Dietary and Nutritional Support in Gastrointestinal Diseases of the Upper Gastrointestinal Tract (I): Esophagus
Abstract
1. Introduction
2. Aim
3. Methods
4. Dietary and Nutritional Support in Esophageal Diseases
4.1. Severe Oropharyngeal Dysphagia
- (1)
- For those patients who do not show adequate tolerance of liquids, the use of certain additives with thickening properties may be helpful in improving their swallowing ability. The European Society for Swallowing Disorders (ESSD) has described the evidence in the literature on the effect that bolus modification has upon the physiology, efficacy, and safety of swallowing in adults with OD of diverse etiologies [11]. These studies show that increasing the viscosity from liquid to nectar and pudding reduces the prevalence of penetrations and aspirations, suggesting that patients with OD do indeed benefit from taking fluids with increased viscosity, which reduces the risk of laryngeal penetration and/or aspiration [11,36,37];
- (2)
- The transfer of the food bolus can be improved if the mouthfuls are small in volume;
- (3)
- Alternation of solid and liquid boluses can also facilitate transfer;
- (4)
- For those patients with severe dysphagia of neurological origin, the assistance of a caregiver may be critical, and the meals should be administered during times of maximal attentiveness;
- (5)
- Finally, for those patients who are refractory to all these measures or at high risk for aspiration (e.g., severe neuromuscular dysfunction), enteral nutrition should be provided, preferably by endoscopic, percutaneous gastrostomy. If the patient also has gastroparesis, a double lumen feeding tube can be attempted, whereby one is placed in the stomach to aspirate the gastric remnant (e.g., biliary reflux) and the other in the duodenum for nutrient perfusion.
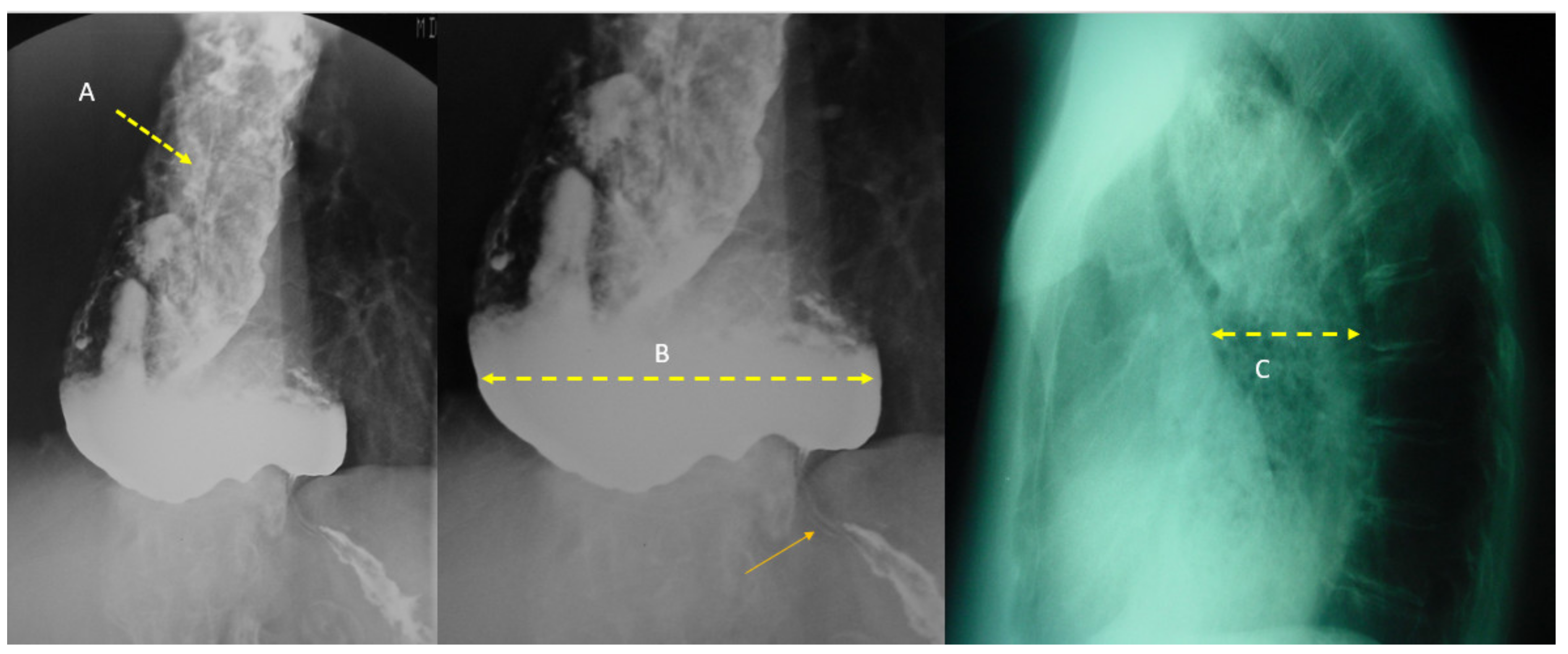
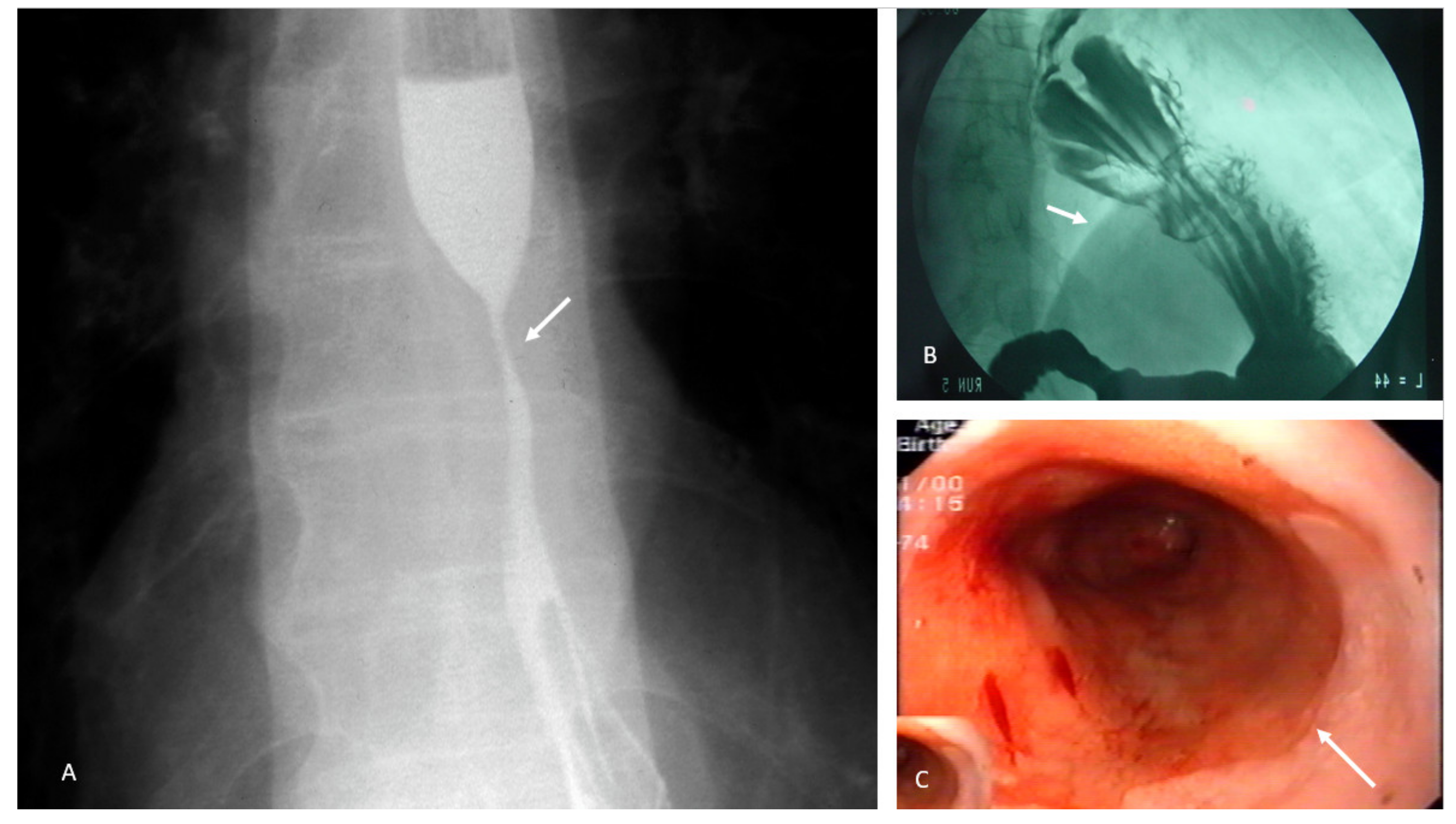
4.2. Achalasia
| Clinical Condition | Percentage | Reference |
|---|---|---|
| Older age | 15–40% | [39,40,41,42] |
| Stroke | 37–78% | [39,40,43,44] |
| Neurodegenerative diseases | ||
| 52–82% | [39,40,45,46] |
| 57–84% | [39,40,47,48,49] |
| 30–100% | [50] |
| Traumatic brain injury | 25 | [40,51,52] |
| Head and neck cancer | 44–50% | [39,53,54] |
- Smooth muscle relaxants;
- Botulinum toxin injections to the lower sphincter;
- Pneumatic dilation;
- Heller myotomy;
- Peroral endoscopic myotomy.
