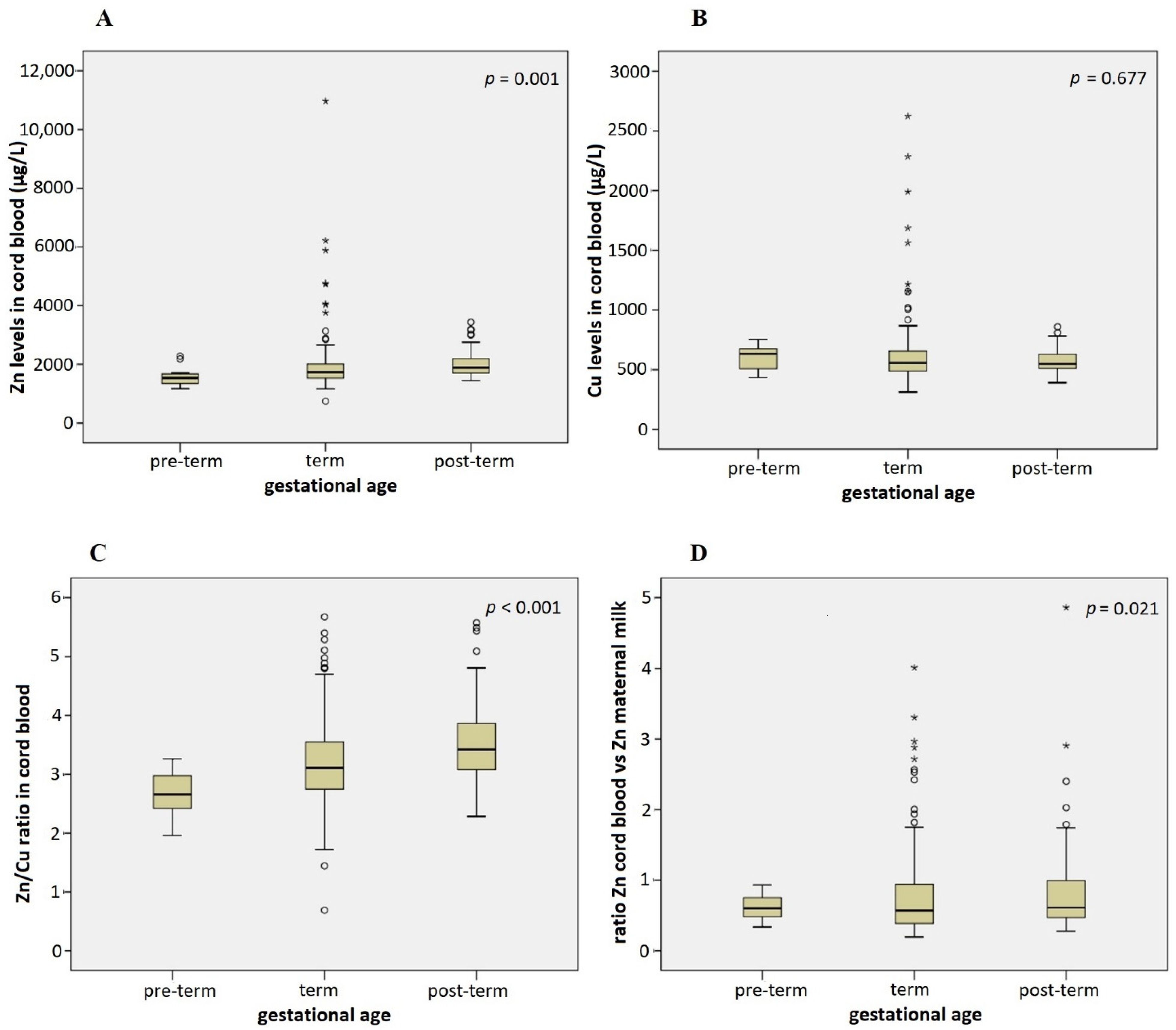
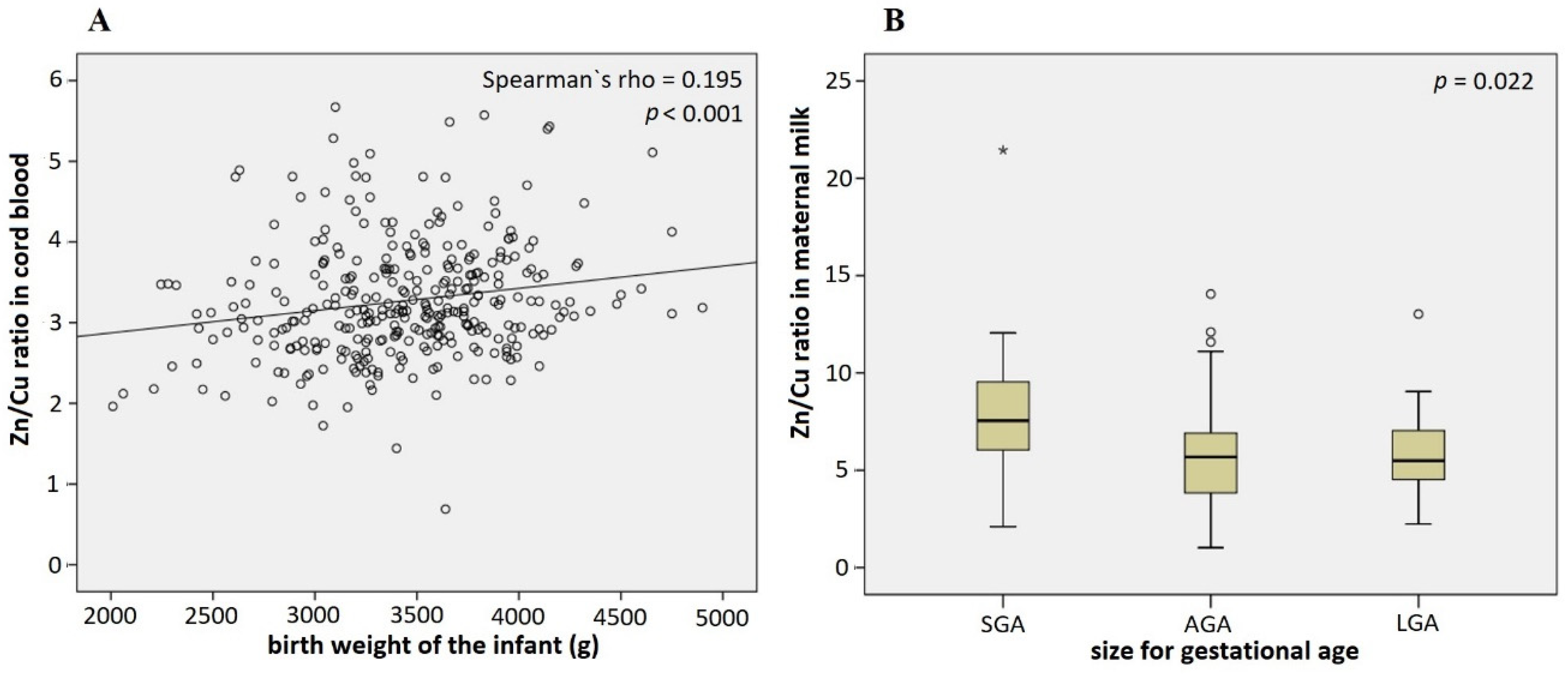
3.3. Association of Infant Characteristics and Pregnancy Outcomes with Cu and Zn Levels in Cord Blood and Maternal Milk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierzchała, O.O.M.; Bednarz, A.; Lenartowicz, M. Structure and function of proteins belonging to the CTR family-membrane transporters of the monovalent metal ions. Post. Epy. Biol. Komórki 2015, 42, 351–374. [Google Scholar]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host. Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serdar, Z.; Gur, E.; Develioglu, O. Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochem. Funct. 2006, 24, 209–215. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Aspects. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Classen, H.G.; Grober, U.; Low, D.; Schmidt, J.; Stracke, H. Zinc deficiency. Symptoms, causes, diagnosis and therapy. Med. Monatsschr. Pharm. 2011, 34, 87–95. [Google Scholar]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Sandstead, H.H. Understanding zinc: Recent observations and interpretations. J. Lab. Clin. Med. 1994, 124, 322–327. [Google Scholar]
- Stern, B.R.; Solioz, M.; Krewski, D.; Aggett, P.; Aw, T.C.; Baker, S.; Crump, K.; Dourson, M.; Haber, L.; Hertzberg, R.; et al. Copper and human health: Biochemistry, genetics, and strategies for modeling dose-response relationships. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10, 157–222. [Google Scholar] [CrossRef] [PubMed]
- Falchuk, K.H. The molecular basis for the role of zinc in developmental biology. Mol. Cell Biochem. 1998, 188, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, M.; Hackshaw, A.; Wald, N. Neural tube defects and serum zinc. Br. J. Obstet. Gynaecol. 1993, 100, 746–749. [Google Scholar] [CrossRef]
- Keen, C.L.; Uriu-Hare, J.Y.; Hawk, S.N.; A Jankowski, M.; Daston, G.P.; Kwik-Uribe, C.L.; Rucker, R.B. Effect of copper deficiency on prenatal development and pregnancy outcome. Am. J. Clin. Nutr. 1998, 67, 1003S–1011S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Record, I.R. Zinc deficiency and the developing embryo. NeuroToxicology 1987, 8, 369–378. [Google Scholar] [PubMed]
- Simmer, K.; Lort-Phillips, L.; James, C.; Thompson, R.P. A double-blind trial of zinc supplementation in pregnancy. Eur. J. Clin. Nutr. 1991, 45, 139–144. [Google Scholar]
- Wastney, M.E.; Angelus, P.; Barnes, R.M.; Subramanian, K.N. Zinc kinetics in preterm infants: A compartmental model based on stable isotope data. Am. J. Physiol. Integr. Comp. Physiol. 1996, 271, R1452–R1459. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F. Zinc in the Fetus and Neonate. In Fetal Neonatal Physiology; Elsevier Saunders: Philadelphia, PA, USA, 2004; pp. 342–347. [Google Scholar]
