Small-Scale Randomized Controlled Trial to Explore the Impact of β-Hydroxy-β-Methylbutyrate Plus Vitamin D3 on Skeletal Muscle Health in Middle Aged Women
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Overview of Study Design
2.3. Randomization and Study Blind
2.4. Dietary Assessment
2.5. Body Composition and Sample Collection
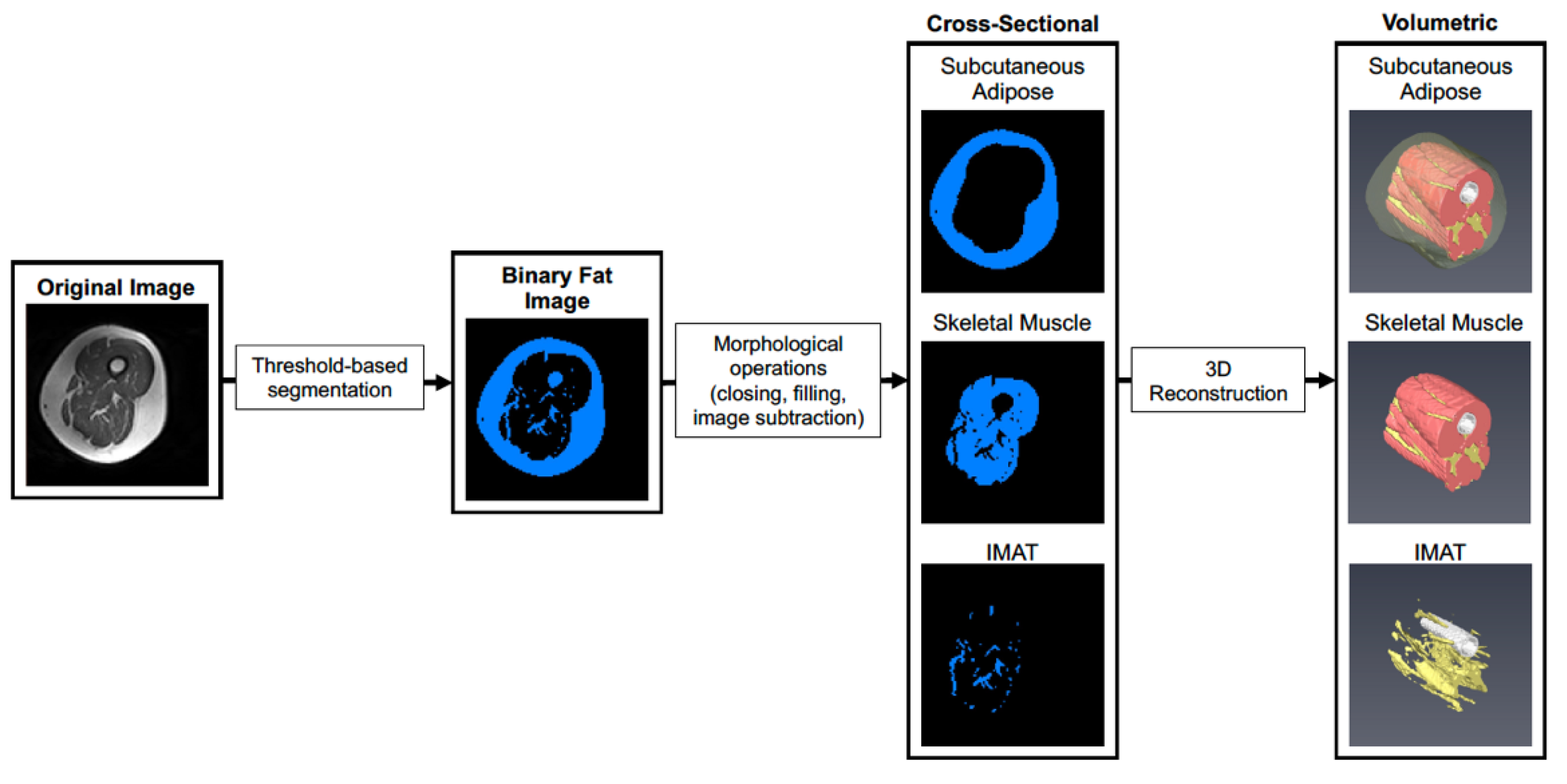
2.6. Magnetic Resonance Imaging
2.7. Muscle Function Testing
2.8. Resistance Exercise Training Program or Sedentary Control
2.9. Supplementation
2.10. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Whole Body and Regional Tissue Mass
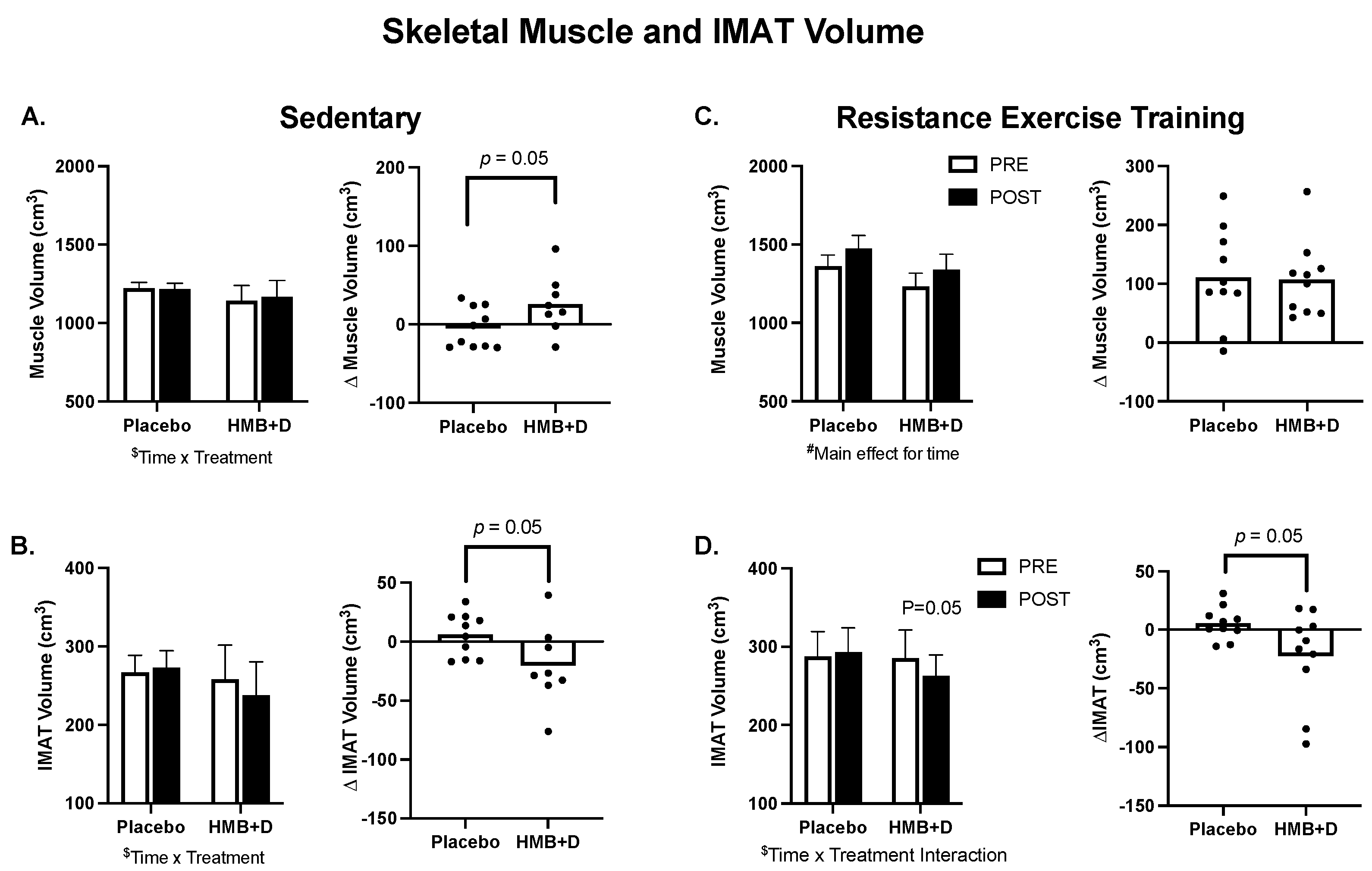
3.3. Skeletal Muscle Size and IMAT
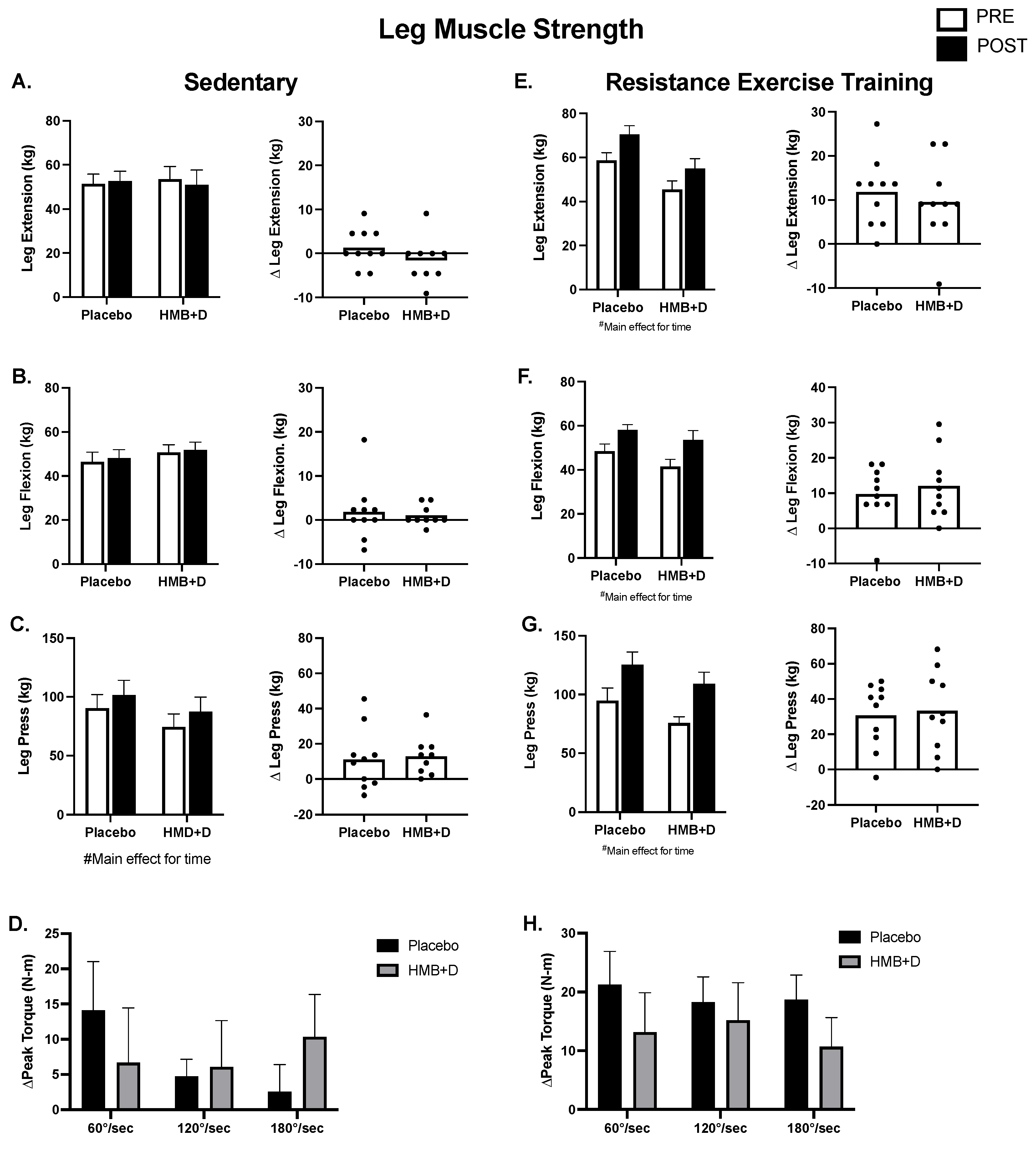
3.4. Muscle Function
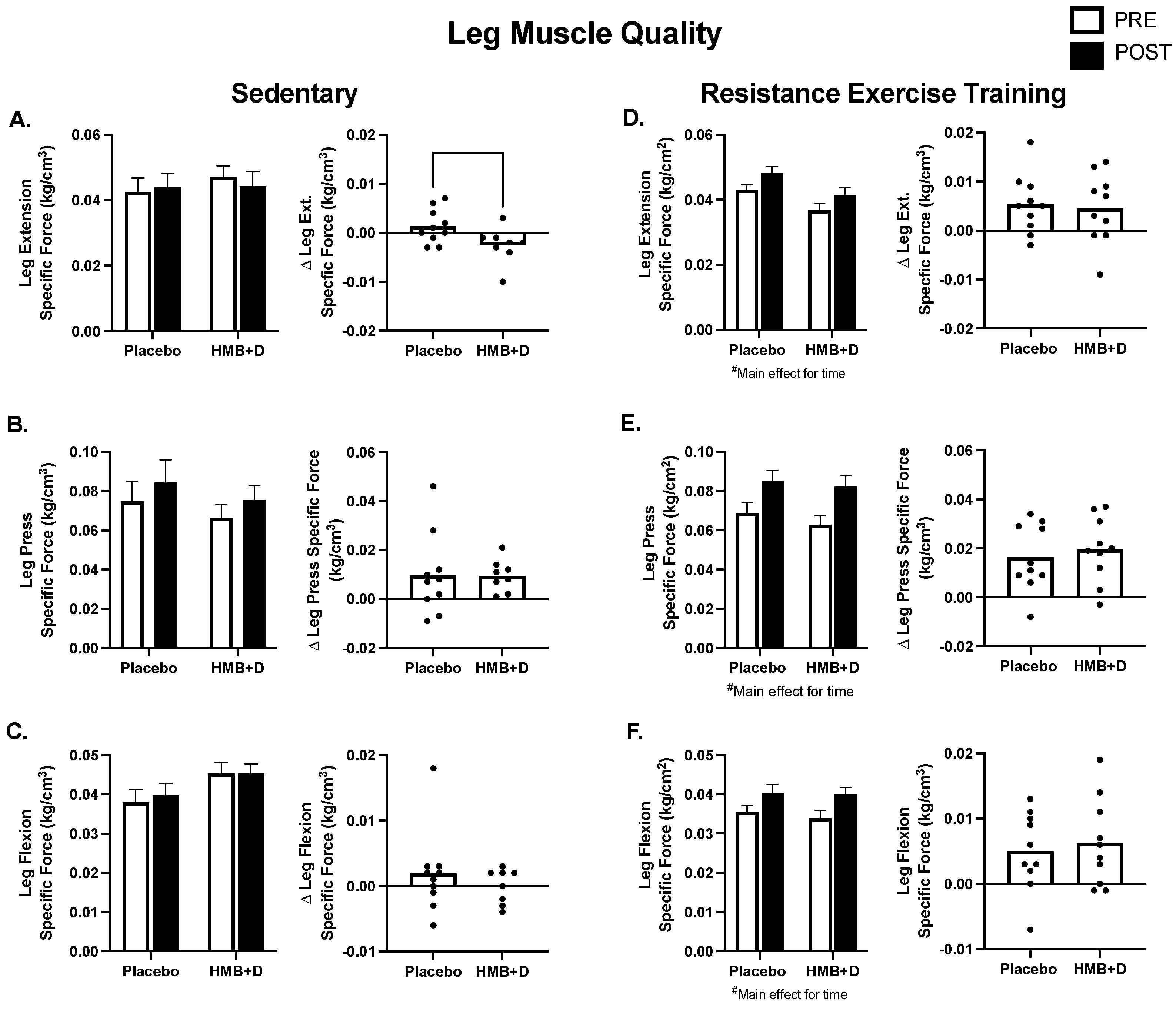
3.5. Muscle Quality
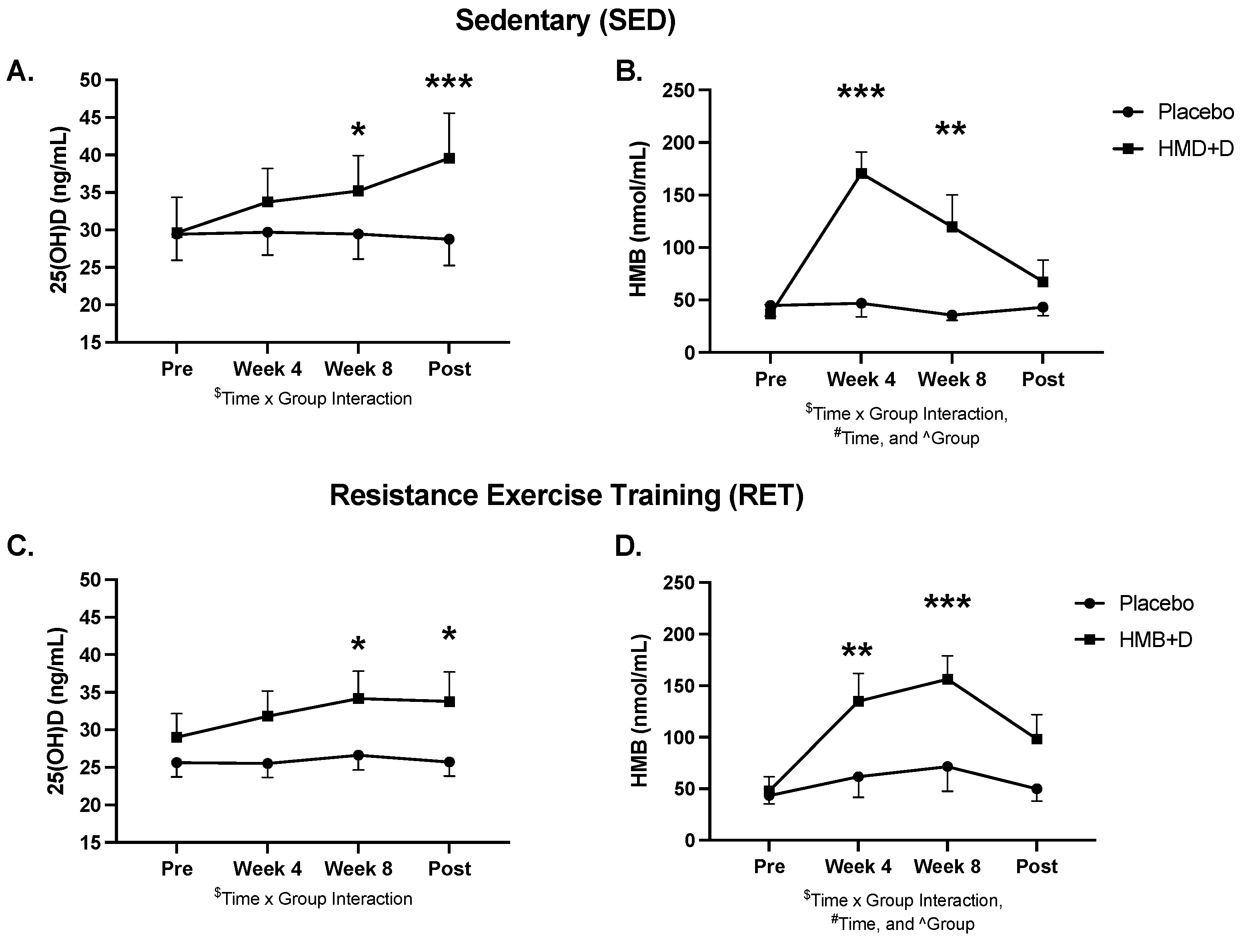
3.6. Exploratory Analysis: Role of Vitamin D3 Sufficiency on the Impact of HMB on IMAT
4. Discussion
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal Study of Muscle Strength, Quality, and Adipose Tissue Infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buford, T.W.; Lott, D.J.; Marzetti, E.; Wohlgemuth, S.E.; Vandenborne, K.; Pahor, M.; Leeuwenburgh, C.; Manini, T.M. Age-Related Differences in Lower Extremity Tissue Compartments and Associations with Physical Function in Older Adults. Exp. Gerontol. 2012, 47, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, B.H.; Chomentowski, P.; Ward, B.K.; Rossi, A.; Glynn, N.W.; Delmonico, M.J.; Kritchevsky, S.B.; Pahor, M.; Newman, A.B. Effects of Physical Activity on Strength and Skeletal Muscle Fat Infiltration in Older Adults: A Randomized Controlled Trial. J. Appl. Physiol. 2008, 105, 1498–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of Skeletal Muscle and Strength in the Elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.L.; Addison, O.; LaStayo, P.C. Intramuscular Adipose Tissue Attenuates Gains in Muscle Quality in Older Adults at High Risk for Falling. A Brief Report. J. Nutr. Health Aging 2013, 17, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Wolff, C.A.; Suer, M.K.; Harber, M.P. Relationship between Intermuscular Adipose Tissue Infiltration and Myostatin before and after Aerobic Exercise Training. Am. J. Physiol. Regul. Integr. Comp. Physiol 2018, 315, R461–R468. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.-F.; Karlamangla, A.S. Changes in Body Composition and Weight during the Menopause Transition. JCI Insight 2019, 4, e124865. [Google Scholar] [CrossRef]
