Chitosan Nanogel with Mixed Food Plants and Its Relation to Blood Glucose in Type 2 Diabetes: A Systematic and Meta-Analysis Review of Observational Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection, Inclusion as Well as Exclusion Criteria
2.3. Information Extraction
2.4. Assessing the Risk Bias
2.5. Strategy for Information Investigation
2.6. Patients as Well as General Participation
3. Outcomes (Results)
3.1. Explored Outcomes
3.2. Features of Involved Investigation as Well as Valuation of Intervention: T2D
3.3. The Bias Risk across Investigations
3.4. Decrease in Blood Glucose Level
4. Discussion
4.1. Principal Findings
4.2. Quality of Evidence
4.3. Limitation
5. Conclusions and Future Implication
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization, Global Report on Diabetes; WHO: Geneva, Switzerland, 2016.
- Odebode, F.D.; Ekeleme, O.T.; Ijarotimi, O.S.; Malomo, S.A.; Idowu, A.O.; Badejo, A.A.; Adebayo, I.A.; Fagbemi, T.N. Nutritional composition, antidiabetic and antilipidemic potentials of flour blends made from unripe plantain, soybean cake, and rice bran. J. Food Biochem. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Shodehinde, S.A.; Ademiluyi, A.O.; Oboh, G.; Akindahunsi, A.A. Contribution of Musa paradisiaca in the inhibition of α-amylase, α-glucosidase and Angiotensin-I converting enzyme in streptozotocin induced rats. Life Sci. 2015, 133, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Iroaganachi, M.; Eleazu, C.O.; Okafor, P.N.; Nwaohu, N. Effect of Unripe Plantain (Musa paradisiaca) and Ginger (Zingiber officinale) on Blood Glucose, Body Weight and Feed Intake of Streptozotocin-induced Diabetic Rats. Biochem. J. 2015, 9, 1–6. [Google Scholar]
- Eleazu, C.O.; Okafor, P. Use of unripe plantain (Musa paradisiaca) in the management of diabetes and hepatic dysfunction in streptozotocin induced diabetes in rats. Interv. Med. Appl. Sci. 2015, 7, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Olabiy, A.A. Nutritional, antioxidant and inhibitory properties of cocoa powder enriched wheat-plantain biscuits on key enzymes linked to type 2 diabetes. Int. Food Res. J. 2018, 25, 793–803. [Google Scholar]
- Sarafidis, P.; Ferro, C.J.; Morales, E.; Ortiz, A.; Malyszko, J.; Hojs, R.; Khazim, K.; Ekart, R.; Valdivielso, J.; Fouque, D.; et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial. Transplant. 2019, 34, 208–230. [Google Scholar] [CrossRef]
- Dabhi, A.S.; Bhatt, N.R.; Shah, M.J. Voglibose: An alpha glucosidase inhibitor. J. Clin. Diagn. Res. 2013, 7, 3023–3027. [Google Scholar] [CrossRef]
- Sabitha, V.; Panneerselvam, K.; Ramachandran, S. In vitro α–glucosidase and α–amylase enzyme inhibitory effects in aqueous extracts of Abelmoscus esculentus (L.) moench. Asian Pac. Trop. Biomed. 2012, 2, 162–164. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, X.; Wang, O.; Liu, J.; Cai, S.; Wang, R.; Zhou, F.; Ji, B. Anti-diabetic effects of the ethanol extract of a functional formula diet in mice fed with a fructose/fat-rich combination diet. J. Sci. Food Agric. 2015, 95, 401–408. [Google Scholar] [CrossRef]
- Oboh, G.; Adefegha, S.A.; Ademosun, A.O.; Unu, D. Effects of hot water treatment on the phenolic phytochemicals and antioxidant activities of lemon grass (Cymbopogon citratus). Elec. J. Agric. Food Chem. 2010, 9, 503–513. [Google Scholar]