4.3. Eosinophilic Esophagitis
- (1)
- The patient or their caregivers should be informed about the pros and cons of each available option before planning;
- (2)
- When available, a registered dietician or nutritionist who is familiar with food allergies is a very valuable part of the patient’s care team;
- (3)
- A diet based on the empirical elimination of six foods (milk, wheat, egg, soy/legumes, nuts, fish/seafood) (6-FED) was initially considered the gold standard for the management of EoE [76,77,78]. The 6-FED followed by an endoscopic procedure before reintroducing a food and after checking histological healing showed that cow milk (especially in children < 10 years old), wheat, egg, legumes and, to a lesser extent, soy were the most common food triggers for EoE in both children and adults [78]. Nevertheless, this dietary approach did not become popular for patients and caregivers due to the need for numerous endoscopies and the high level of restriction for almost a year [76];
- (4)
- (5)
- Studies of 4-FED demonstrated that around half of responders had one or two food triggers (usually milk and wheat) [79]. Following these findings, some authors advocate starting with a two-food (cow milk and wheat) elimination diet, and in the case of nonresponse, sequentially escalating the diet to 4-FED and then 6-FED. This strategy achieves 43%, 60%, and 75% histologic remission rates, respectively [81]. Therefore, an empirical staged elimination diet, starting with one or two food groups, represents a pragmatic dietary approach for children and adult patients with EoE [58];
- (6)
- Of interest, Spanish authors have investigated the tolerance of sterilized cow milk (boiled instead of UHT-processing) regarding maintenance of EoE remission, health-related quality of life (HRQoL), nutritional intake, and allergic sensitization in patients of all ages with milk-triggered EoE. Notably, the results of this elegant study demonstrate that sterilized milk did not trigger EoE in two-thirds of patients with documented milk-induced EoE in either the short or long term [82];
- (7)
- The highest success rates in symptomatic and histologic improvement are seen with the elemental diet. However, even in children, this diet is the most difficult to follow. This diet should be restricted to patients who have not responded to any other approach, when seeking a nutrient supply that cannot be achieved by any other means, or the patient manifests a desire to initiate treatment in this way and if resources allow it.
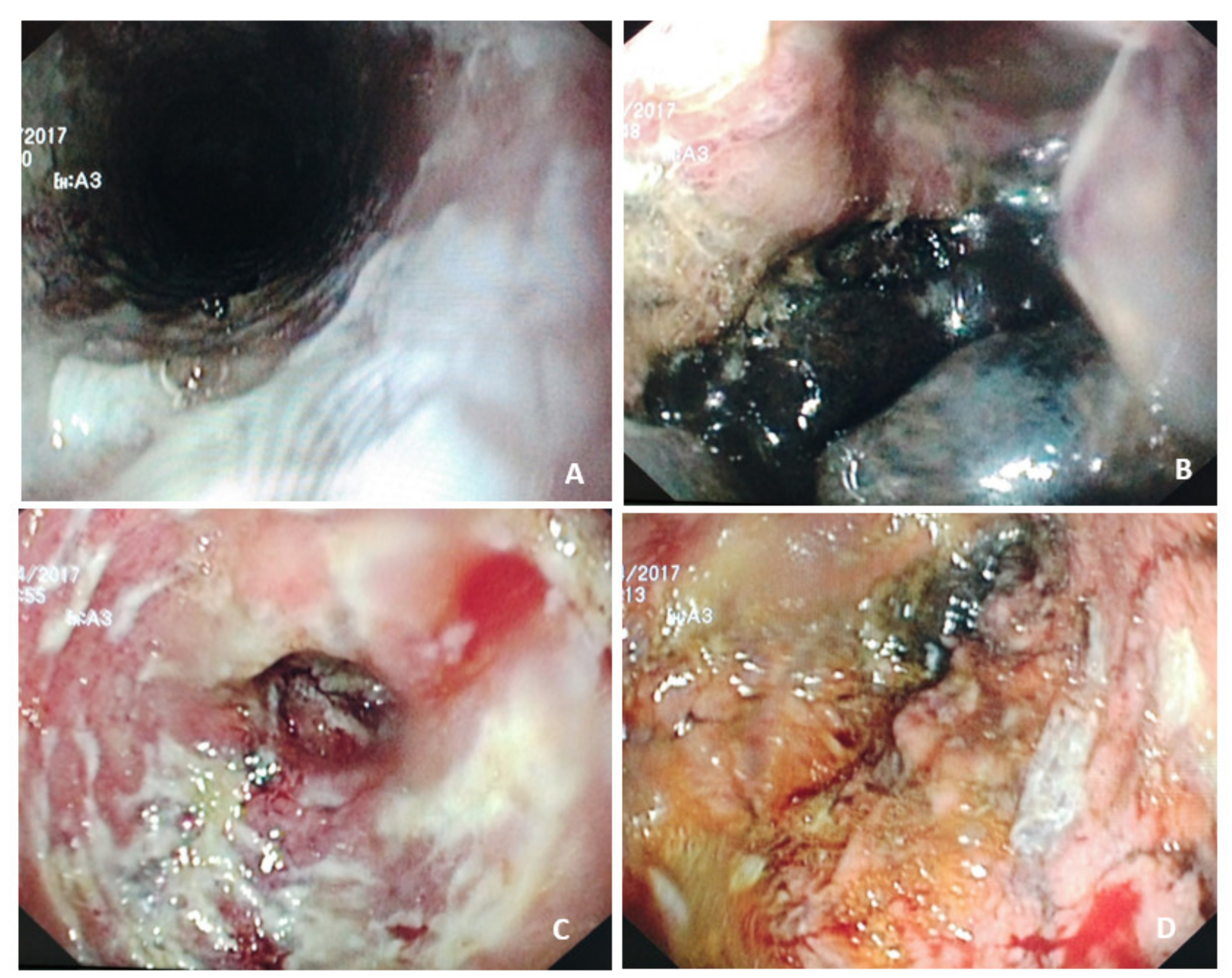
4.4. Caustic Injuries
| Therapy | Further Information |
|---|---|
| Proton Pump Inhibitors (PPIs) | |
| 1 Increase the dose up to twice daily in the absence of symptomatic relief after 4 weeks. Alternatively, initiate PPI with a twice-daily dose. |
| Fluticasone propionate | |
| 1 Patients should not eat or drink for 30 min following administration remission. 2 For patients with episodic or seasonal flares, fluticasone may be administered on request rather than as daily therapy. |
| Budesonide [55,65,66,67,68,69,70,71,72,73] | |
| 1 Viscous budesonide can be compounded by mixing two or four 0.5 mg/2 mL Pulmicort Respules with sucralose (Splenda; 10 × 1 g packets per 1 mg of budesonide, creating a volume of approximately 8 mL) [55,65]. 2 Limited availability [55,67]. 3 After 12 weeks. 4 After 48 weeks. |
| Mometasone furoate1 Dose: | |
| 1 Has only been tested in children. Because of its lower bioavailability, it has potentially fewer adverse effects than other steroids [85]. |
| Ciclesonide 1 Dose: | |
| 1 Has only been tested in children. Because of its lower bioavailability, it has potentially fewer adverse effects than other steroids [85] |
- (1)
- In asymptomatic patients without oral burns and a history of low-volume, accidental ingestion of low-concentration acid or alkali, upper endoscopy is not necessary. Such patients may be discharged from the hospital, and a diet based on soft foods or liquids for the first 24–48 h is recommended;
- (2)
- Patients who have ingested a substance with a high risk of esophageal injury (high-concentration acid or alkali or a high volume (>200 mL) of a low-concentration acid or alkali) should be hospitalized. Nutritional support should be initiated with hemodynamic stabilization and the restoration of fluids, electrolytes, and acid–base balance [92];
- (3)
- Corrosive ingestion injuries up to Zargar 2A-grade 1-CT (low-grade injuries) do not cause long-term sequelae and do not require advanced nutrition. Oral feeding should be reintroduced as soon as patients are swallowing normally, and they should be discharged quickly from the hospital (usually within the first 24–48 h) [92,93];
- (4)
- Patients with grade 2A-CT esophageal injuries have a low risk (<20%) of stricture formation [94]. Oral nutrition is usually well tolerated and should be introduced as soon as pain diminishes, and patients can swallow. Oral liquids are allowed after the first 48 h if the patient is able to swallow saliva. If patients are unable to tolerate oral liquids, early enteral feeding is provided through a nasojejunal tube or jejunostomy;