- Liang, J.; Bennett, J.M.; Shaw, B.A.; Quiñones, A.R.; Ye, W.; Xu, X.; Ofstedal, M.B. Gender Differences in Functional Status in Middle and Older Age: Are There Any Age Variations? J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2008, 63, S282–S292. [Google Scholar] [CrossRef] [Green Version]
- Jindai, K. Multimorbidity and Functional Limitations among Adults 65 or Older, NHANES 2005–2012. Prev. Chronic Dis. 2016, 13, E151. [Google Scholar] [CrossRef] [Green Version]
- Freedman, V.A.; Wolf, D.A.; Spillman, B.C. Disability-Free Life Expectancy Over 30 Years: A Growing Female Disadvantage in the US Population. Am. J. Public Health 2016, 106, 1079–1085. [Google Scholar] [CrossRef]
- Harber, M.P.; Konopka, A.R.; Douglass, M.D.; Minchev, K.; Kaminsky, L.A.; Trappe, T.A.; Trappe, S. Aerobic Exercise Training Improves Whole Muscle and Single Myofiber Size and Function in Older Women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1452–R1459. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Konopka, A.R.; Undem, M.K.; Hinkley, J.M.; Minchev, K.; Kaminsky, L.A.; Trappe, T.A.; Trappe, S. Aerobic Exercise Training Induces Skeletal Muscle Hypertrophy and Age-Dependent Adaptations in Myofiber Function in Young and Older Men. J. Appl. Physiol. 2012, 113, 1495–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, L.M. Exercise Training Response Heterogeneity: Physiological and Molecular Insights. Diabetologia 2017, 60, 2329–2336. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite β-Hydroxy-β-Methylbutyrate on Human Skeletal Muscle Protein Metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Limb, M.C.; Phillips, B.E.; Lund, J.; Williams, J.P.; Brook, M.S.; Cegielski, J.; Philp, A.; Ashcroft, S.; et al. Impact of the Calcium Form of β-Hydroxy-β-Methylbutyrate upon Human Skeletal Muscle Protein Metabolism. Clin. Nutr. 2018, 37, 2068–2075. [Google Scholar] [CrossRef] [Green Version]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling Pathways Initiated by Beta-Hydroxy-Beta-Methylbutyrate to Attenuate the Depression of Protein Synthesis in Skeletal Muscle in Response to Cachectic Stimuli. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Attenuation of Depression of Muscle Protein Synthesis Induced by Lipopolysaccharide, Tumor Necrosis Factor, and Angiotensin II by Beta-Hydroxy-Beta-Methylbutyrate. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1409–E1416. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Lean Body Mass during 10 Days of Bed Rest in Older Adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef]
- Hao, Y.; Jackson, J.R.; Wang, Y.; Edens, N.; Pereira, S.L.; Alway, S.E. β-Hydroxy-β-Methylbutyrate Reduces Myonuclear Apoptosis during Recovery from Hind Limb Suspension-Induced Muscle Fiber Atrophy in Aged Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R701–R715. [Google Scholar] [CrossRef]
- Prado, C.M.; Orsso, C.E.; Pereira, S.L.; Atherton, P.J.; Deutz, N.E.P. Effects of β-Hydroxy β-Methylbutyrate (HMB) Supplementation on Muscle Mass, Function, and Other Outcomes in Patients with Cancer: A Systematic Review. J. Cachexia Sarcopenia Muscle 2022, 13, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Smith-Ryan, A.E.; Fukuda, D.H.; Kendall, K.L.; Moon, J.R.; Hoffman, J.R.; Wilson, J.M.; Oliver, J.S.; Mustad, V.A. Effect of Calcium β-Hydroxy-β-Methylbutyrate (CaHMB) with and without Resistance Training in Men and Women 65+yrs: A Randomized, Double-Blind Pilot Trial. Exp. Gerontol. 2013, 48, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, P.M.; Carrithers, J.A.; Godard, M.P.; Schulze, K.E.; Trappe, S.W. Beta-Hydroxy-Beta-Methylbutyrate Ingestion, Part I: Effects on Strength and Fat Free Mass. Med. Sci. Sports Exerc. 2000, 32, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Lau, K.J.; D’Souza, A.C.; Nunes, E.A. An Umbrella Review of Systematic Reviews of β-Hydroxy-β-Methyl Butyrate Supplementation in Ageing and Clinical Practice. J. Cachexia Sarcopenia Muscle 2022, 13, 2265–2275. [Google Scholar] [CrossRef]
- Jakubowski, J.S.; Wong, E.P.T.; Nunes, E.A.; Noguchi, K.S.; Vandeweerd, J.K.; Murphy, K.T.; Morton, R.W.; McGlory, C.; Phillips, S.M. Equivalent Hypertrophy and Strength Gains in β-Hydroxy-β-Methylbutyrate- or Leucine-Supplemented Men. Med. Sci. Sports Exerc. 