- Adefegha, S.A.; Oboh, G. Enhancement of total phenolics and antioxidant properties of some tropical green leafy vegetables by steam cooking. J. Food Process Pres. 2011, 35, 615–622. [Google Scholar] [CrossRef]
- Sabitha, V.; Ramachandran, S.; Naveen, K.R.; Panneerselvam, K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) moench. In streptozotocin-induced diabetic rats. J. Pharm. Bioallied. Sci. 2011, 3, 397–402. [Google Scholar] [PubMed]
- Sabitha, V.; Ramachandran, S.; Naveen, K.R.; Panneerselvam, K. Investigation of in vivo antioxidant property of Abelmoschus esculentus (L.) moench. Fruit seed and peel powders in streptozotocin-induced diabetic rats. J. Ayurveda Integr. Med. 2012, 3, 188–193. [Google Scholar] [PubMed]
- Huang, C.; Wang, C.; Lin, C.; Lin, H.; Peng, C. The nutraceutical benefits of subfractions of Abelmoschus esculentus in treating type 2 diabetes mellitus. PLoS ONE. 2017, 12, e0189065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martirosyan, D.; Singh, J. A new definition of functional food by FFC: What makes a new definition unique? Funct. Food Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Sathish, K.; Eswar, T.; Praveen, K.; Ashok, K.; Bramha, S.; Ramarao, N. A review on: Abelmoschus esculentus (okra). IRJP 2013, 3, 129–132. [Google Scholar]
- Dike, L.P.; Obembe, O.O.; Adebiyi, E.F. Ethnobotanical survey for potential anti-malarial plants in south-western Nigeria. J. Ethnopharmacol. 2012, 144, 618–626. [Google Scholar] [CrossRef]
- Egbuonu, A.C.C.; Nzewi, D.C.; Egbuonu, O.N.C. Functional Properties of Bitter Yam (Dioscorea dumetorum) as Influenced by Soaking Prior to Oven-drying. Am. J. Food Technol. 2014, 9, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Chem, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Nimenibo-Uadia, R.; Oriakhi, A. Proximate, Mineral and Phytochemical Composition of Dioscorea dumetorum Pax. J. Appl. SCI. Environ. Manag. 2017, 21, 771. [Google Scholar]
- Famakin, O.; Fatoyinbo, A.; Ijarotimi, O.S.; Badejo, A.A.; Fagbemi, T.N. Assessment of nutritional quality, glycaemic index, antidiabetic and sensory properties of plantain (Musa paradisiaca)-based functional dough meals. J. Food Sci. Technol. 2016, 53, 3865–3875. [Google Scholar] [CrossRef]
- Nguekouo, P.T.; Kuate, D.; Kengne, A.P.N.; Woumbo, C.Y.; Tekou, F.A.; Oben, J.E. Effect of boiling and roasting on the antidiabetic activity of Abelmoschus esculentus (Okra) fruits and seeds in type 2 diabetic rats. J. Food Biochem. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Go, H.; Rahman, M.M.; Kim, G.; Na, C.; Song, C.; Kim, J.; Kim, S.; Kang, H. Antidiabetic Effects of Yam (Dioscorea batatas) and Its Active Constituent, Allantoin, in a Rat Model of Streptozotocin-Induced Diabetes. Nutrients 2015, 7, 8532–8544. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.; Wu, X.; Liu, R. Antioxidant and anti-proliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Saha, R.; Pal, P. Arsenic Uptake and Accumulation in Okra (Abelmoschus esculentus) as Affected by Different Arsenical Speciation. Bull. Environ. Contam. Toxicol. 2016, 96, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Thulé, P.M.; Umpierrez, G. Sulfonylureas: A new look at old therapy. Curr. Diab. Rep. 2014, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Lee, C.; Choi, J.S.; Kim, I.; Byeon, H.J.; Kim, T.H.; Oh, K.T.; Lee, E.S.; Lee, K.C.; Youn, Y.S. Decanoic acid-modified glycol chitosan hydrogels containing tightly adsorbed palmityl-acylated exendin-4 as a long-acting sustained-release anti-diabetic system. Acta Biomater. 2014, 10, 812–820. [Google Scholar] [CrossRef]