- (5)
- Patients with grade 2-CT lesions will develop stricture in 80% of cases. Pain, sialorrhea, and odynophagia are frequent during the acute phase and can severely limit swallowing, making oral feeding impossible. Such cases may benefit from nutritional support by the nasoenteral route, jejunostomy and, as a last resort, exclusive parenteral nutrition. The decision to adopt one procedure over another is dependent on how long before the patient is expected to be able to restart feeding and their tolerance [91,94]. Kochhar et al. compared the nutritional parameters of 53 and 43 patients with severe acute corrosive injury supplied with nasoenteral tube (NETF) or jejunostomy feeding (JF), respectively. NETF was found to be as effective as JF in maintaining nutrition, and the rate of complications was similar (including the development of strictures). However, NETF provided a lumen for dilatation that was useful as a guide for performing the procedure [95];
- (6)
- Signs of perforation (e.g., mediastinitis, peritonitis), major metabolic disorders, and CT evidence of transmural necrosis of the esophagus or stomach (grade 3-CT) in patients are indications for emergency surgery. In all these cases, the surgeon disrupts the continuity of the gastrointestinal tract to save the patient’s life, making oral feeding virtually impossible. The most common interventions in this scenario are:
- ▪
- Esophagogastrectomy through a combined abdominal cervical approach. After surgery, patients are left with a cervical esophagostomy (spit fistula), a defunctionalized duodenum, and a feeding jejunostomy [96,97]. One-stage reconstruction after emergency esophagectomy is not advisable because the subsequent development of pharyngeal strictures might compromise outcomes [98]. Whenever necrosis in the upper two-thirds of the esophagus is seen, tracheobronchial endoscopy must be performed before surgery for the detection of tracheobronchial necrosis, which would alter the surgical management (e.g., pulmonary patch repair, typically through a right thoracotomy approach) [99];
- ▪
- If necrosis is confined to the stomach, total gastrectomy with preservation of the native esophagus should be considered. Although immediate esophagojejunostomy reconstruction has been shown to be safe in a high-volume referral center, with leaks in 5–8% of cases [100], other surgeons prefer to leave protective jejunostomy until after definitive reconstruction by means of a retrosternal ileocolonic esophagoplasty 4 to 8 months after the initial operation. In the interim, the patient can receive their nutritional needs through jejunostomy;
- ▪
- Concomitant necrosis in about 20% of patients undergoing esophagogastrectomy for causative ingestion requires the excision of additional abdominal organs such as the spleen, colon, small bowel, duodenum, or pancreas. In some patients, it is necessary to perform a proximal pancreatoduodenectomy for duodenal or pancreatic necrosis. The main complication related to this procedure is pancreatic fistula, which may be medically treated [100]. The most experienced surgeons seal the main pancreatic duct and avoid pancreatojejunostomy because of the combined presence of soft healthy pancreatic tissue, peritoneal inflammation, and frequent hemodynamic instability in the postoperative period. Such patients may be nourished by temporarily employing a jejunostomy [93,100];
- ▪
- ▪
- In summary, the construction of a feeding jejunostomy at the end of surgery (irrespective of the conducted procedure) enables early enteral nutrition in patients with compromised digestive function [93].
- (7)
- Esophageal strictures are the most common complication of caustic esophageal ingestion and can affect the esophagus, stomach, and other locations in the digestive tract. They usually develop within 2 months (3 weeks to 1 year) and multiple strictures appear in some cases [98]. Again, nutritional support plays a role in management. Exclusive enteral nutrition is indicated in the following contexts:
- ▪
- In patients with esophageal strictures, when endoscopic dilatation is complicated by perforation (4−17%). Esophageal perforations in this context are usually contained and can benefit from non-operative management [98]. In such cases, the nasoenteral route or jejunostomy can be used depending on the patient’s clinical condition;
- ▪
- Patients with multiple failed attempts at endoscopic dilatations should be considered for reconstructive surgery, usually by elective esophageal resection with esophagogastric anastomosis or colonic interposition;
- ▪
- Patients with pharyngoesophageal strictures (0.7–6%) that require retrograde or anterograde dilation and/or surgical reconstruction with colonic interposition and/or myocutaneous flap inlay;
- ▪
- Patients with gastric strictures (75–89% located in the antrum) and who present a perforation complicating the outcome of endoscopic dilatation (3.4–46%) or after stent implantation [101,102]. Many surgeons prefer to perform resection or bypass, which are associated with very low morbidity and mortality rates [103].

