Effects of Green Kiwifruit Peel Extract on Sleep-Wake Profiles in Mice: A Polysomnographic Study Based on Electroencephalogram and Electromyogram Recordings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GKPEE
2.3. Animals
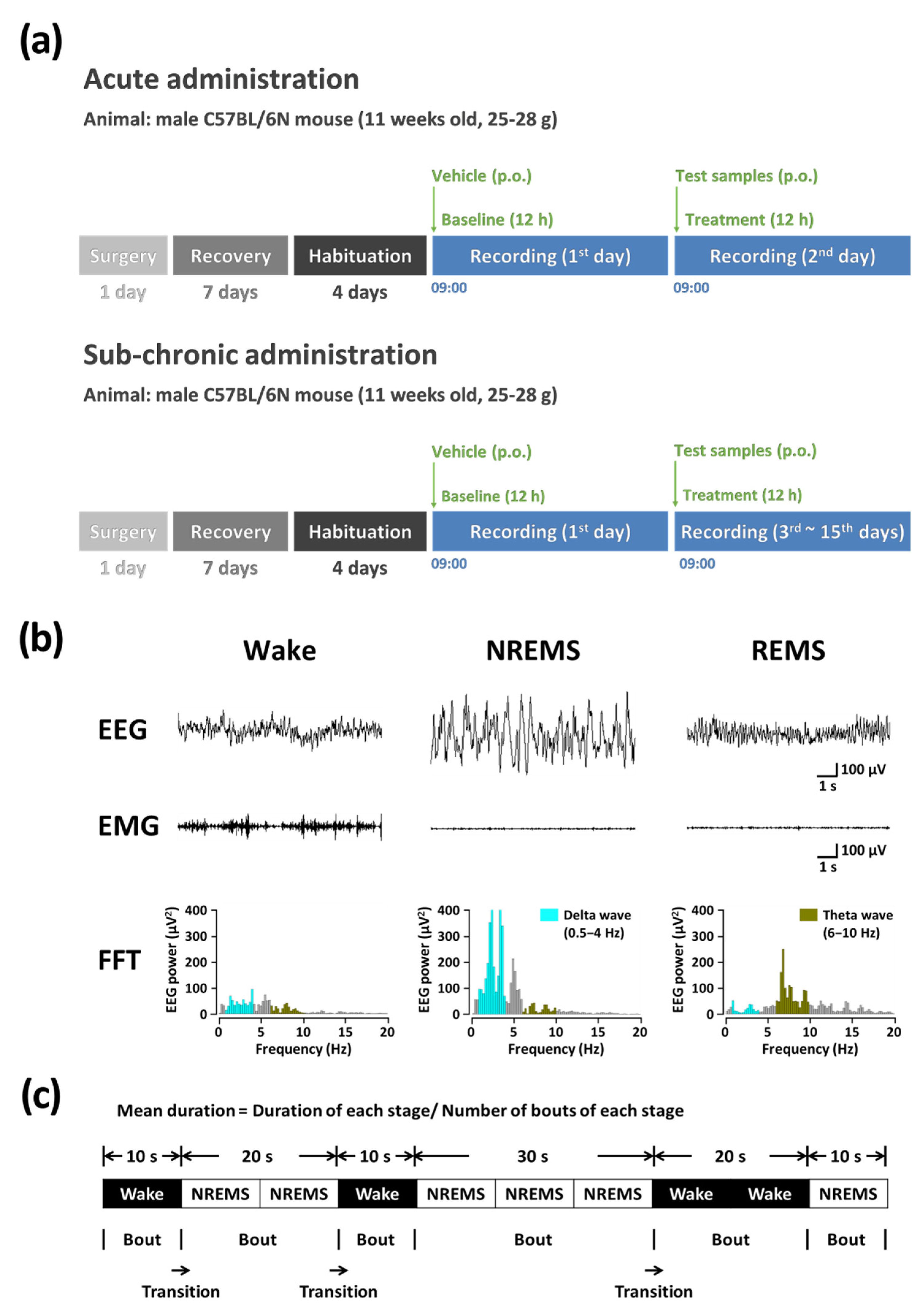
2.4. Analysis of Sleep Architecture
2.4.1. Acute Administration
2.4.2. Sub-Chronic Administration
2.4.3. Polysomnographic Recordings and Vigilance State
2.5. Statistical Analysis
3. Results
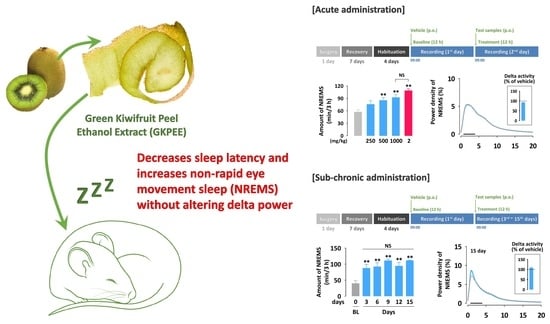
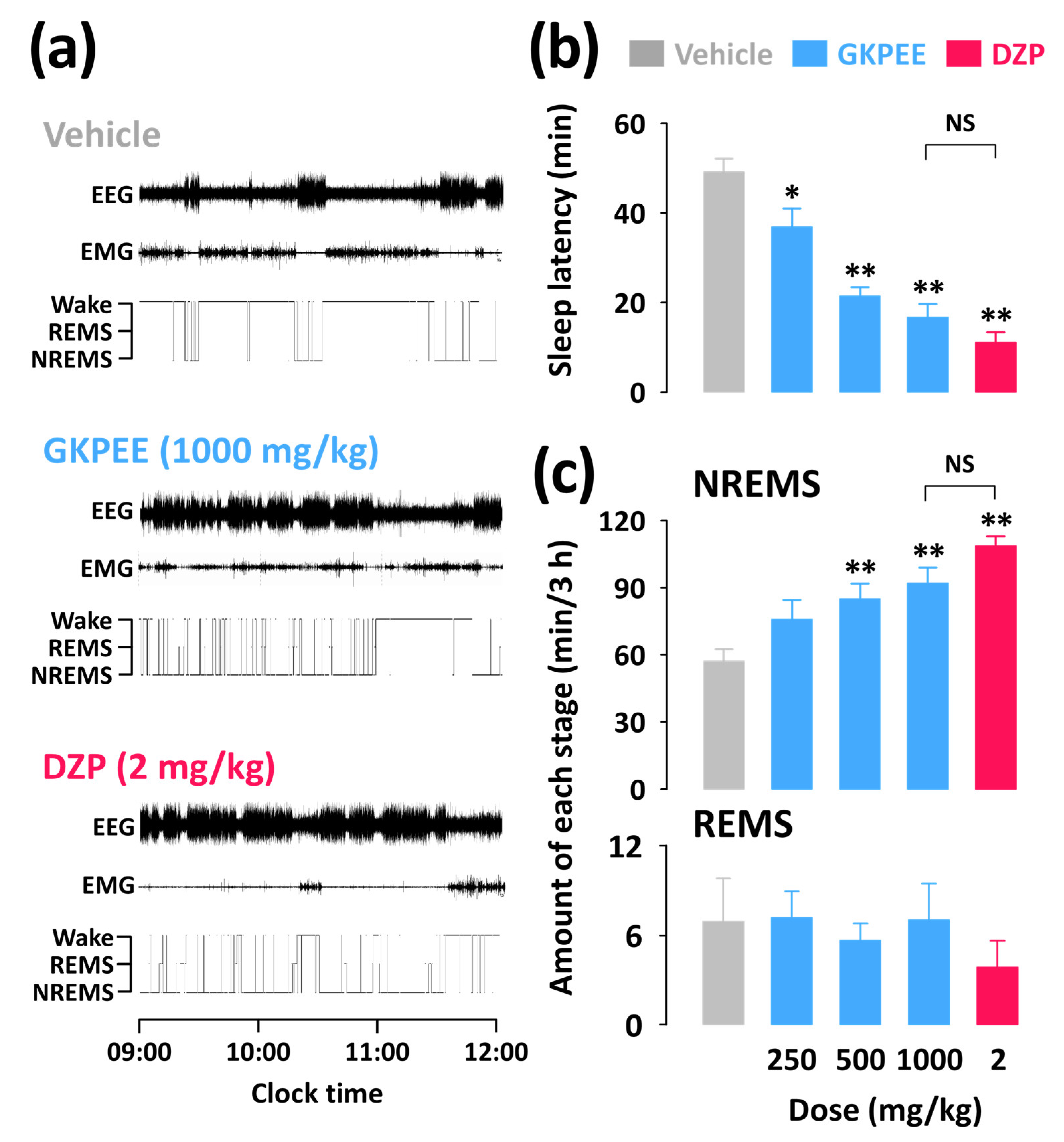
3.1. Effects of Acute Administration of GKPEE on Sleep-Wake Profiles
3.2. Effects of Acute Administration of GKPEE on Time-Course Changes in Each Sleep Stage
3.3. Effects of Acute Administration of GKPEE on Sleep-Wake Episode and Delta Activity
3.4. Effects of Sub-Chronic Administration of GKPEE on Sleep-Wake Profiles and Delta Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larocca, M.; Rossano, R.; Riccio, P. Analysis of green kiwi fruit (Actinidia deliciosa cv.Hayward) proteinases by two-dimensional zymography and direct identification of zymographic spots by mass spectrometry. J. Sci. Food Agric. 