- Song, L.; Zhi, Z.; Pickup, J.C. Nanolayer encapsulation of insulin- chitosan complexes improves efficiency of oral insulin delivery. Int. J. Nanomed. 2014, 9, 2127–2136. [Google Scholar]
- Jo, S.; Ha, K.; Moon, K.; Kim, J.; Oh, C.; Kim, Y.; Apostolidis, E.; Kwon, Y. Molecular Weight Dependent Glucose Lowering Effect of Low Molecular Weight Chitosan Oligosaccharide (GO2KA1) on Postprandial Blood Glucose Level in SD Rats Model. Int. J. Mol. Sci. 2013, 14, 14214–14224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekalska, M.; Sosnowska, K.; Zakrzeska, A.; Kasacka, I.; Lewandowska, A.; Winnicka, K. The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride—In Vitro and in Vivo Evaluation. Molecules 2017, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, O.T.; Sunday, B.E.; Folaranmi, O.A. Nutrient composition and contribution of plantain (Musa paradisiacea) products to dietary diversity of Nigerian consumers. Afi. J. Biotechnol. 2012, 11, 13601–13605. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Oloyede, H.O.B.; Salawu, M.O. Antihyperglycemic and antidyslipidemic activity of Musa paradisiaca-based diet in alloxan- induced diabetic rats. Food Sci. Nutr. 2018, 6, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Eleazu, C.O.; Iroaganachi, M.; Eleazu, K.C. Ameliorative Potentials of Cocoyam (Colocasia esculenta L.) and Unripe Plantain (Musa paradisiaca L.) on the Relative Tissue Weights of Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; IntHout, J.; Ritskes-Hoitinga, M.; Rovers, M.M. Meta-Analyses of Animal Studies: An Introduction of a Valuable Instrument to Further Improve Healthcare. ILAR J. 2014, 55, 418–426. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Beyene, F. Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. J. Food Qual. 2014, 33, 87–96. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.; Kim, T.H.; Lee, E.S.; Shin, B.S.; Chi, S.C.; Park, E.; Lee, K.C.; Youn, Y.S. Self-assembled glycol chitosan nanogels containing palmityl-acylated exendin-4 peptide as a long-acting anti-diabetic inhalation system. J. Control. Release 2012, 161, 728–734. [Google Scholar] [CrossRef]
- Raafat, K.; Samy, W. Amelioration of Diabetes and Painful Diabetic Neuropathy by Punica granatum L. Extract and Its Spray Dried Biopolymeric Dispersions. Evid. Based Compl. Alt. 2014, 2014, 180495. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.; Lee, I.; Lee, E.; Kim, H.; Kim, Y.; Jon, S. Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J. Control. Release 2013, 170, 226–232. [Google Scholar] [CrossRef]
- Lopes, M.; Aniceto, D.; Abrantes, M.; Simões, S.; Branco, F.; Vitória, I.; Botelho, M.F.; Seiça, F.; Veiga, F.; Ribeiro, A. In vivo biodistribution of antihyperglycemic biopolymer-based nanoparticles for the treatment of type 1 and type 2 diabetes. Eur. J. Pharm. Biopharma. 2017, 113, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Modified for use The PRISMA Group; Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement 2009. PLoS Med. 2009, 6, e1000097. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC. Med. Res. Methodol. 