- Bax, C.M.R.; Bloxam, D.L. Two major pathways of zinc(II) acquisition by human placental syncytiotrophoblast. J. Cell. Physiol. 1995, 164, 546–554. [Google Scholar] [CrossRef]
- Sur, D.; Gupta, D.N.; Mondal, S.K.; Ghosh, S.; Manna, B.; Rajendran, K.; Bhattacharya, S.K. Impact of Zinc Supplementation on Diarrheal Morbidity and Growth Pattern of Low Birth Weight Infants in Kolkata, India: A Randomized, Double-Blind, Placebo-Controlled, Community-Based Study. Pediatrics 2003, 112, 1327–1332. [Google Scholar] [CrossRef]
- Terrin, G.; Canani, R.B.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; de Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef] [Green Version]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2015, 2015, CD000230. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Rossipal, E.; Krachler, M.; Li, F.; Micetic-Turk, D. Investigation of the transport of trace elements across barriers in humans: Studies of placental and mammary transfer. Acta Paediatr. 2000, 89, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Schramel, P.; Lill, G.; Hasse, S.; Klose, B.J. Mineral- and trace element concentrations in human breast milk, placenta, maternal blood, and the blood of the newborn. Biol. Trace Elem. Res. 1988, 16, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Krachler, M.; Rossipal, E.; Micetic-Turk, D. Trace element transfer from the mother to the newborn--investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr. 1999, 53, 486–494. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Ashworth, C.J. Micronutrients in fetal growth and development. Br. Med. Bull. 1999, 55, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Prohaska, J.R.; Thiele, D.J. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6842–6847. [Google Scholar] [CrossRef] [Green Version]
- Sharp, P.A. Ctr1 and its role in body copper homeostasis. Int. J. Biochem. Cell Biol. 2003, 35, 288–291. [Google Scholar] [CrossRef]
- Wee, N.K.; Weinstein, D.C.; Fraser, S.T.; Assinder, S.J. The mammalian copper transporters CTR1 and CTR2 and their roles in development and disease. Int. J. Biochem. Cell. Biol. 2013, 45, 960–963. [Google Scholar] [CrossRef]
- McArdle, H.J.; Andersen, H.S.; Jones, H.; Gambling, L. Copper and iron transport across the placenta: Regulation and interactions. J. Neuroendocrinol. 2008, 20, 427–431. [Google Scholar] [CrossRef]
- Kelner, G.S.; Lee, M.; Clark, M.E.; Maciejewski, D.; McGrath, D.; Rabizadeh, S.; Lyons, T.; Bredesen, D.; Jenner, P.; Maki, R.A. The copper transport protein Atox1 promotes neuronal survival. J. Biol. Chem. 2000, 275, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Petris, M.J.; Smith, K.; Lee, J.; Thiele, D.J. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J. Biol. Chem. 2003, 278, 9639–9646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonnerdal, B. Trace element transport in the mammary gland. Annu. Rev. Nutr. 2007, 27, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Zapata, C.L.; Melo, M.R.; Donangelo, C.M. Maternal, placental and cord zinc components in healthy women with different levels of serum zinc. Biol. Neonatol. 1997, 72, 84–93. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Enhanced zinc utilization during lactation may reduce maternal and infant zinc depletion. Am. J. Clin. Nutr. 2002, 75, 2–3. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, J.P.; Bobo, J.A.; Cui, L.; McMahon, R.J.; Cousins, R.J. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J. Nutr. 2003, 133, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Vargas Zapata, C.L.; Trugo, N.M.; Donangelo, C.M. Zinc uptake by human placental microvillous membrane vesicles: Effects of gestational age and maternal serum zinc levels. Biol. Trace Elem. Res. 2000, 73, 127–137. [Google Scholar] [CrossRef]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Kondoh, M.; Ebihara, C.; Fujii, M.; Nakanishi, T.; Utoguchi, N.; Enomoto, S.; Tanaka, K.; Watanabe, Y. Induction of zinc transporters by forskolin in human trophoblast BeWo cells. Reprod. Toxicol. 2006, 21, 285–291. [Google Scholar] [CrossRef]
- Helston, R.M.; Phillips, S.R.; McKay, J.A.; Jackson, K.A.; Mathers, J.C.; Ford, D. Zinc transporters in the mouse placenta show a coordinated regulatory response to changes in dietary zinc intake. Placenta 2007, 28, 437–444. [Google Scholar] [CrossRef]
- Donangelo, C.M.; King, J.C. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients 2012, 4, 782–798. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, S.L.; Lopez, V.; Lonnerdal, B.; Dufner-Beattie, J.; Andrews, G.K. Zip3 (Slc39a3) functions in zinc reuptake from the alveolar lumen in lactating mammary gland. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R194–R201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadem, N.; Mohammadzadeh, A.; Farhat, A.S.; Valaee, L.; Khajedaluee, M.; Parizadeh, S.M. Relationship between Low Birth Weight Neonate and Maternal Serum Zinc Concentration. Iran. Red. Crescent. Med. J. 2012, 14, 240–244. [Google Scholar]
- Shen, P.J.; Gong, B.; Xu, F.Y.; Luo, Y. Four trace elements in pregnant women and their relationships with adverse pregnancy outcomes. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4690–4697. [Google Scholar]
- Wang, H.; Hu, Y.F.; Hao, J.H.; Chen, Y.H.; Su, P.Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.Y.; Zhang, C.; et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: A population-based birth cohort study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef] [Green Version]