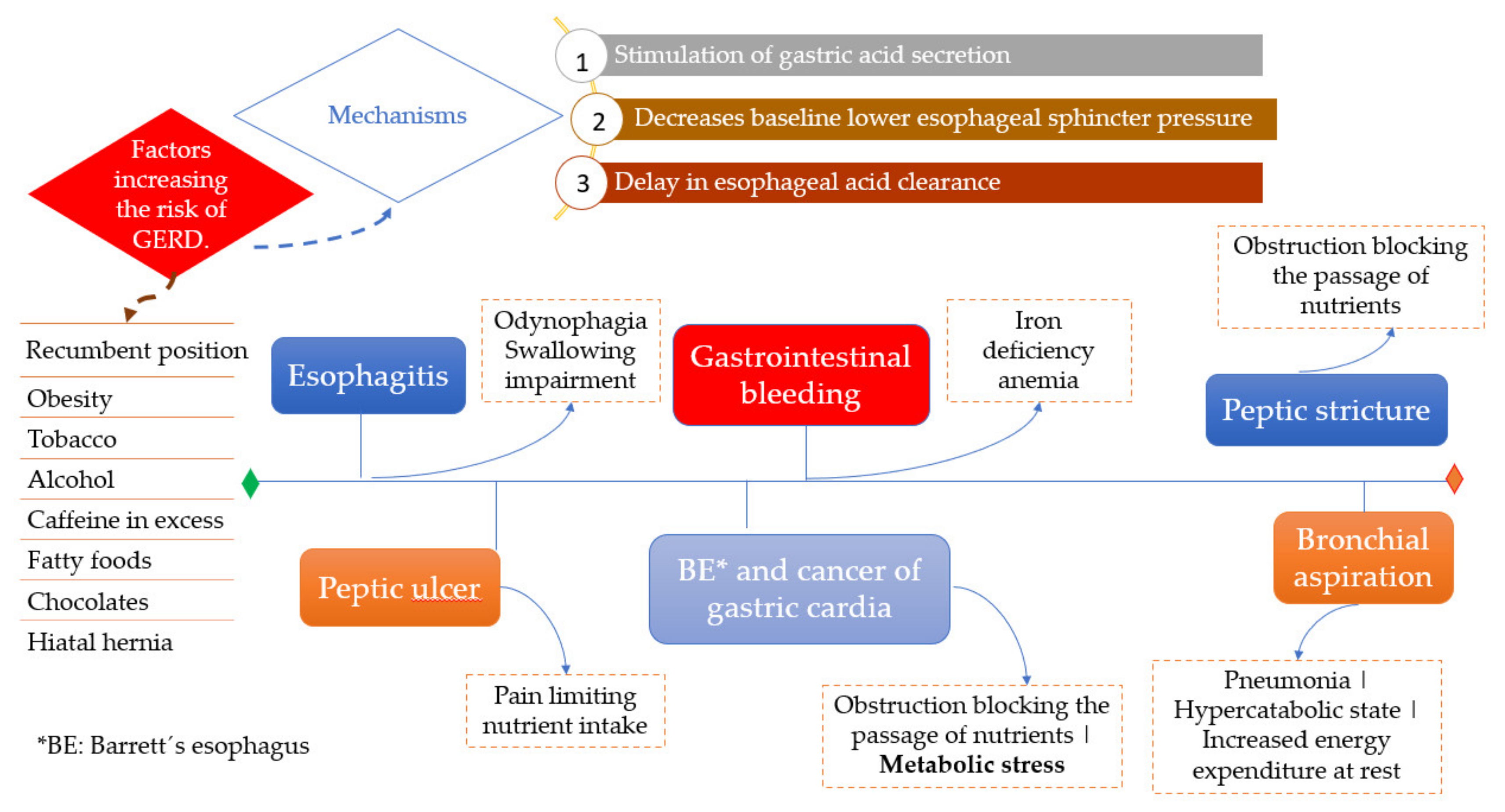
4.5. Gastroesophageal Reflux Disease
4.5.1. Foods That Contribute to the Triggering or Worsening of Symptoms
4.5.2. Overweight and Obesity
4.5.3. Food and Reflux Symptoms during Sleep
- ○
- ○
- ○
- ○
- ○
- Elevating the head of the bed may be useful for relieving acid regurgitation among esophageal cancer patients after surgery [141];
- ○
- The use of a wedge-shaped pillow (WSP) alleviates reflux symptoms in patients with esophageal cancer following esophagectomy and reconstruction. Likewise, the combined treatment (antisecretory drugs + WSP) also reduces the severity of esophagitis [143];
- ○
- Several studies show that sleeping with the head of the bed elevated or on a wedge reduces GER and lying left-side down reduces GER versus lying right-side down and supine [144]. The left lateral position is a suitable alternative to prone for the postural management of infants with symptomatic GER [145];
- ○
- Finally, bed head elevation by reducing the time of acid exposure also alleviates the consequences of nocturnal supraesophageal reflux, including perennial nasopharyngitis, cough, and asthma [146].
4.5.4. Dietary, Barrett’s Esophagus, and Cancer Risk
4.5.5. Complications Contributing to Malnutrition
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASPEN | American Society for Parenteral and Enteral Nutrition |
| BE | Barrett’s esophagus |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| BSPGHAN | British Society of Paediatric Gastroenterology, Hepatology and Nutrition |
| CT | Computed tomography |
| DXA | Dual-energy X-ray absorptiometry |
| EoE | Eosinophilic esophagitis |
| EOS | Eosinophils |
| ESPEN | European Society for Nutrition and Metabolism (ESPEN) |
| ESSD | European Society for Swallowing Disorders |
| FED | Food elimination diet |
| GER | Gastroesophageal reflux |
| GERD | Gastroesophageal reflux disease |
| GI | Gastrointestinal |
| HPF | High-power field |
| HRM | High-resolution manometry |
| HRQoL | Health-related quality of life |
| IGF-1 | Insulin-like growth factor |
| IGFBP3 | Insulin-like growth binding protein 3 |
| INS-R | Insulin resistance |
| IRP | Integrated relaxation pressure |
| JF | Jejunostomy feeding |
| LES | Lower esophageal sphincter |
| MG | Milligrams |
| ML | Milliliters |
| NETF | Nasoenteral tube feeding |
| OD | Oropharyngeal dysphagia |
| PPIs | Proton pump inhibitors |
| RYGB | Roux-en-Y gastric bypass |
| UHT | Ultra-High-Temperature |
| WHO | World Health Organization |
| WSP | Wedge-shaped pillow |
References
- Madanick, R.; Orlando, R.C. Anatomy, Histology, Embryology, and Developmental anomalies of the esophagus. In Sleisenger and Fordtran´s. Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, and Management; Feldman, M., Friedman, L., Brandt, L.J., Eds.; Saunders & Elsevier: Philadelphia, PA, USA, 2016; pp. 689–700. [Google Scholar]
- Pandolfino, J.E.; Kharilas, P.J. Esophageal Neuromuscular Function and Motility Disorders. In Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, and Management; Feldman, M., Friedman, L., Brandt, L.J., Eds.; Saunders & Elsevier: Philadelphia, PA, USA, 2016; pp. 732–761. [Google Scholar]
- Parkman, H.P. Modern Approaches for Evaluation and Treatment of GI Motility Disorders. In Gastroenterology Clinics: Esophagus; Elsevier: Philadelphia, PA, USA, 2020; pp. 1–498. [Google Scholar]
- Gavaghan, M. Anatomy and physiology of the esophagus. AORN J. 1999, 69, 370–386. [Google Scholar] [CrossRef]
- Jones, G.; Macaninch, E.; Mellor, D.D.; Spiro, A.; Martyn, K.; Butler, T.; Johnson, A.; Moore, J.B. Putting nutrition education on the table: Development of a curriculum to meet future doctors’ needs. Br. J. Nutr. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, K.; Haller, E. Nutrition Tools for the practicing gastroenterologist. Gastroenterol. Clin. N. Am. 2021, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fain, C.; Bull-Henry, K.; Abdi, M. Nutritional considerations in the hospital setting. Gastroenterol. Clin. N. Am. 2021, 50, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Martindale, R.G.; Patel, J.J.; Herron, T.J.; Codner, P.A. Sepsis and critical illness. In The ASPEN Adult Nutrition Support Core Curriculum, 3rd ed.; Mueller, C.M., Lord, L.M., Marian, M., McClave, S.A., Miller, S., Eds.; ASPEN: Silver Spring, MD, USA, 2017; pp. 457–472. [Google Scholar]
- Mueller, C.M.; Lord, L.M.; Marian, M.; McClave, S.A.; Miller, S.J. The Aspen Adult Nutrition Support Core Curriculum, 3rd ed.; ASPEN: Silver Spring, MD, USA, 2017; pp. 1–829. [Google Scholar]
- Sobotka, L.; Allison, S.P.; Forbes, A.; Meier, R.F.; Schnneider, S.M.; Soeters, P.B.; Stanga, Z.; Gossum, A.V. Basics in Clinical Nutrition, 5th ed.; ESPEN: Prague, Czech Republic, 2019; pp. 1–654. [Google Scholar]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249. [Google Scholar] [CrossRef]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A Systematic Review of the Prevalence of Oropharyngeal Dysphagia in Stroke, Parkinson’s Disease, Alzheimer’s Disease, Head Injury, and Pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef]
- Barczi, S.R.; Sullivan, P.A.; Robbins, J.A. How should dysphagia care of older adults differ? Establishing optimal practice patterns. Semin. Speech Lang. 2000, 21, 347–364. [Google Scholar] [CrossRef]
- Ekberg, O. Dysphagia: Diagnosis and Treatment; Springer Publishing: Berlin, Germany, 2012. [Google Scholar]
- Daniels, S.K.; Brailey, K.; Priestly, D.H.; Herrington, L.R.; Weisberg, L.A.; Foundas, A.L. Aspiration in patients with acute stroke. Arch. Phys. Med. Rehabil. 1998, 79, 14–19. [Google Scholar] [CrossRef]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef]
- Kalf, J.; de Swart, B.; Bloem, B.; Munneke, M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Park. Relat. Disord. 2012, 18, 311–315. [Google Scholar] [CrossRef]