2014, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hak, T.; Van Rhee, H.J.; Suurmond, R. How to Interpret Results of Meta-Analysis? Version 1.3; Erasmus Rotterdam Institute of Management: Rotterdam, The Netherlands, 2016. [Google Scholar]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adefegha, S.A.; Oboh, G. Inhibition of Key Enzymes Linked to Type 2 Diabetes and Sodium Nitroprusside-Induced Lipid Peroxidation in Rat Pancreas by Water Extractable Phytochemicals from Some Tropical Spices. Pharm. Biol. 2012, 50, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Mostafa-Hedeab, G.; El-Shetry, E.S. Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 2021, 768, 145288. [Google Scholar] [CrossRef]
- Van Rhee, H.J.; Suurmond, R.; Hak, T. User Manual for Meta-Essentials: Workbooks for Meta-Analysis; Version 1.4; Erasmus Research Institute of Management: Rotterdam, The Netherlands, 2015. [Google Scholar]
- Adamson, S.S.; Craniyu, O. Aqueous Extracts from Unripe Plantain (Musa paradisiaca) Products Inhibit Key Enzymes Linked with Type 2 Diabetes and Hypertension in vitro. Jordan J. Biol. Sci. 2012, 54, 239–246. [Google Scholar]
- Sun, Q.; Spiegelman, D.; Van Dam, R.M.; Holmes, M.D.; Malik, V.S.; Willett, W.C.; Hu, F.B. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch. Intern. Med. 2010, 170, 961–969. [Google Scholar] [CrossRef]

| Class of Antidiabetic Drug | Specific Mechanism of Action | Adverse Effect | Reference |
|---|---|---|---|
| Acabose—Used for treating T2D | Acarbose works by slowing down the action of certain chemicals that break down food to release glucose into the blood; slow food digestion helps to keep blood glucose from rising high after any meal. | Hyperglycemia, Shaking, Dizziness, Sweating, Irritability, Mood change, Headache, Numbness, Weakness, Pale skin, Hunger, Clumsy, Confusion, Seizures, Loss of consciousness, Extreme thirst, Frequent urination, Blurred vision, Dry mouth, Stomach upset, Vomiting, Shortness of breath, Breath that smells fruity and decreased consciousness. | [6] |
| Voglibose—Used as an α-glucosidase inhibitor that manages postprandial blood sugar in T2D. | α-glucosidase inhibitor; the saccharides, acting as competitive inhibitor of enzymes needed to digest carbohydrate specifically the α–glucosidases enzymes present in brush border of small intestine. | Seen in about 25% of users. Adverse effects include: Soft stool, Diarrhoea, Flatulence, Bloating, Abdominal pain or fullness and nausea. | [7] |
| Glyset (Miglitol)—A drug employed to treat symptoms of T2D. It can be used alone or in combination with this class of drugs. It belongs to a class of drugs referred to as Antidiabetics, α-glucosidase inhibitors. | Unlike Sulonylureas, Glyset (Miglitol) does not enhance insulin secretion. Antihyperglycemia action of Miglitol results from a reversible inhibition of membrane-bound intestinal α-glucosidase hydrolase enzymes. | Hives, Difficulty breathing, swelling of the face, Lips, Tongue, or throat, Severe diarrhea, Constipation, Bloody or tarry stools, rectal bleeding and diarrhea that contains blood or mucus. | [8] |
| Biguanides—This refers to a group of oral diabetes drugs that work by preventing the production of sugar in the liver, improving the body sensitivity towards insulin and reducing the amount of sugar absorbed by the intestines. | It works by preventing the liver from converting fat and amino acids into sugar. They also activate an enzyme which helps cells respond more effectively to insulin and takes in sugar from the blood. It is used by obese people as it promotes weight loss. | Hypoglycemia results very rarely, weight gain and digestive adverse reactions. | [9] |