2019, 51, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of Beta-Hydroxy-Beta-Methylbutyrate, Arginine, and Lysine Supplementation on Strength, Functionality, Body Composition, and Protein Metabolism in Elderly Women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Peng, L.-N.; Cheng, Y.-C.; Yu, P.-C.; Lee, W.-J.; Lin, M.-H.; Chen, L.-K. Oral Nutritional Supplement with β-Hydroxy-β-Methylbutyrate (HMB) Improves Nutrition, Physical Performance and Ameliorates Intramuscular Adiposity in Pre-Frail Older Adults: A Randomized Controlled Trial. J. Nutr. Health Aging 2021, 25, 767–773. [Google Scholar] [CrossRef]
- Lowery, R.P.; Joy, J.M.; Rathmacher, J.A.; Baier, S.M.; Fuller, J.C.J.; Shelley, M.C.I.; Jäger, R.; Purpura, M.; Wilson, S.M.C.; Wilson, J.M. Interaction of Beta-Hydroxy-Beta-Methylbutyrate Free Acid and Adenosine Triphosphate on Muscle Mass, Strength, and Power in Resistance Trained Individuals. J. Strength Cond. Res. 2016, 30, 1843–1854. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Stubbs, N.B.; Bohlken, R.M. Body Composition in 70-Year-Old Adults Responds to Dietary Beta-Hydroxy-Beta-Methylbutyrate Similarly to That of Young Adults. J. Nutr. 2001, 131, 2049–2052. [Google Scholar] [CrossRef] [Green Version]
- Guedes, J.M.; Peluzio, M.d.C.G.; Rathmacher, J.A.; Leal, T.F.; Júnior, M.A.C.; de Carvalho, D.M.; de Oliveira, L.L.; Natali, A.J. β-Hydroxy-β-Methylbutyrate Supplementation Benefits the Effects of Resistance Training on Body Fat Reduction via Increased Irisin Expression in White Adipose Tissue. Biol. Sport. 2021, 38, 113–121. [Google Scholar] [CrossRef]
- Sohl, E.; van Schoor, N.M.; de Jongh, R.T.; Visser, M.; Deeg, D.J.H.; Lips, P. Vitamin D Status Is Associated with Functional Limitations and Functional Decline in Older Individuals. J. Clin. Endocrinol. Metab. 2013, 98, E1483–E1490. [Google Scholar] [CrossRef]
- Gilsanz, V.; Kremer, A.; Mo, A.O.; Wren, T.A.L.; Kremer, R. Vitamin D Status and Its Relation to Muscle Mass and Muscle Fat in Young Women. J. Clin. Endocrinol. Metab. 2010, 95, 1595–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, J.C.; Baier, S.; Flakoll, P.; Nissen, S.L.; Abumrad, N.N.; Rathmacher, J.A. Vitamin D Status Affects Strength Gains in Older Adults Supplemented with a Combination of β-Hydroxy-β-Methylbutyrate, Arginine, and Lysine: A Cohort Study. JPEN J. Parenter. Enteral Nutr. 2011, 35, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.; Chanet, A.; Giraudet, C.; Patrac, V.; Pierre, P.; Jourdan, M.; Luiking, Y.C.; Verlaan, S.; Migné, C.; Boirie, Y.; et al. 1,25(OH)2-Vitamin D3 Enhances the Stimulating Effect of Leucine and Insulin on Protein Synthesis Rate through Akt/PKB and MTOR Mediated Pathways in Murine C2C12 Skeletal Myotubes. Mol. Nutr. Food Res. 2013, 57, 2137–2146. [Google Scholar] [CrossRef]
- Rathmacher, J.A.; Pitchford, L.M.; Khoo, P.; Angus, H.; Lang, J.; Lowry, K.; Ruby, C.; Krajek, A.C.; Fuller, J.C.; Sharp, R.L. Long-Term Effects of Calcium β-Hydroxy-β-Methylbutyrate and Vitamin D3 Supplementation on Muscular Function in Older Adults with and Without Resistance Training: A Randomized, Double-Blind, Controlled Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 2089–2097. [Google Scholar] [CrossRef]
- Konopka, A.R.; Douglass, M.D.; Kaminsky, L.A.; Jemiolo, B.; Trappe, T.A.; Trappe, S.; Harber, M.P. Molecular Adaptations to Aerobic Exercise Training in Skeletal Muscle of Older Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopka, A.R.; Trappe, T.A.; Jemiolo, B.; Trappe, S.W.; Harber, M.P. Myosin Heavy Chain Plasticity in Aging Skeletal Muscle with Aerobic Exercise Training. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 835–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopka, A.R.; Suer, M.K.; Wolff, C.A.; Harber, M.P. Markers of Human Skeletal Muscle Mitochondrial Biogenesis and Quality Control: Effects of Age and Aerobic Exercise Training. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Konopka, A.R.; Laurin, J.L.; Musci, R.V.; Wolff, C.A.; Reid, J.J.; Biela, L.M.; Zhang, Q.; Peelor, F.F.; Melby, C.L.; Hamilton, K.L.; et al. Influence of Nrf2 Activators on Subcellular Skeletal Muscle Protein and DNA Synthesis Rates after 6 Weeks of Milk Protein Feeding in Older Adults. GeroScience 2017, 39, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Konopka, A.R.; Laurin, J.L.; Schoenberg, H.M.; Reid, J.J.; Castor, W.M.; Wolff, C.A.; Musci, R.V.; Safairad, O.D.; Linden, M.A.; Biela, L.M.; et al. Metformin Inhibits Mitochondrial Adaptations to Aerobic Exercise Training in Older Adults. Aging Cell 2019, 18, e12880. [Google Scholar] [CrossRef]