2010, 90, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Rajoka, M.S.R.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Ilie, G.-I.; Milea, Ș.-A.; Râpeanu, G.; Cîrciumaru, A.; Stănciuc, N. Sustainable Design of Innovative Kiwi Byproducts-Based Ingredients Containing Probiotics. Foods 2022, 11, 2334. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Shirataki, Y.; Kawase, M.; Tani, S.; Sakagami, H.; Satoh, K.; Kurihara, T.; Nakashima, H.; Wolfard, K.; Miskolci, C. Biological activity of kiwifruit peel extracts. Phytother. Res. 2001, 15, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Scognamiglio, M.; Monaco, P. Identification and assessment of antioxidant capacity of phytochemicals from kiwi fruits. J. Agric. Food Chem. 2009, 57, 4148–4155. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Cordiner, S.B.; Sawyer, G.M.; McGhie, T.K.; Espley, R.V.; Allan, A.C.; Hurst, R.D. Kiwifruit with high anthocyanin content modulates NF-κB activation and reduces CCL11 secretion in human alveolar epithelial cells. J. Funct. Foods 2020, 65, 103734. [Google Scholar] [CrossRef]
- Jung, K.-A.; Song, T.-C.; Han, D.; Kim, I.-H.; Kim, Y.-E.; Lee, C.-H. Cardiovascular protective properties of kiwifruit extracts in vitro. Biol. Pharm. Bull. 2005, 28, 1782–1785. [Google Scholar] [CrossRef] [Green Version]
- Pastorello, E.A.; Conti, A.; Pravettoni, V.; Farioli, L.; Rivolta, F.; Ansaloni, R.; Ispano, M.; Incorvaia, C.; Giuffrida, M.G.; Ortolani, C. Identification of actinidin as the major allergen of kiwi fruit. J. Allergy Clin. Immunol. 1998, 101, 531–537. [Google Scholar] [CrossRef]
- Sanz, V.; López-Hortas, L.; Torres, M.; Domínguez, H. Trends in kiwifruit and byproducts valorization. Trends Food Sci. Technol. 2021, 107, 401–414. [Google Scholar] [CrossRef]
- Borja, N.L.; Daniel, K.L. Ramelteon for the treatment of insomnia. Clin Ther. 2006, 28, 1540–1555. [Google Scholar] [CrossRef]
- Doghramji, K. The epidemiology and diagnosis of insomnia. Am. J. Manag. Care 2006, 12, S214. [Google Scholar]
- National Sleep Foundation. 2011 Sleep in America Poll: Communications Technology in the Bedroom. Available online: https://teensneedsleep.files.wordpress.com/2011/05/national-sleep-foundation-2011-sleep-in-america-poll-communications-technology-in-the-bedroom.pdf (accessed on 27 September 2022).