- Simons, J.A. Swallowing Dysfunctions in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 1207–1238. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Hirano, H.; Watanabe, Y.; Edahiro, A.; Sato, K.; Yamane, G.; Katakura, A. Detecting signs of dysphagia in patients with Alzheimer’s disease with oral feeding in daily life. Geriatr. Gerontol. Int. 2014, 14, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Langmore, S.E.; Olney, R.K.; Lomen-Hoerth, C.; Miller, B.L. Dysphagia in patients with frontotemporal lobar dementia. Arch. Neurol. 2007, 64, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Homer, J.; Alberts, M.J.; Dawson, D.V.; Cook, G.M. Swallowing in Alzheimerʼs disease. Alzheimer Dis. Assoc. Disord. 1994, 8, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, L.J.; Appel, V.; Appel, S.H. Natural history of amyotrophic lateral sclerosis in a database population Validation of a scoring system and a model for survival prediction. Brain 1995, 118, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.E.; Morgan, A.S.; Bernstein, B.A. Swallowing disorders in severe brain injury: Risk factors affecting return to oral intake. Arch. Phys. Med. Rehabil. 1999, 80, 365–371. [Google Scholar] [CrossRef]
- Mélotte, E.; Maudoux, A.; Panda, R.; Kaux, J.-F.; Lagier, A.; Herr, R.; Belorgeot, M.; Laureys, S.; Gosseries, O. Links Between Swallowing and Consciousness: A Narrative Review. Dysphagia 2022, 30, 1–23. [Google Scholar] [CrossRef] [PubMed]
- García-Peris, P.; Parón, L.; Velasco, C.; de la Cuerda, C.; Camblor, M.; Bretón, I.; Herencia, H.; Verdaguer, J.; Navarro, C.; Clavé, P. Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: Impact on quality of life. Clin. Nutr. 2007, 26, 710–717. [Google Scholar] [CrossRef]
- Berg, M.G.A.V.D.; Rütten, H.; Rasmussen-Conrad, E.L.; Knuijt, S.; Takes, R.P.; Van Herpen, C.M.L.; Wanten, G.J.A.; Kaanders, J.H.A.M.; Merkx, M.A.W. Nutritional status, food intake, and dysphagia in long-term survivors with head and neck cancer treated with chemoradiotherapy: A cross-sectional study. Head Neck 2014, 36, 60–65. [Google Scholar] [CrossRef]
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Romea, M.; Palomera, E.; Almirall, J.; Cabré, M.; Serra-Prat, M.; Clavé, P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol. Motil. 2010, 22, 851-e230. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, N.K.; Chan, W.W. Assessing Upper Esophageal Sphincter Function in Clinical Practice: A Primer. Curr. Gastroenterol. Rep. 2016, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Cock, C.; Omari, T. Diagnosis of Swallowing Disorders: How We Interpret Pharyngeal Manometry. Curr. Gastroenterol. Rep. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Omari, T.I.; Ciucci, M.; Gozdzikowska, K.; Hernández, E.; Hutcheson, K.; Jones, C.; Maclean, J.; Nativ-Zeltzer, N.; Plowman, E.; Rogus-Pulia, N.; et al. High-Resolution Pharyngeal Manometry and Impedance: Protocols and Metrics—Recommendations of a High-Resolution Pharyngeal Manometry International Working Group. Dysphagia 2019, 35, 281–295. [Google Scholar] [CrossRef]
- Boaden, E.; Nightingale, J.; Bradbury, C.; Hives, L.; Georgiou, R. Clinical practice guidelines for videofluoroscopic swallowing studies: A systematic review. Radiography 2020, 26, 154–162. [Google Scholar] [CrossRef]
- Luan, S.; Wu, S.-L.; Xiao, L.-J.; Yang, H.-Y.; Liao, M.-X.; Wang, S.-L.; Fan, S.-N.; Ma, C. Comparison studies of ultrasound-guided botulinum toxin injection and balloon catheter dilatation in the treatment of neurogenic cricopharyngeal muscle dysfunction. NeuroRehabilitation 2021, 49, 629–639. [Google Scholar] [CrossRef]
- Wei, P. Botulinum Toxin Injection for the Treatment of Upper Esophageal Sphincter Dysfunction. Toxins 2022, 14, 321. [Google Scholar] [CrossRef]
- Hammond, C.A.S.; Goldstein, L.B. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia: ACCP evidence-based clinical practice guide-lines. Chest 2006, 129, 154S–168S. [Google Scholar] [CrossRef]
- Trimble, J.; Patterson, J. Cough reflex testing in acute stroke: A survey of current UK service provision and speech and language therapist perceptions. Int. J. Lang. Commun. Disord. 2020, 55, 899–916. [Google Scholar] [CrossRef]
- Blonski, W.; Slone, S.; Richter, J.E. Update on the Diagnosis and Treatment of Achalasia. Dysphagia 2022, 18, 1–13. [Google Scholar] [CrossRef]
- Sadowski, D.C.; Ackah, F.; Jiang, B.; Svenson, L. Achalasia: Incidence, prevalence and survival. A population-based study. Neurogastroenterol. Motil. 2010, 22, e256–e261. [Google Scholar] [CrossRef] [PubMed]
- Podas, T.; Eaden, J.; Mayberry, M.; Mayberry, J. Achalasia: A critical review of epidemiological studies. Am. J. Gastroenterol. 1998, 93, 2345–71998. [Google Scholar] [CrossRef] [PubMed]
- Marlais, M.; Fishman, J.R.; Fell, J.M.E.; Haddad, M.J.; Rawat, D.J. UK incidence of achalasia: An 11-year national epidemiological study. Arch. Dis. Child. 2011, 96, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, P.M.; Raz, D.; Palazzo, F.; Niponmick, I.; Patti, M.G. Clinical, radiological, and manometric profile in 145 patients with untreated achalasia. World J. Surg. 2008, 32, 1974–1979. [Google Scholar] [CrossRef]
- A Patel, D.; Naik, R.; Slaughter, J.C.; Higginbotham, T.; Silver, H.; Vaezi, M.F. Weight loss in achalasia is determined by its phenotype. Dis. Esophagus 2018, 31, doy046. [Google Scholar] [CrossRef]
- Surdea-Blaga, T.; David, L.; Pop, A.; Tantau, M.; Dumitrascu, D.L. Clinical and Manometric Characteristics of Patients with Achalasia: One Disease with Three Presentations or Three Diseases with One Presentation? J. Gastrointest. Liver Dis. 2020, 29, 501–508. [Google Scholar] [CrossRef]
- Patel, D.A.; Yadlapati, R.; Vaezi, M.F. Esophageal Motility Disorders: Current Approach to Diagnostics and Therapeutics. Gastroenterology 2022, 162, 1617–1634. [Google Scholar] [CrossRef]
- Arcerito, M.; Jamal, M.M.; Perez, M.G.; Kaur, H.; Sundahl, A.; Moon, J.T. Esophageal Achalasia: From Laparoscopic to Robotic Heller Myotomy and Dor Fundoplication. JSLS J. Soc. Laparoendosc. Surg. 2022, 26, e2022.00027. [Google Scholar] [CrossRef]