| Thiazolidinedione (TZDs)—These are insulin sensitizers that act on intercellular metabolic pathways to enhance insulin action and increase sensitivity in critical tissues. | TZDs act by activating peroxisome proliferator-activated receptors. They are also agonist. The endogenous ligands for those receptors which are fatty acids and eicosanoids. This binds DNA when receptors activated. | Increase hepatitis and possible liver failure, Edema, Heart failure, Coronary Heart Disease (CHD), Plaque progression, Myocardia | [9] |
| Sulfonylureas—Stimulates the release of insulin from pancreatic Beta-cells and have a number of extra-pancreatic effects | Induces sugar independent-insulin release from Beta-cells by inhibiting potassium flux through Adenosine Triphosphate (ATP) dependent potassium channels. | Hypoglycemia, Induces hyponatremia, Edema, Induces alcohol flushing. | [10] |
| Meglitinides—these are oral drugs used for T2D. They work by triggering production of insulin. | Meglitinide (Rpaglinide)—This is an insulin secretagogue meaning that it binds to receptors on pancreatic beta-cells and stimulates insulin release. Repaglinide binds to an ATP-dependent potassium channel on beta-cells. | Hypoglycemia (low blood sugar) is associated with increased mortality and weight gain | [9] |
| Food Plant and Chitosan | Therapeutic Use | Benefits/Nutritional Value | Reference |
|---|---|---|---|
| Unripe plantain (Musa paradisiaca) |
|
| [3,34] |
| Unripe plantain (Musa paradisiaca); soya bean cake and cassava fibre. |
|
| [23,34] |
| Unripe plantain (Musa paradisiaca) and (Dioscorea rotundata) |
|
| [22,34,35] |
| Unripe plantain (Musa paradisiaca) |
|
| [5,34] |
| Unripe plantain (Musa paradisiaca L.); cocoyam (Colocasia esculenta L.). |
|
| [34,36] |
| Unripe plantain (Musa paradisiaca L.); soya bean cake and rice bran. |
|
| [27,34] |
| Unripe plantain (Musa paradisiaca L.), Ginger (Zingber officinale). |
|
| [4,34] |
| Bitter yam (Dioscorea batatas) |
|
| [22,37] |
| Okra (Abelmoschus esculentus) |
|
| [24,38] |
| Okra (Abelmoschus esculentus) |
|
| [15,38] |
| Chitosan |
|
| [39] |
| Chitosan |
|
| [40] |
| Chitosan |
|
| [41] |
| Chitosan |
|
| [42] |
| Chitosan |
|
| [30] |
| Chitosan |
|
| [31] |
| Chitosan |
|
| [32] |
| Chitosan |
|
| [33] |
| Item | Type of Bias | Domain | Description of Domain | Review Author’s Judgement |
|---|---|---|---|---|
| 1 | Selection bias | Sequence generation | There is direct evidence that cases and controls were similar, recruited within the same time frame, and controls are described as having no history of the outcome. | Yes * |
| 2 | Selection bias | Baseline characteristics | There is direct evidence that appropriate adjustments were made for covariates and confounders in the final analyses through the use of statistical models to reduce research-specific bias including standardization, matching of cases and controls, adjustment in multivariate model, stratification, propensity scoring, or other methods were appropriately justified. | Yes |
| 3 | Selection bias | Allocation concealment | There is insufficient information on concealment in the allocation of the animals into groups and subgroups. | No * |