- Lin, H.-H.; Tsai, P.-S.; Fang, S.-C.; Liu, J.-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174. [Google Scholar]
- Nødtvedt, Ø.O.; Hansen, A.L.; Bjorvatn, B.; Pallesen, S. The effects of kiwi fruit consumption in students with chronic insomnia symptoms: A randomized controlled trial. Sleep Biol. Rhythm. 2017, 15, 159–166. [Google Scholar] [CrossRef]
- Yang, H.; Lee, Y.-C.; Han, K.-S.; Singh, H.; Yoon, M.; Park, J.-H.; Cho, C.-W.; Cho, S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013, 136, 160–163. [Google Scholar] [CrossRef]
- Kohtoh, S.; Taguchi, Y.; Matsumoto, N.; Wada, M.; Huang, Z.-L.; Urade, Y. Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol. Rhythm. 2008, 6, 163–171. [Google Scholar] [CrossRef]
- Tobler, I.; Kopp, C.; Deboer, T.; Rudolph, U. Diazepam-induced changes in sleep: Role of the α1 GABAA receptor subtype. Proc. Natl. Acad. Sci. USA 2001, 98, 6464–6469. [Google Scholar] [CrossRef] [Green Version]
- Askari, V.R.; Rahimi, V.B.; Ghorbani, A.; Rakhshandeh, H. Hypnotic Effect of Ocimum basilicum on Pentobarbital-Induced Sleep in Mice. Iran. Red. Crescent. Med. J 2016, 18, e24261. [Google Scholar] [CrossRef] [Green Version]
- Rakhshandeh, H.; Heidari, A.; Pourbagher-Shahri, A.M.; Rashidi, R.; Forouzanfar, F. Hypnotic Effect of A. absinthium Hydroalcoholic Extract in Pentobarbital-Treated Mice. Neurol. Res. Int. 2021, 2021, 5521019. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Tajani, A.S.; Hosseini, A.; Rakhshandeh, H. Evaluation of the Sleep-Prolonging Effect of Lagenaria vulgaris and Cucurbita pepo Extracts on Pentobarbital-Induced Sleep and Possible Mechanisms of Action. Medicina 2018, 54, 55. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Shimizu, M. Natural sleep aids and polyphenols as treatments for insomnia. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 141–151. [Google Scholar]
- Johnston, G.A. GABAA receptor channel pharmacology. Curr. Pharm. Des. 2005, 11, 1867–1885. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Tan, R.; Qu, W.; Wu, Z.; Wang, Y.; Urade, Y.; Huang, Z. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, exerts antiepileptic effects via the GABA/benzodiazepine receptor complex in mice. Br. J. Pharmacol. 2011, 164, 1534–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Obara, Y.; Kamei, C. Effects of some antipsychotics and a benzodiazepine hypnotic on the sleep-wake pattern in an animal model of schizophrenia. J. Pharmacol. Sci. 2009, 111, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.A.; Smith, P.F.; Darlington, C.L. The behavioural and neuronal effects of the chronic administration of benzodiazepine anxiolytic and hypnotic drugs. Prog. Neurobiol. 1996, 49, 73–97. [Google Scholar] [CrossRef]
- Ebert, B.; Anderson, N.J.; Cremers, T.I.; Rasmussen, S.; Vogel, V.; Fahey, J.M.; Sánchez, C. Gaboxadol—A different hypnotic profile with no tolerance to sleep EEG and sedative effects after repeated daily dosing. Pharmacol. Biochem. Behav. 2008, 90, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Drake, C. Evolution of insomnia: Current status and future direction. Sleep Med. 2004, 5, S23–S30. [Google Scholar] [CrossRef]
- Fang, X.S.; Hao, J.; Zhou, H.; Zhu, L.; Wang, J.; Song, F. Pharmacological studies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine 2010, 17, 75–80. [Google Scholar] [CrossRef]