- Upadhyaya, C.; Mishra, S.; Ajmera, P.; Sharma, P. Serum iron, copper and zinc status in maternal and cord blood. Indian J. Clin. Biochem. 2004, 19, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motas, M.; Jimenez, S.; Oliva, J.; Camara, M.A.; Perez-Carceles, M.D. Heavy Metals and Trace Elements in Human Breast Milk from Industrial/Mining and Agricultural Zones of Southeastern Spain. Int. J. Environ. Res. Public Health 2021, 18, 9289. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Giacconi, R.; Piacenza, F.; Santarelli, L.; Cipriano, C.; Costarelli, L.; Tesei, S.; Pierpaoli, S.; Basso, A.; Galeazzi, R.; et al. Plasma copper/zinc ratio: An inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology 2010, 11, 309–319. [Google Scholar] [CrossRef]
- Leone, N.; Courbon, D.; Ducimetiere, P.; Zureik, M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006, 17, 308–314. [Google Scholar] [CrossRef]
- Emokpae, M.A.F.E.B. Cu/ZnRatio as an Inflammatory Marker in Patients with Sickle Cell Disease. Science 2020, 2, 89. [Google Scholar] [CrossRef]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef]
- Miklavcic, A.; Cuderman, P.; Mazej, D.; Snoj Tratnik, J.; Krsnik, M.; Planinsek, P.; Osredkar, J.; Horvat, M. Biomarkers of low-level mercury exposure through fish consumption in pregnant and lactating Slovenian women. Environ. Res. 2011, 111, 1201–1207. [Google Scholar] [CrossRef]
- Miklavcic, A.; Casetta, A.; Snoj Tratnik, J.; Mazej, D.; Krsnik, M.; Mariuz, M.; Sofianou, K.; Spiric, Z.; Barbone, F.; Horvat, M. Mercury, arsenic and selenium exposure levels in relation to fish consumption in the Mediterranean area. Environ. Res. 2013, 120, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Snoj Tratnik, J.; Falnoga, I.; Trdin, A.; Mazej, D.; Fajon, V.; Miklavcic, A.; Kobal, A.B.; Osredkar, J.; Sesek Briski, A.; Krsnik, M.; et al. Prenatal mercury exposure, neurodevelopment and apolipoprotein E genetic polymorphism. Environ. Res. 2017, 152, 375–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbone, F.; Rosolen, V.; Mariuz, M.; Parpinel, M.; Casetta, A.; Sammartano, F.; Ronfani, L.; Vecchi Brumatti, L.; Bin, M.; Castriotta, L.; et al. Prenatal mercury exposure and child neurodevelopment outcomes at 18 months: Results from the Mediterranean PHIME cohort. Int. J. Hyg. Environ. Health 2019, 222, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Trdin, A.; Snoj Tratnik, J.; Stajnko, A.; Marc, J.; Mazej, D.; Sesek Briski, A.; Kastelec, D.; Prpic, I.; Petrovic, O.; Spiric, Z.; et al. Trace elements and APOE polymorphisms in pregnant women and their new-borns. Environ. Int. 2020, 143, 105626. [Google Scholar] [CrossRef] [PubMed]
- Valent, F.; Horvat, M.; Sofianou-Katsoulis, A.; Spiric, Z.; Mazej, D.; Little, D.; Prasouli, A.; Mariuz, M.; Tamburlini, G.; Nakou, S.; et al. Neurodevelopmental effects of low-level prenatal mercury exposure from maternal fish consumption in a Mediterranean cohort: Study rationale and design. J. Epidemiol. 2013, 23, 146–152. [Google Scholar] [CrossRef]