| 4 | Performance bias | Random housing | There is direct evidence that the housing of animals was not random as they were kept in cages in the animal house. | No |
| 5 | Performance bias | Blinding | There is direct evidence that caregivers and researchers were not blinded or information was not provided. | No |
| 6 | Detection bias | Random outcome assessment | There is indirect evidence that it was possible for outcome assessors to infer the exposure level prior to reporting outcomes. | No |
| 7 | Detection bias | Blinding | Investigators also served as outcome assessors. There is direct evidence that exposure was consistently assessed using well-established methods that directly measure exposure like the blood glucose levels. | Yes |
| 8 | Attrition bias | Incomplete outcome data | There is no information provided on subject removal or exclusion from the study. | No * |
| 9 | Reporting bias | Selective outcome reporting | There is direct evidence that all of the study’s criteria were measured in the protocol, such as methods, abstract and introduction have been reported. | Yes * |
| 10 | Others | Other sources of bias | There is direct evidence that the other bias like “Units” was reported. Appropriate units such as mg/dL and mmol/L were assigned. | Yes * |
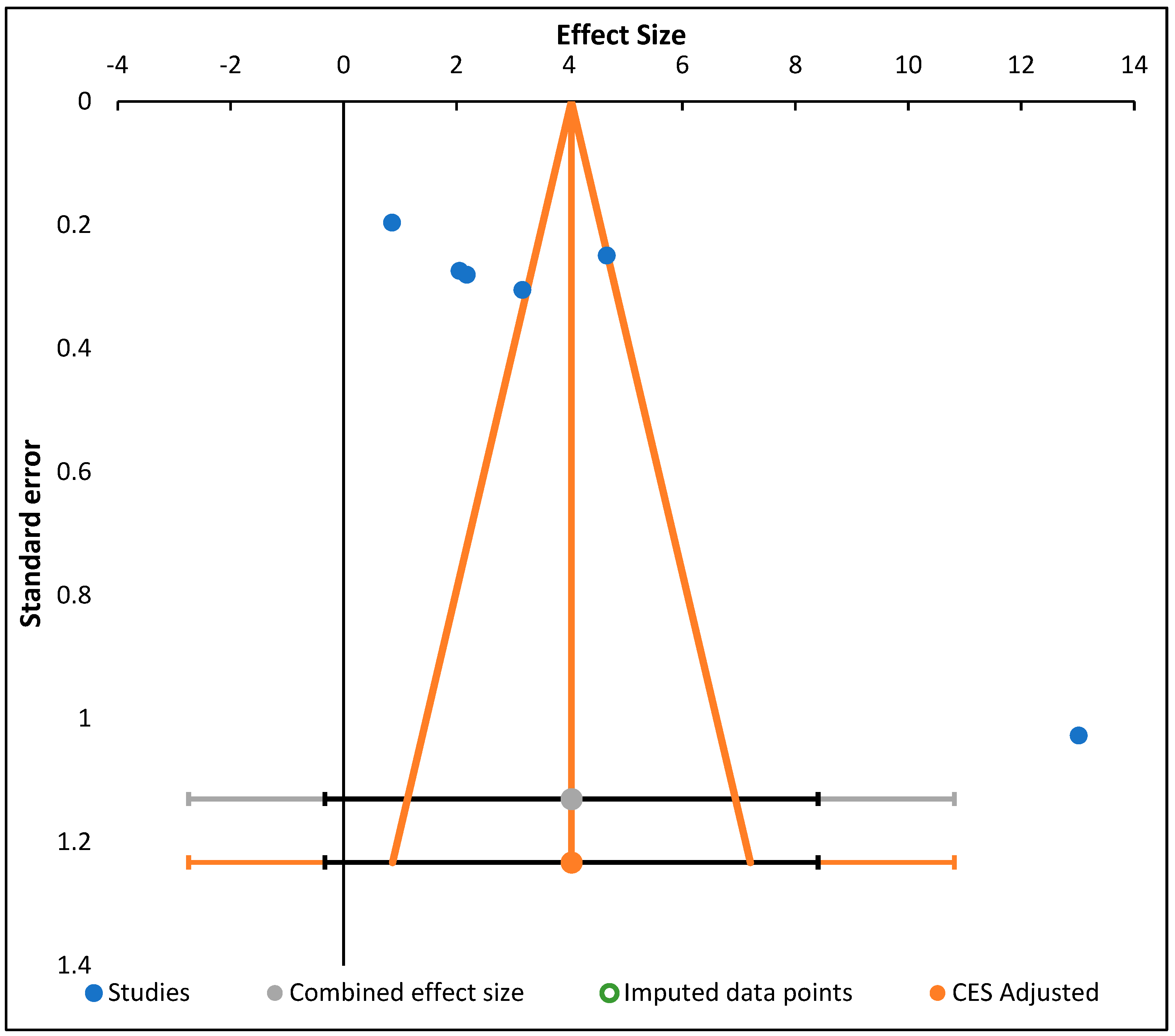
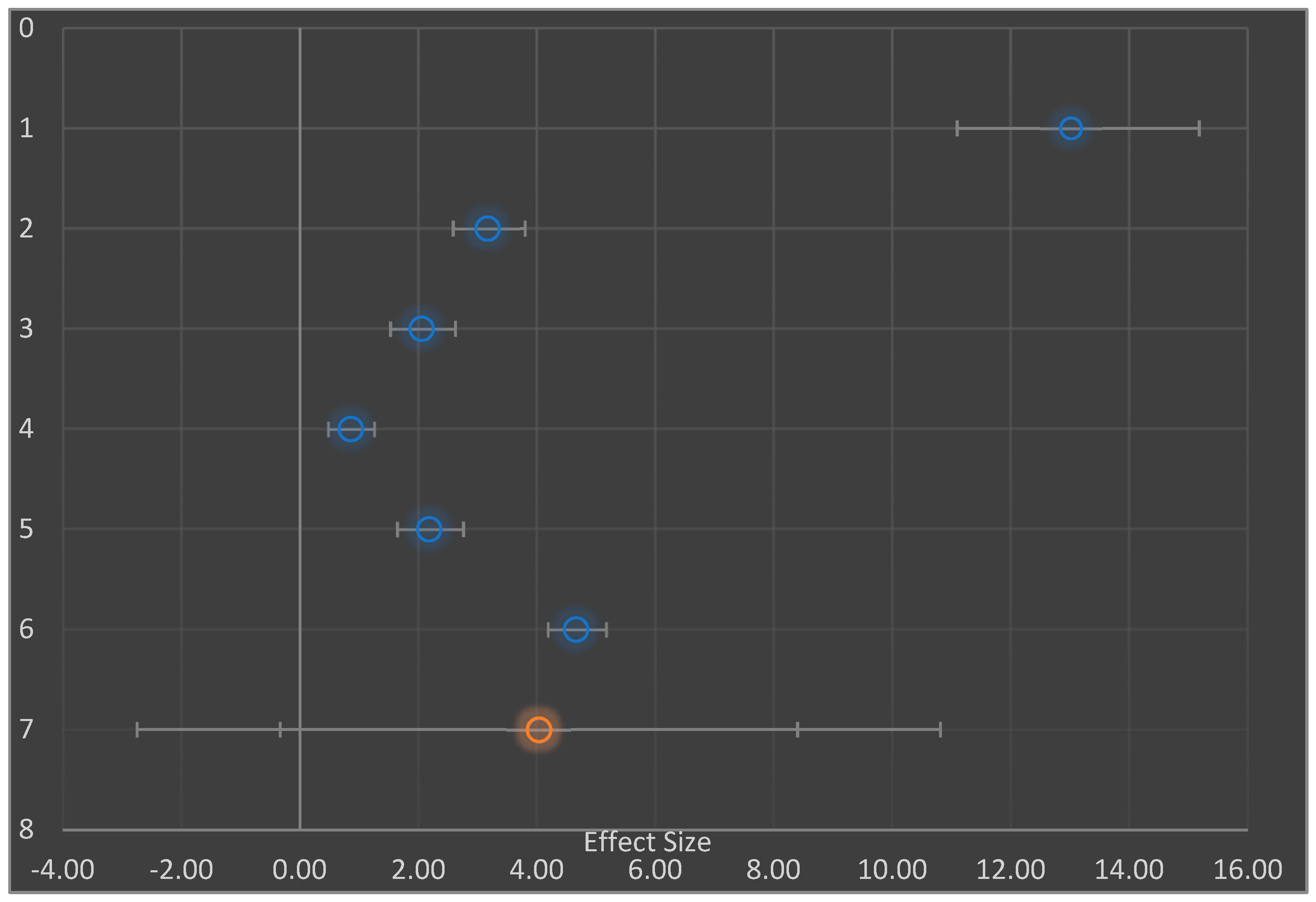
| # | Study Name/Subgroup Name | Effect Size | CI Lower Limit | CI Upper Limit | Weight | Q | PQ | I2 | T2 | T | PI Lower Limit | PI Upper Limit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shodehinde et al., 2015 [3] | 13.02 | 10.98 | 15.06 | 21.82% | |||||||
| 2 | Eleazu and Okafor; 2015 [5] | 3.17 | 2.56 | 3.78 | 25.94% | |||||||
| 3 | Eleazu et al., 2013 [36] | 2.06 | 1.51 | 2.60 | 26.03% | |||||||
| 4 | Huang et al., 2017 [15] | 0.86 | 0.47 | 1.25 | 26.22% | |||||||
| 5 | AA | 4.42 | −0.82 | 9.66 | 17.78% | 162.77 | 0.000 | 98.16% | 5.01 | 2.24 | −6.65 | 15.50 |
| 6 | Lopes et al., 2017 [42] | 2.18 | 1.62 | 2.74 | 49.87% | |||||||
| 7 | Raafat and Samy, 2014 [40] | 4.66 | 4.17 | 5.15 | 50.13% | |||||||
| 8 | BB | 3.43 | 0.99 | 5.86 | 82.22% | 43.54 | 0.000 | 97.70% | 3.00 | 1.73 | −23.65 | 30.50 |
| 9 | Combined Effect Size | 3.60 | 1.03 | 6.17 | 258.29 | 0.000 | 98.06% | 4.07 | 2.02 | 1.03 | 6.17 |
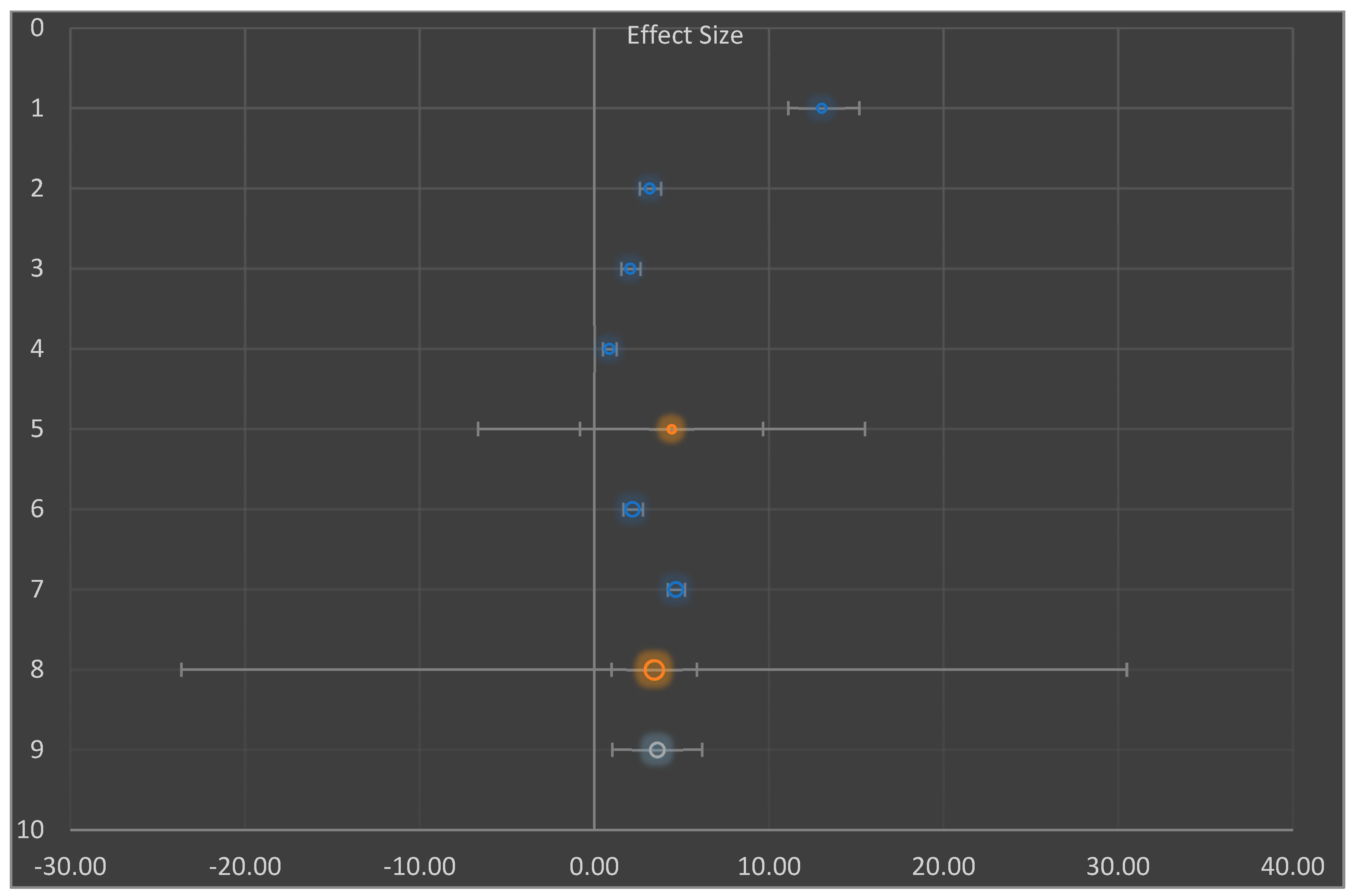
| Combined Effect Size | Observed | Heterogeneity | |
|---|---|---|---|
| Effect Size | 4.03 | Q | 258.29 |
| Standard error | 1.70 | PQ | 0.000 |
| CI Lower limit | −0.33 | I2 | 98.06% |
| CI Upper limit | 8.40 | T2 | 4.07 |
| PI Lower limit | −2.75 | T | 2.02 |
| PI Upper limit | 10.82 | ||
| Combined effect size | Adjusted | Trim and Fill | On |
| Effect Size | 4.03 | Estimator for missing studies | Leftmost Run/Rightmost run |
| Standard error | 1.70 | Search from mean | Left |
| CI Lower limit | −0.33 | Number of missing studies | 0 |
| CI Upper limit | 8.40 | ||
| PI Lower limit | −2.75 | ||
| PI Upper limit | 10.82 |
| Stydy/Country | N, Characteristics [No. of Cells-In Vitro or No of Rats-In Vivo, Weight (Wt.) of Rats (Rts) No of Group (Grp)] | Study Duration | FP or Material and Family/BM/T | Reagent for Induction of Diabetes/SL(Average) Post Induction | Description of the Study | Outcome Measures with the Use of FP |
|---|---|---|---|---|---|---|
| Shodehinde, S.A. et al. Life Sci. 2015 [3]/ Nigeria | 42 male rats (in vivo), Wt = 200 g, Grp-7 |