- Konopka, A.R.; Esponda, R.R.; Robinson, M.M.; Johnson, M.L.; Carter, R.E.; Schiavon, M.; Cobelli, C.; Wondisford, F.E.; Lanza, I.R.; Nair, K.S. Hyperglucagonemia Mitigates the Effect of Metformin on Glucose Production in Prediabetes. Cell Rep. 2016, 15, 1394–1400. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Zeng, L.; Deng, J.; Duan, Y.; Li, F. β-Hydroxy-β-Methylbutyrate (HMB) Improves Mitochondrial Function in Myocytes through Pathways Involving PPARβ/δ and CDK4. Nutrition 2019, 60, 217–226. [Google Scholar] [CrossRef] [PubMed]
- da Justa Pinheiro, C.H.; Gerlinger-Romero, F.; Guimarães-Ferreira, L.; de Souza, A.L., Jr.; Vitzel, K.F.; Nachbar, R.T.; Nunes, M.T.; Curi, R. Metabolic and Functional Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation in Skeletal Muscle. Eur. J. Appl. Physiol. 2012, 112, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The Vitamin D Receptor Regulates Mitochondrial Function in C2C12 Myoblasts. Am. J. Physiol. Cell Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef] [PubMed]
- Sachs, S.; Zarini, S.; Kahn, D.E.; Harrison, K.A.; Perreault, L.; Phang, T.; Newsom, S.A.; Strauss, A.; Kerege, A.; Schoen, J.A.; et al. Intermuscular Adipose Tissue Directly Modulates Skeletal Muscle Insulin Sensitivity in Humans. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E866–E879. [Google Scholar] [CrossRef] [Green Version]
- Sparks, L.M.; Goodpaster, B.H.; Bergman, B.C. The Metabolic Significance of Intermuscular Adipose Tissue: Is IMAT a Friend or a Foe to Metabolic Health? Diabetes 2021, 70, 2457–2467. [Google Scholar] [CrossRef]
- Pedroso, M.G.; de Almeida, A.C.; Aily, J.B.; de Noronha, M.; Mattiello, S.M. Fatty Infiltration in the Thigh Muscles in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Rheumatol. Int. 2019, 39, 627–635. [Google Scholar] [CrossRef]
- Snow, B.J.; Wilcox, J.J.; Burks, R.T.; Greis, P.E. Evaluation of Muscle Size and Fatty Infiltration with MRI Nine to Eleven Years Following Hamstring Harvest for ACL Reconstruction. J. Bone Jt. Surg. Am. 2012, 94, 1274–1282. [Google Scholar] [CrossRef]
- Gerber, C.; Schneeberger, A.G.; Hoppeler, H.; Meyer, D.C. Correlation of Atrophy and Fatty Infiltration on Strength and Integrity of Rotator Cuff Repairs: A Study in Thirteen Patients. J. Shoulder Elbow Surg. 2007, 16, 691–696. [Google Scholar] [CrossRef]
- Kamigaichi, A.; Harada, H.; Shibata, S. Muscle Quality Predicts Outcomes after Surgery for Early-Stage Non-Small-Cell Lung Cancer. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 262–270. [Google Scholar] [CrossRef]