- Verdenik, I. Slovene reference standards for weight, length and head circumference at birth for given gestational age of popualtion born in years 1987–96. Slov. Med. J. 2000, 69, 153–156. [Google Scholar]
- Ellingsen, D.G.; Aaseth, B.M.L.; Copper, J. Handbook of the Toxicology of Metals. Volume II: Specific Metals; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Pluhator, M.M.T.A.; Fedorak, R.N. Clinical Aspects of Trace Elements: Zinc in Human Nutrition—Assessment of Zinc Status. Can. J. Gastroenterol. Hepatol. 1996, 10, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clin. Toxicol. 2011, 3, 0495. [Google Scholar] [CrossRef] [Green Version]
- Craciun, E.C.; Bjorklund, G.; Tinkov, A.A.; Urbina, M.A.; Skalny, A.V.; Rad, F.; Dronca, E. Evaluation of whole blood zinc and copper levels in children with autism spectrum disorder. Metab. Brain Dis. 2016, 31, 887–890. [Google Scholar] [CrossRef]
- Barany, E.; Bergdahl, I.A.; Schuetz, A.; Skerfving, S.; Oskarsson, A. Inductively coupled plasma mass spectometry for direct multi-element analysis of diluted human blood and serum. J. Anal. At. Spectrom. 1997, 12, 1005–1009. [Google Scholar] [CrossRef]
- Jagodič, M.; Snoj Tratnik, J.; Mazej, D.; Stajnko, A.; Pavlin, M.; Krsnik, M.; Kobal, A.B.; Kononenko, L.; Osland, J.O.; Horvat, M. Birth weight in relation to maternal blood levels of selected elements in slovenian populations: A cross-sectional study. J. Health Sci. 2017, 5, 95–106. [Google Scholar]
- Potočnik, D.; Nečemer, M.; Mazej, D.; Jaćimović, R.; Ogrinc, N. Multi-elemental composition of Slovenian milk: Analytical approach and geographical origin determination. Acta Imeko 2016, 5, 15–21. [Google Scholar] [CrossRef]
- Galinier, A.; Periquet, B.; Lambert, W.; Garcia, J.; Assouline, C.; Rolland, M.; Thouvenot, J.P. Reference range for micronutrients and nutritional marker proteins in cord blood of neonates appropriated for gestational ages. Early Hum. Dev. 2005, 81, 583–593. [Google Scholar] [CrossRef]
- Leotsinidis, M.; Alexopoulos, A.; Kostopoulou-Farri, E. Toxic and essential trace elements in human milk from Greek lactating women: Association with dietary habits and other factors. Chemosphere 2005, 61, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosta, L.; Byrne, A.R.; Dermelj, M. Trace elements in some human milk samples by radiochemical neutron activation analysis. Sci. Tot. Environ. 1983, 29, 261–268. [Google Scholar] [CrossRef]
- Iyengar, G.V. Reevaluation of the trace element content in Reference Man. Radiat. Phys. Chem. 1998, 51, 545–560. [Google Scholar] [CrossRef]