|
| Streptozotocin/≥250 mg/dL | The effect of the diets on the blood glucose level, pancreatic α-amylase, intestinal and α-glucosidase content of the unripe plantain products was determined. |
|
| Famakin, O. et al. J. Food Sci. Technol. 2016 [23]/Nigeria | 60 Wistar albino, Wt = 150 g Grp-6 |
|
|
|
| Blood glucose change from 355 ± 43 to 103 ± 14 mg/dL (p < 0.05). Weight change noted |
| Ajiboye, B.O. et al., Food Sci Nutr. 2018 [35] Nigeria | 48 Albino rat, Wt = 150 ± 20 gGrp- 4 |
|
|
|
| Blood glucose change from 350 to 100 mg/dL (p < 0.05). Physique heaviness as well as hyperglycemia was measured |
| Eleazu, C.O.; Okafor, P. Interv. Med. Appl. Sci. 2015 [5] Nigeria | 48 male albino, Wt = 243 g, Grp-3 |
|
|
|
|
|
| Eleazu, C.O.; et al. J. Diabetes Res. 2013 [36] Nigeria | 40 male albino, Wt= 288.74 g |
|
|
|
| Hyperglycemia as well as heaviness change Decreased of SL by 38.13% (p < 0.05)
|
| Sukanya, C. et al. Glyset (Miglitol). Bull Environ Contam Toxicol. 2016 [27]. Nigeria | 35 Wistar albino, Wt = 150 g, Grp-5. |
|
|
|
|
|
| Iroaganachi, M.; et al., Biochem. J. 2015 [4]. Nigeria | 30 male albino, Wt = 232.91 g, Grp-4 |
|
|
|
|
|
| Hooijmans, C.R.;et al., ILAR J. (2014), [37]. Nimenibo-Uadia, R. et al., Pa. J. Appl. SCI. Environ Manag. 2017 [22]. Republic of Korea |
Grp-5 |
|
|
| Suspended crude yam powder-treated diabetic; water extract of yam-treated diabetic and allantoin-treated diabetic group normal control, STZ-induced diabetic control |
|
| Nguekouo, P.T.;et al., J. Food Biochem. 2018 [24]. Thule, Umpierrez. Curr Diab Rep, 2014, 14 [28]. Cameroon |
Grp-6 |
|
|
|
| Blood glucose change from 333 ± 20 to 119 ± 18 mg/dl (p < 0.05) |
| Huang, C.; et al., PLoS One. 2017 [15]. South Africa |
Wt = 250 ± 20 g Grp10. |
|
|
|
| Blood Glucose change from 500 to 120 mg/dL
|
| Lee, J.; et al., J. Control Release. 2012 [39]. Republic of Korea | 48 male db/db mice, Wt unspecified, Grp-8, |
|
|
|
| Blood Glucose change from 150 to 57 mg/dL (p < 0.05)
|
| Raafat, K.; et al., Evid. Based Compl. Alt. 2014 [40]. Lebanon | 112 Swiss webster mice, Wt = 280 gGrp-16. |
|
|
|
|
|
| 112 Swiss, webster mice, Wt= 280 g, Grp-16 |
|
| Alloxan monohydrate, 200 mg/dL |
|
| |
| Ahn, S.; et al., J. Control Release, 2013 [41]. Republic of Korea | Mouse, Pancreatic Cell lines (INS-1), 18 male db/db mice, Wt unspecified Grp-3) |
|
|
|
|
|
| Lopes, M.;et al.; Eur. J. Pharm. Biopharma. 2017 [42]. Portugal | 36 male wistar, Wt= 350 g, Grp-3 |
|
| Streptozotocin, ≥14 mM Subcutaneous (S.C.)
|
The oral administration of 50 IU/kg insulin-loaded nanoparticles to Type 1 diabetic rats resulted in extended period of antihyperglycemic effects up to 12 h and relative pharmacological availability of 5.04% comparing to the subcutaneous administration. |
|
|
|
|
|
| Sustained drug-release time 10 days | |
| Lee, C; et al., Acta Biomater. 2014 [30]. Republic of Korea | Murine, melanoma B16F10 cell lines (20,000 cells seeded), 18 male db/db mice, Grp-3 Wt not specified |
|
|
|
Blank DA-GC hydrogels were also administered as controls.
|
|
| Song, L.; et al., Int. J. Nanomedicine. 2014 [31]. United Kindom | 30 male mice, Wt= 30 g Grp-5 |
|
|
|
| Blood glucose change from 60 to 20 mMolL (p < 0.05) and sustained drug release dosage. |
| Jo, S.; et al., Int. J. Mol. Sci. 2013 [32]. Republic of Korea | 30 male Sprague Dawley (SD), Wt= 200 g, Grp-6 |