| Sedentary | Resistance Exercise Training | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 10) | HMB + D (n = 9) | Placebo (n = 10) | HMB + D (n = 10) | |||||
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| Calories (kcal/day | 1766 ± 462 | 1856 ± 487 | 1482 ± 441 | 1523 ± 363 | 1581 ± 195 | 1531 ± 436 | 1511 ± 549 | 1728 ± 436 |
| Protein (g/day) | 74 ± 29 | 75 ± 19 | 64 ± 13 | 64 ± 13 | 76 ± 32 | 70 ± 16 | 71 ± 22 | 73 ± 20 |
| Carbohydrate (g/day) | 232 ± 64 | 229 ± 64 | 157 ± 60 | 172 ± 52 | 169 ± 37 | 164 ± 79 | 144 ± 56 | 191 ± 68 |
| Fat (g/day) | 60 ± 16 | 73 ± 25 | 65 ± 32 | 66 ± 19 | 62 ± 16 | 62 ± 16 | 66 ± 32 | 75 ± 31 |
| Sedentary | Resistance Exercise Training | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 10) | HMB + D (n = 9) | Placebo (n = 10) | HMB + D (n = 10) | |||||
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| Age (years) | 53 ± 1 | 53 ± 1 | 52 ± 1 | 51 ± 1 | ||||
| BMI (kg/m2) | 28 ± 1 | 28 ± 1 | 26 ± 2 | 26 ± 2 | 27 ± 1 | 27 ± 1 | 25 ± 2 | 25 ± 2 |
| Total Body Mass (kg) | 73 ± 4 | 73 ± 4 | 68 ± 5 | 68 ± 5 | 75 ± 4 | 76 ± 3 | 68 ± 5 | 69 ± 5 |
| Whole Body Lean Mass (kg) † | 41 ± 2 | 41 ± 2 | 40 ± 2 | 40 ± 2 | 41 ± 2 | 42 ± 1 | 38 ± 2 | 39 ± 2 |
| Appendicular Lean Mass (kg) † | 17 ± 3 | 17 ± 3 | 17 ± 3 | 16 ± 3 | 17 ± 0.7 | 18 ± 0.6 | 15 ± 0.8 | 16 ± 0.9 |
| Sarcopenic Index (kg/m2) † | 6.2 ± 0.8 | 6.2 ± 0.8 | 6.0 ± 0.7 | 5.9 ± 0.7 | 6.3 ± 0.3 | 6.6 ± 0.3 | 5.9 ± 0.3 | 6.3 ± 0.3 |
| Leg Lean Mass (kg) † | 13.3 ± 0.6 | 13.5 ± 0.6 | 13.0 ± 0.8 | 13.3 ± 0.9 | 13.6 ± 0.5 | 14.3 ± 0.5 | 12.1 ± 0.7 | 12.8 ± 0.8 |
| Arm Lean Mass (kg) $,† | 4.0 ± 0.8 | 3.7 ± 0.8 * | 3.7 ± 0.5 | 3.8 ± 0.6 | 3.7 ± 0.2 | 3.9 ± 0.2 | 3.3 ± 0.2 | 3.5 ± 0.2 |
| Body Fat (%) | 45 ± 2 | 44 ± 2 | 43 ± 2 | 42 ± 2 | 43 ± 1 | 43 ± 1 | 40 ± 1 | 40 ± 1 |
| Fat Mass (kg) | 32.3 ± 2.5 | 31.7 ± 2.3 | 28.5 ± 3.9 | 28.8 ± 4.0 | 33.4 ± 2.8 | 33.7 ± 2.8 | 29.5 ± 3.4 | 29.0 ± 3.2 |
| Trunk Fat Mass (kg) | 15.0 ± 1.5 | 14.9 ± 1.4 | 13.8 ± 2.1 | 14.0 ± 2.3 | 16.0 ± 1.6 | 16.0 ± 1.6 | 13.7 ± 1.8 | 13.4 ± 1.8 |
| Leg Fat Mass (kg) | 12.6 ± 0.9 | 12.4 ± 0.9 | 10.8 ± 1.5 | 10.8 ± 1.5 | 12.4 ± 1.1 | 12.6 ± 1.2 | 11.4 ± 1.2 | 11.5 ± 1.3 |