- Hew, S.; Bechara, R.; Patti, M.G.; Fisichella, P.M.; Nabi, Z.; Ramchandani, M.; Reddy, D.N.; Werner, Y.B.; Rösch, T. Endoscopic or Surgical Myotomy in Achalasia. N. Engl. J. Med. 2020, 382, 1376–1377. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, P.M.; Patti, M.G. From Heller to POEM (1914–2014): A 100-year history of surgery for Achalasia. J. Gastrointest. Surg. 2014, 18, 1870–1875. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; Von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Dias, J.A.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Hruz, P.; Straumann, A.; Bussmann, C.; Heer, P.; Simon, H.-U.; Zwahlen, M.; Beglinger, C.; Schoepfer, A.M.; Swiss EoE Study Group. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J. Allergy Clin. Immunol. 2011, 128, 1349–1350.e5. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Arias, Á.; Arias-González, L.; Laserna-Mendieta, E.J.; Ruiz-Ponce, M.; Lucendo, A.J. Systematic review with meta-analysis: The growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment. Pharmacol. Ther. 2019, 49, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; Fairley, S.K.; Masson, J.W.; Chong, A.K.; Whitaker, D.A.; Kanowski, P.A.; Walker, N.I. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest. Endosc. 2003, 58, 516–522. [Google Scholar] [CrossRef]
- Hurrell, J.M.; Genta, R.M.; Dellon, E.S. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am. J. Gastroenterol. 2012, 107, 698–706. [Google Scholar] [CrossRef]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Safroneeva, E.; Bussmann, C.; Kuchen, T.; Portmann, S.; Simon, H.; Straumann, A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013, 145, 1230–1236.e2. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/product-information/jorveza-epar-product-information_en.pdf (accessed on 14 January 2020).
- Warners, M.J.; Nijhuis, R.O.; De Wijkerslooth, L.R.H.; Smout, A.J.P.M.; Bredenoord, A.J. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am. J. Gastroenterol. 2018, 113, 836–844. [Google Scholar] [CrossRef]
- Molina-Infante, J. Nutritional and Psychological Considerations for Dietary Therapy in Eosinophilic Esophagitis. Nutrients 2022, 14, 1588. [Google Scholar] [CrossRef]
- Furuta, G.T.; Katzka, D.A. Eosinophilic Esophagitis. N. Engl. J. Med. 2015, 373, 1640–1648. [Google Scholar] [CrossRef]
- Hirano, I.; Furuta, G.T. Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Gastroenterology 2020, 158, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Arias, Á.; Molina-Infante, J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 13–22.e1. [Google Scholar] [CrossRef] [PubMed]
- Laserna-Mendieta, E.J.; Casabona, S.; Guagnozzi, D.; Savarino, E.; Perelló, A.; Guardiola-Arévalo, A.; Barrio, J.; Pérez-Martínez, I.; Krarup, A.P.S.; Alcedo, J.; et al. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: Results from the EoE connect registry. Aliment. Pharmacol. Ther. 2020, 52, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.R.; Gupta, A.; Attar, B.M.; Ravi, V.; Koduru, P. Topical steroids in eosinophilic esophagitis: Systematic review and meta-analysis of placebo-controlled randomized clinical trials. J. Gastroenterol. Hepatol. 2016, 31, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Kia, L.; Nelson, M.; Zalewski, A.; Gregory, D.; Gonsalves, N.; Straumann, A.; Hirano, I. Oral delivery of fluticasone powder improves esophageal eosinophilic inflammation and symptoms in adults with eosinophilic esophagitis. Dis. Esophagus 2018, 31, doy098. [Google Scholar] [CrossRef] [PubMed]
- Eluri, S.; Selitsky, S.R.; Perjar, I.; Hollyfield, J.; Betancourt, R.; Randall, C.; Rusin, S.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Clinical and Molecular Factors Associated With Histologic Response to Topical Steroid Treatment in Patients With Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2019, 17, 1081–1088.e2. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, X.; Liu, D.; Tan, C. A meta-analysis on randomized controlled trials of treating eosinophilic esophagitis with budesonide. Ann. Med. 2022, 54, 2078–2088. [Google Scholar] [CrossRef]
- Syverson, E.P.; Hait, E. Update on Emerging Pharmacologic Therapies for Patients With Eosinophilic Esophagitis. Gastroenterol. Hepatol. 2022, 18, 207–212. [Google Scholar]
- Hirano, I.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Morey, R.; Desai, N.K.; Lan, L.; Williams, J.; Dellon, E.S. Budesonide Oral Suspension Improves Outcomes in Patients With Eosinophilic Esophagitis: Results From a Phase 3 Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 525–534.e10. [Google Scholar] [CrossRef]
- Dohil, R.; Newbury, R.; Fox, L.; Bastian, J.; Aceves, S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 2010, 139, 418–429.e1. [Google Scholar] [CrossRef]
- Dellon, E.S.; Woosley, J.T.; Arrington, A.; McGee, S.J.; Covington, J.; Moist, S.E.; Gebhart, J.H.; Tylicki, A.E.; Shoyoye, S.O.; Martin, C.F.; et al. Efficacy of Budesonide vs Fluticasone for Initial Treatment of Eosinophilic Esophagitis in a Randomized Controlled Trial. Gastroenterology 2019, 157, 65–73.e5. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Schlag, C.; Biedermann, L.; Vaquero, C.S.; de Los Rios, C.C.; Schmoecker, C.; Madisch, A.; et al. Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients With Eosinophilic Esophagitis. Gastroenterology 2020, 159, 1672–1685.e5. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Godat, A.; Ringel, A.; Almonte, H.S.; Schupack, D.; Mendoza, G.; McCright-Gill, T.; Dellon, E.S.; Hirano, I.; Alexander, J.; et al. Effectiveness and Safety of High- vs Low-Dose Swallowed Topical Steroids for Maintenance Treatment of Eosinophilic Esophagitis: A Multicenter Observational Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2514–2523.e2. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Rossetti, D.; Papoff, P.; Tiberti, A.; Mallardo, S.; Volpe, D.; Ruggiero, C.; Russo, G.; Vezzoli, D.; Isoldi, S.; et al. A 12-Week Maintenance Therapy with a New Prepared Viscous Budesonide in Pediatric Eosinophilic Esophagitis. Am. J. Dig. Dis. 2019, 64, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Dellon, E.S.; Hamilton, J.D.; Collins, M.H.; Peterson, K.; Chehade, M.; Schoepfer, A.M.; Safroneeva, E.; Rothenberg, M.E.; Falk, G.W.; et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis. Gastroenterology 2020, 158, 111–122.e10. [Google Scholar] [CrossRef] [PubMed]