|
|
|
| Blood Glucose change From193 to 152 mg/dL (p < 0.5), α-amylase and α-glucosidase inhibition at sustained time. |
| Szekalska, M.; et al., 2017 [33]. Poland | 30 male Sprague Dawley (SD), Wt = 250 ± 20 g Grp-5. |
|
|
|
|
|
| Selection Bias | Selection Bias | Selection Bias | Performance Bias | Performance Bias | Detection Bias | Detection Bias | Attrition Bias | Reporting Bias | Others | Overall Review Author’s Judgement | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [3] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [23] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [35] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [5] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [36] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [2] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [4] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [37] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [24] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [15] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [39] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [40] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [41] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [30,42] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [31] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [32] |
| Low risk | Low risk | High risk | High risk | High risk | High risk | Low risk | High risk | Low risk | Low risk | Unclear * | [33] |
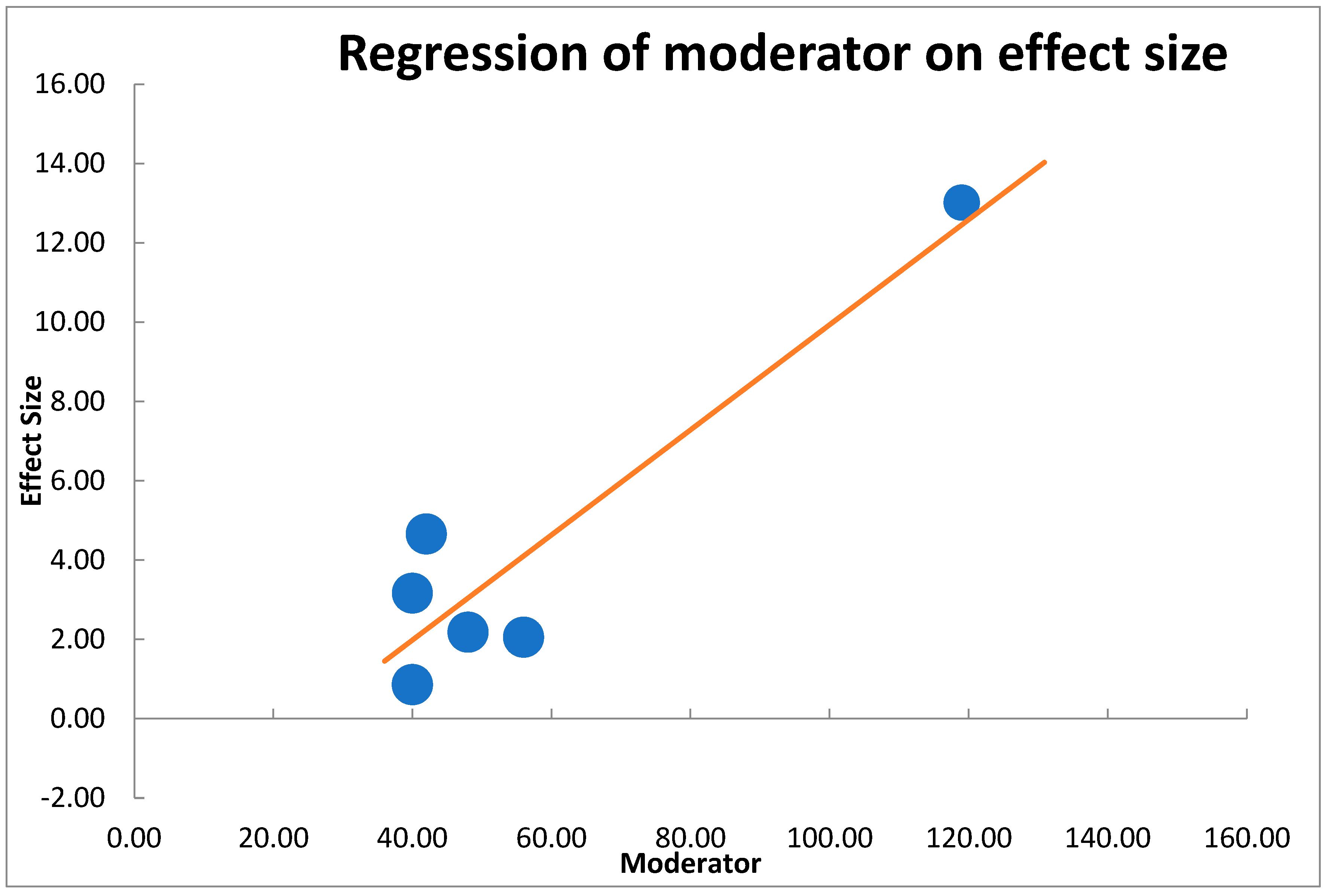
| B | SE | CI LL | CI UL | β | Z-Value | p-Value | |
|---|---|---|---|---|---|---|---|
| Intercept | −3.31791 | 1.81 | −7.98 | 1.34 | −1.83 | 0.067 | |
| Moderator | 0.13252 | 0.03 | 0.06 | 0.21 | 0.91 | 4.45 | 0.000 |
| Analysis of variance | Sum of squares (Q*) | df | p | Mean square | F-Value | p-value | |
| Model | 19.79 | 1 | 0.000 | 19.79 | 19.75 | 0.011 | |
| Residual | 4.01 | 4 | 0.405 | 1.00 | |||
| Total | 23.80 | 5 | 0.000 | ||||
| Combined effect size | 3.98 | ||||||
| T2 (method of moments estimation) | 3.34 | ||||||
| R2 | 83.16% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloysius, M.; Felekkis, K.N.; Petrou, C.; Papandreou, D.; Andreou, E. Chitosan Nanogel with Mixed Food Plants and Its Relation to Blood Glucose in Type 2 Diabetes: A Systematic and Meta-Analysis Review of Observational Studies. Nutrients 2022, 14, 4710. https://doi.org/10.3390/nu14224710
Aloysius M, Felekkis KN, Petrou C, Papandreou D, Andreou E. Chitosan Nanogel with Mixed Food Plants and Its Relation to Blood Glucose in Type 2 Diabetes: A Systematic and Meta-Analysis Review of Observational Studies. Nutrients. 2022; 14(22):4710. https://doi.org/10.3390/nu14224710
Chicago/Turabian StyleAloysius, Morris, Kyriacos N. Felekkis, Christos Petrou, Dimitrios Papandreou, and Eleni Andreou. 2022. "Chitosan Nanogel with Mixed Food Plants and Its Relation to Blood Glucose in Type 2 Diabetes: A Systematic and Meta-Analysis Review of Observational Studies" Nutrients 14, no. 22: 4710. https://doi.org/10.3390/nu14224710
APA StyleAloysius, M., Felekkis, K. N., Petrou, C., Papandreou, D., & Andreou, E. (2022). Chitosan Nanogel with Mixed Food Plants and Its Relation to Blood Glucose in Type 2 Diabetes: A Systematic and Meta-Analysis Review of Observational Studies. Nutrients, 14(22), 4710. https://doi.org/10.3390/nu14224710