- Snoj Tratnik, J.; Falnoga, I.; Mazej, D.; Kocman, D.; Fajon, V.; Jagodic, M.; Stajnko, A.; Trdin, A.; Slejkovec, Z.; Jeran, Z.; et al. Results of the first national human biomonitoring in Slovenia: Trace elements in men and lactating women, predictors of exposure and reference values. Int. J. Hyg. Environ. Health 2019, 222, 563–582. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Mirabella, F.; Martinez, M.A.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part B: Predictors of exposure. Environ. Res. 2020, 182, 109108. [Google Scholar] [CrossRef]
- Awadallah, S.M.; Abu-Elteen, K.H.; Elkarmi, A.Z.; Qaraein, S.H.; Salem, N.M.; Mubarak, M.S. Maternal and Cord Blood Serum Levels of Zinc, Copper, and Iron in Healthy Pregnant Jordanian Women. J. Trace Elem. Exp. Med. 2004, 17, 1–8. [Google Scholar] [CrossRef]
- Ozturk, P.; Kurutas, E.; Ataseven, A.; Dokur, N.; Gumusalan, Y.; Gorur, A.; Tamer, L.; Inaloz, S. BMI and levels of zinc, copper in hair, serum and urine of Turkish male patients with androgenetic alopecia. J. Trace Elem. Med. Biol. 2014, 28, 266–270. [Google Scholar] [CrossRef]
- Ghayour-Mobarhan, M.; Taylor, A.; Kazemi-Bajestani, S.M.; Lanham-New, S.; Lamb, D.J.; Vaidya, N.; Livingstone, C.; Wang, T.; Ferns, G.A. Serum zinc and copper status in dyslipidaemic patients with and without established coronary artery disease. Clin. Lab. 2008, 54, 321–329. [Google Scholar] [PubMed]
- Obeid, O.; Elfakhani, M.; Hlais, S.; Iskandar, M.; Batal, M.; Mouneimne, Y.; Adra, N.; Hwalla, N. Plasma copper, zinc, and selenium levels and correlates with metabolic syndrome components of lebanese adults. Biol. Trace Elem. Res. 2008, 123, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Durazzo, M.; Gambino, R.; Berutti, C.; Milanesio, N.; Caropreso, A.; Gentile, L.; Cassader, M.; Cavallo-Perin, P.; Pagano, G. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J. Nutr. 2008, 138, 305–310. [Google Scholar] [CrossRef]
- Hytten, F.; Chamberlain, G. (Eds.) Clinical Physiology in Obstetrics; Blackwell Scientific Publications: Oxford, UK, 1980. [Google Scholar]
- Nwagha, U.I.; Iyare, E.E.; Ogbodo, S.O.; Agu, P.U.; Olobobokun, T.H.; Ezeonu, P.O.; Onyebuchi, A.K. Parity-related changes in body weight may influence the zinc and copper status in urban pregnant women: A report from South Eastern Nigeria. J. Basic Clin. Reprod. Sci. 2013, 2, 31–37. [Google Scholar] [CrossRef] [Green Version]
- NHMRC. Nutrient Reference Values for Australia and New Zealand; NHMRC: Canberra, Australia, 2005. [Google Scholar]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc; The National Academic Press: Washington, DC, USA, 2001. [Google Scholar]
- Jasinska-Starczewska, M.; Szydlowska, I.; Mroczek, B.; Laszczynska, M.; Chlubek, D.; Kemicer-Chmielewska, E.; Chelstowski, K.; Karakiewicz, B.; Ciecwiez, S.; Starczewski, A. The Influence of Cigarette Smoke Exposure on the Copper Concentration in the Serum Depending on the Use of Menopausal Hormone Therapy. Biomed. Res. Int. 2017, 2017, 5732380. [Google Scholar] [CrossRef]