- Barnes, J.; Eugene, M. Intracellular trafficking of GABAA receptors. Life Sci. 2000, 66, 1063–1070. [Google Scholar] [CrossRef]
- Lou, S.-N.; Hsu, Y.-S.; Ho, C.-T. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J. Food Drug Anal. 2014, 22, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yang, Y.-f.; Zhou, Z.-q. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.; Tsim, K.W. A review of dietary Ziziphus jujuba fruit (Jujube): Developing health food supplements for brain protection. Evid.-Based Complement. Altern. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.-R.; Kim, Y.; Jo, A.; Choi, E.J.; Oh, K.-N.; Kim, J.; Kang, H.; Kim, Y.R.; Choi, C.Y. Sedative and hypnotic effects of Vaccinium bracteatum Thunb. through the regulation of serotonegic and GABAA-ergic systems: Involvement of 5-HT1A receptor agonistic activity. Biomed. Pharmacother. 2019, 109, 2218–2227. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, L.; Qi, Y.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Antioxidative Properties and Phenolic Profile of the Core, Pulp and Peel of Commercialized Kiwifruit by LC-ESI-QTOF-MS/MS. Processes 2022, 10, 1811. [Google Scholar] [CrossRef]
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of bioactive compounds extracted from Hayward kiwifruit pomace by subcritical water extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]




| Method | Pentobarbital-Induced Sleep Test | Polygraphic Recordings | ||
|---|---|---|---|---|
| Animal | ICR mouse | C57BL/6N mouse | ||
| Measurements | Righting reflex | EEG and EMG | ||
| Advantages | Short assay time, possible to screen many samples | Assessment of both sleep quantity and quality | ||
| Disadvantages | Impossible to evaluate sleep quality | Long assay time, high cost | ||
| Evaluation markers and hypnotic effects of the kiwifruit peel extract | Sleep latency Sleep duration | Significant decrease [15] Significant increase [15] | Sleep latency NREMS amount REMS amount Delta activity | None None None None |
| Method | Pentobarbital-Induced Sleep Test | Polygraphic Recordings | ||
|---|---|---|---|---|
| Hypnotic agents | DZP | GKPEE | DZP | GKPEE |
| Sleep quantity | Increase | Increase | Increase | Increase |
| Sleep intensity | None | None | Decrease | No effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Yoon, M.; Kim, S.; Um, M.Y.; Cho, S. Effects of Green Kiwifruit Peel Extract on Sleep-Wake Profiles in Mice: A Polysomnographic Study Based on Electroencephalogram and Electromyogram Recordings. Nutrients 2022, 14, 4732. https://doi.org/10.3390/nu14224732
Kim D, Yoon M, Kim S, Um MY, Cho S. Effects of Green Kiwifruit Peel Extract on Sleep-Wake Profiles in Mice: A Polysomnographic Study Based on Electroencephalogram and Electromyogram Recordings. Nutrients. 2022; 14(22):4732. https://doi.org/10.3390/nu14224732
Chicago/Turabian StyleKim, Duhyeon, Minseok Yoon, Seonghui Kim, Min Young Um, and Suengmok Cho. 2022. "Effects of Green Kiwifruit Peel Extract on Sleep-Wake Profiles in Mice: A Polysomnographic Study Based on Electroencephalogram and Electromyogram Recordings" Nutrients 14, no. 22: 4732. https://doi.org/10.3390/nu14224732
APA StyleKim, D., Yoon, M., Kim, S., Um, M. Y., & Cho, S. (2022). Effects of Green Kiwifruit Peel Extract on Sleep-Wake Profiles in Mice: A Polysomnographic Study Based on Electroencephalogram and Electromyogram Recordings. Nutrients, 14(22), 4732. https://doi.org/10.3390/nu14224732