- Eluri, S.; Runge, T.M.; Cotton, C.C.; Burk, C.M.; Wolf, W.A.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest. Endosc. 2016, 83, 1142–1148. [Google Scholar] [CrossRef]
- Kagalwalla, A.F.; Sentongo, T.A.; Ritz, S.; Hess, T.; Nelson, S.P.; Emerick, K.M.; Melin–Aldana, H.; Li, B. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2006, 4, 1097–1102. [Google Scholar] [CrossRef]
- Gonsalves, N.; Yang, G.; Doerfler, B.; Ritz, S.; Ditto, A.M.; Hirano, I. Elimination Diet Effectively Treats Eosinophilic Esophagitis in Adults; Food Reintroduction Identifies Causative Factors. Gastroenterology 2012, 142, 1451–1459.e1. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Arias, A.; González-Cervera, J.; Yagüe-Compadre, J.L.; Guagnozzi, D.; Angueira, T.; Jiménez-Contreras, S.; González-Castillo, S.; Rodríguez-Domíngez, B.; De Rezende, L.C.; et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: A prospective study on the food cause of the disease. J. Allergy Clin. Immunol. 2013, 131, 797–804. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Arias, A.; Barrio, J.; Rodríguez-Sánchez, J.; Sanchez-Cazalilla, M.; Lucendo, A.J. Four-food group elimination diet for adult eosinophilic esophagitis: A prospective multicenter study. J. Allergy Clin. Immunol. 2014, 134, 1093–1099.e1. [Google Scholar] [CrossRef]
- Kagalwalla, A.F.; Wechsler, J.B.; Amsden, K.; Schwartz, S.; Makhija, M.; Olive, A.; Davis, C.M.; Manuel-Rubio, M.; Marcus, S.; Shaykin, R.; et al. Efficacy of a 4-Food Elimination Diet for Children With Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1698–1707.e7. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Arias, A.; Alcedo, J.; Garcia-Romero, R.; Casabona-Frances, S.; Prieto-Garcia, A.; Modolell, I.; Gonzalez-Cordero, P.L.; Perez-Martinez, I.; Martin-Lorente, J.L.; et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2-4-6 study. J. Allergy Clin. Immunol. 2018, 141, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Aceves, S.S.; Alexander, J.A.; Baron, T.H.; Bredenoord, A.J.; Day, L.; Dellon, E.S.; Falk, G.W.; Furuta, G.T.; Gonsalves, N.; Hirano, I.; et al. Endoscopic approach to eosinophilic esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference. Gastrointest. Endosc. 2022, 96, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Haboubi, H.N.; Attwood, S.E.; Auth, M.K.H.; Dunn, J.M.; Sweis, R.; Morris, D.; Epstein, J.; Novelli, M.R.; Hunter, H.; et al. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut 2022, 71, 1459–1487. [Google Scholar] [CrossRef]
- González-Cervera, J.; Arias, A.; Navarro, P.; Juárez-Tosina, R.; Cobo-Palacios, M.; Olalla, J.M.; Angueira-Lapeña, T.; Lucendo, A.J. Tolerance to sterilised cow’s milk in patients with eosinophilic oesophagitis triggered by milk. Aliment. Pharmacol. Ther. 2022, 56, 957–967. [Google Scholar] [CrossRef]
- Contini, C.S.S. Caustic injury of the upper gastrointestinal tract: A comprehensive review. World J. Gastroenterol. 2013, 19, 3918–3930. [Google Scholar] [CrossRef]
- Cabral, C.; Chirica, M.; de Chaisemartin, C.; Gornet, J.-M.; Munoz-Bongrand, N.; Halimi, B.; Cattan, P.; Sarfati, E. Caustic injuries of the upper digestive tract: A population observational study. Surg. Endosc. 2012, 26, 214–221. [Google Scholar] [CrossRef]
- Kluger, Y.; Ben Ishay, O.; Sartelli, M.; Katz, A.; Ansaloni, L.; Gomez, C.A.; Biffl, W.; Catena, F.; Fraga, G.P.; Di Saverio, S.; et al. Caustic ingestion management: World society of emergency surgery preliminary survey of expert opinion. World J. Emerg. Surg. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Mowry, J.B.; Spyker, D.A.; Cantilena, L.R., Jr.; McMillan, N.; Ford, M. Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin. Toxicol. 2014, 52, 1032–1283. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A. Esophagogastric lesions caused by caustics. Gastroenterol. Hepatol. 2000, 23, 436–447. [Google Scholar]
- Ryu, H.H.; Jeung, K.W.; Lee, B.K.; Uhm, J.H.; Park, Y.H.; Shin, M.H.; Kim, H.L.; Heo, T.; Min, Y.I. Caustic injury: Can CT grading system enable prediction of esophageal stricture? Clin. Toxicol. 2010, 48, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.A.; Kochhar, R.; Nagi, B.; Mehta, S.; Mehta, S.K. Ingestion of corrosive acids. Spectrum of injury to upper gastrointes-tinal tract and natural history. Gastroenterology 1989, 97, 702–707. [Google Scholar] [CrossRef]
- A Zargar, S.; Kochhar, R.; Nagi, B.; Mehta, S.K. Ingestion of strong corrosive alkalis: Spectrum of injury to upper gastrointestinal tract and natural history. Am. J. Gastroenterol. 1992, 87, 337–341. [Google Scholar] [PubMed]
- Chibishev, A.; Markoski, V.; Smokovski, I.; Shikole, E.; Stevcevska, A. Nutritional therapy in the treatment of acute corrosive intoxication in adults. Mater. Socio Medica 2016, 28, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Chirica, M.; Resche-Rigon, M.; Zagdanski, A.M.; Bruzzi, M.; Bouda, D.; Roland, E.; Sabatier, F.; Bouhidel, F.; Bonnet, F.; Munoz-Bongrand, N.; et al. Computed Tomography Evaluation of Esophagogastric Necrosis After Caustic Ingestion. Ann. Surg. 2016, 264, 107–113. [Google Scholar] [CrossRef]
- Chirica, M.; Kelly, M.D.; Siboni, S.; Aiolfi, A.; Riva, C.G.; Asti, E.; Ferrari, D.; Leppäniemi, A.; Broek, R.P.G.T.; Brichon, P.Y.; et al. Esophageal emergencies: WSES guidelines. World J. Emerg. Surg. 2019, 14, 26. [Google Scholar] [CrossRef]
- Bruzzi, M.; Chirica, M.; Resche-Rigon, M.; Corte, H.; Voron, T.; Sarfati, E.; Zagdanski, A.-M.; Cattan, P. Emergency computed tomography predicts caustic esophageal stricture formation. Ann. Surg. 2019, 270, 109–114. [Google Scholar] [CrossRef]