| Arm Fat mass (kg) | 3.5 ± 0.3 | 3.3 ± 0.3 | 2.8 ± 0.4 | 2.8 ± 0.4 | 3.9 ± 0.3 | 3.9 ± 0.3 | 3.3 ± 0.4 | 3.1 ± 0.3 |
| Sedentary | Resistance Exercise Training | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 10) | HMB + D (n = 9) | Placebo (n = 10) | HMB + D (n = 10) | |||||
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | |
| Chest Press (kg) ‡,† | 19.7 ± 1.9 | 20.9 ± 2.3 | 18.1 ± 1.5 | 23.5 ± 3.6 * | 20.5 ± 2.3 | 30.1 ± 3.1 | 15.9 ± 1.9 | 24.3 ± 2.9 |
| Shoulder Press (kg) † | 8.6 ± 1.2 | 9.9 ± 1.3 | 9.6 ± 1.3 | 10.1 ± 1.4 | 9.0 ± 1.0 | 13.5 ± 1.3 | 8.2 ± 1.0 | 13.9 ± 1.8 |
| Seated Row (kg) † | 30.0 ± 2.0 | 30.0 ± 2.1 | 29.2 ± 1.5 | 28.5 ± 1.1 | 30.2 ± 1.7 | 38.2 ± 2.0 | 25.8 ± 1.9 | 34.5 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fairfield, W.D.; Minton, D.M.; Elliehausen, C.J.; Nichol, A.D.; Cook, T.L.; Rathmacher, J.A.; Pitchford, L.M.; Paluska, S.A.; Kuchnia, A.J.; Allen, J.M.; et al. Small-Scale Randomized Controlled Trial to Explore the Impact of β-Hydroxy-β-Methylbutyrate Plus Vitamin D3 on Skeletal Muscle Health in Middle Aged Women. Nutrients 2022, 14, 4674. https://doi.org/10.3390/nu14214674
Fairfield WD, Minton DM, Elliehausen CJ, Nichol AD, Cook TL, Rathmacher JA, Pitchford LM, Paluska SA, Kuchnia AJ, Allen JM, et al. Small-Scale Randomized Controlled Trial to Explore the Impact of β-Hydroxy-β-Methylbutyrate Plus Vitamin D3 on Skeletal Muscle Health in Middle Aged Women. Nutrients. 2022; 14(21):4674. https://doi.org/10.3390/nu14214674
Chicago/Turabian StyleFairfield, William D., Dennis M. Minton, Christian J. Elliehausen, Alexander D. Nichol, Taylor L. Cook, John A. Rathmacher, Lisa M. Pitchford, Scott A. Paluska, Adam J. Kuchnia, Jacob M. Allen, and et al. 2022. "Small-Scale Randomized Controlled Trial to Explore the Impact of β-Hydroxy-β-Methylbutyrate Plus Vitamin D3 on Skeletal Muscle Health in Middle Aged Women" Nutrients 14, no. 21: 4674. https://doi.org/10.3390/nu14214674
APA StyleFairfield, W. D., Minton, D. M., Elliehausen, C. J., Nichol, A. D., Cook, T. L., Rathmacher, J. A., Pitchford, L. M., Paluska, S. A., Kuchnia, A. J., Allen, J. M., & Konopka, A. R. (2022). Small-Scale Randomized Controlled Trial to Explore the Impact of β-Hydroxy-β-Methylbutyrate Plus Vitamin D3 on Skeletal Muscle Health in Middle Aged Women. Nutrients, 14(21), 4674. https://doi.org/10.3390/nu14214674