- Kocyigit, A.; Erel, O.; Gur, S. Effects of tobacco smoking on plasma selenium, zinc, copper and iron concentrations and related antioxidative enzyme activities. Clin. Biochem. 2001, 34, 629–633. [Google Scholar] [CrossRef]
- Bermudez, L.; Garcia-Vicent, C.; Lopez, J.; Torro, M.I.; Lurbe, E. Assessment of ten trace elements in umbilical cord blood and maternal blood: Association with birth weight. J. Transl. Med. 2015, 13, 291. [Google Scholar] [CrossRef] [Green Version]
- Dorea, J.G. Iron and copper in human milk. Nutrition 2000, 16, 209–220. [Google Scholar] [CrossRef]
- Elizabeth, K.E.; Krishnan, V.; Vijayakumar, T. Umbilical cord blood nutrients in low birth weight babies in relation to birth weight & gestational age. Indian J. Med. Res. 2008, 128, 128–133. [Google Scholar] [PubMed]
- Gomez, T.; Bequer, L.; Mollineda, A.; Gonzalez, O.; Diaz, M.; Fernandez, D. Serum zinc levels of cord blood: Relation to birth weight and gestational period. J. Trace Elem. Med. Biol. 2015, 30, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, M. Comparison of Maternal Serum and Neonatal Cord Blood Levels of Zinc in Relation to Birth Weight and Period Gestation. Ph.D. Thesis, Rajiv Gandhi University, Arunachal Pradesh, India, 2009. [Google Scholar]
- King, J.C. Determinants of maternal zinc status during pregnancy. Am. J. Clin. Nutr. 2000, 71, 1334S–1343S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, C.; Shoji, H.; Ikeda, N.; Kitamura, T.; Hisata, K.; Shimizu, T. Association of zinc and copper with clinical parameters in the preterm newborn. Pediatr. Int. 2017, 59, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Nanbakhsh, F.; Tabrizi, F.M. Relationship between cord blood and maternal serum zinc levels and birth weight. Iran J. Neonatol. 2017, 8, 6–10. [Google Scholar]
- Harding, J.E.; Cormack, B.E.; Alexander, T.; Alsweiler, J.M.; Bloomfield, F.H. Advances in nutrition of the newborn infant. Lancet 2017, 389, 1660–1668. [Google Scholar] [CrossRef]
- Ustundag, B.; Yilmaz, E.; Dogan, Y.; Akarsu, S.; Canatan, H.; Halifeoglu, I.; Cikim, G.; Aygun, A.D. Levels of Cytokines (IL-1ββ, IL-2, IL-6, IL-8, TNF-αα) and Trace Elements (Zn, Cu) in Breast Milk From Mothers of Preterm and Term Infants. Mediat. Inflamm. 2005, 2005, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Sultana, Z.; Maiti, K.; Aitken, J.; Morris, J.; Dedman, L.; Smith, R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017, 77, e12653. [Google Scholar] [CrossRef] [PubMed]

| Intake/Concentration | Cu | Zn |
|---|---|---|
| Excess | Preterm birth Low birth weight Gestational diabetes | Neuronal defects Teratogenic Lethal |
| Deficiency | Underdevelopment of nervous system Intrauterine growth restriction Spontaneous delivery Spontaneous abortion | Preterm birth Pregnancy induce hypertension Low birth weight Preeclampsia Placental insufficiency |
| Normal range in whole blood | 508–1307 µg/L (8.0–20.6 µmol/L) | 4290–7600 µg/L (66–116 µmol/L) |
| Variable | Mean ± SD or Frequency (%) |
|---|---|
| Age at delivery (years) | 30.05 ± 4.175 |
| Pre-pregnancy BMI (kg/m2) | 23.92 ± 4.31 |
| Pregnancy BMI increase (kg/m2) | 5.10 ± 1.89 |
| Marital status Married/living together: Widow: Divorced/separated: Single: | 314 (96.9) 1 (0.3) 1 (0.3) 8 (2.5) |
| Education None: Elementary school: Vocational school: High school: University degree: | 1 (0.3) 4 (1.2) 31 (9.6) 108 (33.3) 180 (55.6) |
| Socio-economic status Employed: Unemployed: Student: | 285 (88.0) 23 (7.1) 16 (4.9) |
| Smoking status Never smoked: Smoker: Ex-smoker: | 221 (68.2) 45 (13.9) 58 (17.9) |
| Cu (µg/L) | Zn (µg/L) | |
|---|---|---|
| Week of Pregnancy | +/−2 SD | +/−2 SD |
| 26–30 | 32–440 | 914–1519 |
| 31–32 | 0–605 | 752–1980 |
| 33–34 | 0–584 | 778–1718 |
| 35–36 | 121–529 | 917–1647 |
| 37–42 | 159–695 | 823–1635 |
| Variable | Mean ± SD or Frequency (N(%)) |
|---|---|
| Sex Male: Female: | 159 (49.1%) 165 (50.9%) |
| Gestational age (weeks) | 39.52 ± 1.66 |
| Gestational age (categorical) Pre-term (<37 weeks): Term (37–42 weeks): Post-term (>42 weeks): | 11 (3.4%) 214 (66.0%) 99 (30.6%) |
| Birth weight (g) | 3439.10 ± 486.47 |
| Birth length (cm) | 51.75 ± 2.30 |
| Size for gestational age SGA: AGA: LGA: | 23 (7.1%) 261 (80.6%) 40 (12.3%) |
| Delivery type Spontaneous vaginal: Induced with medications: Emergency Caesarean section: Vacuum: Breach/rotation: | 150 (46.3%) 150 (46.3%) 19 (5.9%) 4 (1.2%) 1 (0.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osredkar, J.; Geršak, Ž.M.; Karas Kuželički, N.; Snoj Tratnik, J.; Mazej, D.; Falnoga, I.; Horvat, M.; Geršak, K. Association of Zn and Cu Levels in Cord Blood and Maternal Milk with Pregnancy Outcomes among the Slovenian Population. Nutrients 2022, 14, 4667. https://doi.org/10.3390/nu14214667
Osredkar J, Geršak ŽM, Karas Kuželički N, Snoj Tratnik J, Mazej D, Falnoga I, Horvat M, Geršak K. Association of Zn and Cu Levels in Cord Blood and Maternal Milk with Pregnancy Outcomes among the Slovenian Population. Nutrients. 2022; 14(21):4667. https://doi.org/10.3390/nu14214667
Chicago/Turabian StyleOsredkar, Joško, Živa Miriam Geršak, Nataša Karas Kuželički, Janja Snoj Tratnik, Darja Mazej, Ingrid Falnoga, Milena Horvat, and Ksenija Geršak. 2022. "Association of Zn and Cu Levels in Cord Blood and Maternal Milk with Pregnancy Outcomes among the Slovenian Population" Nutrients 14, no. 21: 4667. https://doi.org/10.3390/nu14214667
APA StyleOsredkar, J., Geršak, Ž. M., Karas Kuželički, N., Snoj Tratnik, J., Mazej, D., Falnoga, I., Horvat, M., & Geršak, K. (2022). Association of Zn and Cu Levels in Cord Blood and Maternal Milk with Pregnancy Outcomes among the Slovenian Population. Nutrients, 14(21), 4667. https://doi.org/10.3390/nu14214667