- Kochhar, R.; Poornachandra, K.S.; Puri, P.; Dutta, U.; Sinha, S.K.; Sethy, P.K.; Wig, J.D.; Nagi, B.; Singh, K. Comparative evaluation of nasoenteral feeding and jejunostomy feeding in acute corrosive injury: A retrospective analysis. Gastrointest. Endosc. 2009, 70, 874–880. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Cheng, C.-L.; Lin, C.-H.; Tang, J.-H.; Chu, Y.-Y.; Liu, N.-J.; Chen, P.-C. Caustic ingestion in adults: The role of endoscopic classification in predicting outcome. BMC Gastroenterol. 2008, 8, 31. [Google Scholar] [CrossRef]
- Chirica, M.; Resche-Rigon, M.; Bongrand, N.M.; Zohar, S.; Halimi, B.; Gornet, J.M.; Sarfati, E.; Cattan, P. Surgery for caustic injuries of the upper gastrointestinal tract. Ann. Surg. 2012, 256, 994–1001. [Google Scholar] [CrossRef]
- Chirica, M.; Bonavina, L.; Kelly, M.D.; Sarfati, E.; Cattan, P. Caustic ingestion. Lancet 2017, 389, 2041–2052. [Google Scholar] [CrossRef]
- Cattan, P.; Munoz-Bongrand, N.; Berney, T.; Halimi, B.; Sarfati, E.; Celerier, M. Extensive abdominal surgery after caustic ingestion. Ann. Surg. 2000, 231, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Chirica, M.; Kraemer, A.; Petrascu, E.; Vuarnesson, H.; Pariente, B.; Halimi, B.; Munoz-Bongrand, N.; Sarfati, E.; Cattan, P. Esophagojejunostomy after total gastrectomy for caustic injuries. Dis. Esophagus 2013, 27, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Liang, C.-M.; Tam, W.; Wu, K.-L.; Lu, L.-S.; Hu, M.-L.; Tai, W.-C.; Chiu, K.-W.; Chuah, S.-K. The effects of endoscopic-guided balloon dilations in esophageal and gastric strictures caused by corrosive injuries. BMC Gastroenterol. 2013, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, R.; Malik, S.; Gupta, P.; Reddy, Y.R.; Dhaka, N.; Sinha, S.K.; Gupta, V.; Noor, M.T.; Mallick, B. Etiological spectrum and response to endoscopic balloon dilation in patients with benign gastric outlet obstruction. Gastrointest. Endosc. 2018, 88, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Bonavina, L.; Chirica, M.; Skrobic, O.; Kluger, Y.; Andreollo, N.A.; Contini, S.; Simic, A.; Ansaloni, L.; Catena, F.; Fraga, G.P.; et al. Foregut caustic injuries: Results of the world society of emergency surgery consensus conference. World J. Emerg. Surg. 2015, 10, 44. [Google Scholar] [CrossRef]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187.e3. [Google Scholar] [CrossRef]
- Gallup Organization. Heartburn across America: A Gallup Organization National Survey; Gallup Organization: Princeton, NJ, USA, 1988. [Google Scholar]
- Nebel, O.T.; Fornes, M.F.; Castell, D.O. Symptomatic gastroesophageal reflux: Incidence and precipitating factors. Am. J. Dig. Dis. 1976, 21, 953–956. [Google Scholar] [CrossRef]
- Manterola, C.; Grande, L.; Bustos, L.; Otzen, T. Prevalence of gastroesophageal reflux disease: A population-based cross-sectional study in southern Chile. Gastroenterol. Rep. 2020, 8, 286–292. [Google Scholar] [CrossRef]
- Chowdhury, S.D.; George, G.; Ramakrishna, K.; Ramadass, B.; Pugazhendhi, S.; Mechenro, J.; Jeyaseelan, L.; Ramakrishna, B.S. Prevalence and factors associated with gastroesophageal reflux disease in southern India: A community-based study. Indian J. Gastroenterol. 2019, 38, 77–82. [Google Scholar] [CrossRef]
- Kaltenbach, T.; Crockett, S.; Gerson, L.B. Are lifestyle measures effective in patients with gastroesophageal reflux disease? Arch. Intern. Med. 2006, 166, 965–971. [Google Scholar] [CrossRef] [PubMed]
- DeVault, K.R.; Castell, D.O. American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am. J. Gastroenterol. 2005, 100, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Dagli, U.; Kalkan, I.H. The role of lifestyle changes in gastroesophageal reflux diseases treatment. Turk. J. Gastroenterol. 2017, 28, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Richter, J.E. Diet and gastroesophageal reflux disease. Curr. Opin. Gastroenterol. 2017, 33, 107–111. [Google Scholar] [CrossRef]
- Ayazi, S.; Hagen, J.A.; Chan, L.S.; Demeester, S.R.; Lin, M.W.; Ayazi, A.; Leers, J.M.; Oezcelik, A.; Banki, F.; Lipham, J.C.; et al. Obesity and gastroesophageal reflux: Quantifying the association between body mass index, esophageal acid exposure, and lower esophageal sphincter status in a large series of patients with reflux symptoms. J. Gastrointest. Surg. 2009, 13, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.V.; Yurenev, G.L.; Mironova, E.M.; Yureneva-Thorzhevskaya, T.V. Phenotype of obesity and gastroesophageal reflux disease in the context of comorbidity in patients with cardiovascular diseases. Ter. Arkh. 2019, 91, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barazzoni, R.; Busetto, L.; Campmans-Kuijpers, M.; Cardinale, V.; Chermesh, I.; Eshraghian, A.; Kani, H.T.; Khannoussi, W.; Lacaze, L.; et al. European guideline on obesity care in patients with gastrointestinal and liver diseases—Joint European Society for Clinical Nutrition and Metabolism / United European Gastroenterology guideline. United Eur. Gastroenterol. J. 2022, 10, 663–720. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Friedenberg, F. Obesity and GERD. Gastroenterol. Clin. N. Am. 2014, 43, 161–173. [Google Scholar] [CrossRef]
- A Corley, D.; Kubo, A. Body mass index and gastroesophageal reflux disease: A systematic review and meta-analysis. Am. J. Gastroenterol. 2006, 101, 2619–2628. [Google Scholar] [CrossRef]
- Hampel, H.; Abraham, N.S.; El-Serag, H.B. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 2005, 143, 199. [Google Scholar] [CrossRef]
- Nam, S.Y.; Choi, I.J.; Ryu, K.H.; Park, B.J.; Kim, H.B.; Nam, B. Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women. Gastroenterology 2010, 139, 1902–1911.e2. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, D.; Park, M.J.; Kim, Y.S.; Kim, J.S.; Jung, H.C.; Song, I.S. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: A cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut 2008, 57, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Nadaleto, B.F.; Herbella, F.A.; Patti, M.G. Gastroesophageal reflux disease in the obese: Pathophysiology and treatment. Surgery 2016, 159, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, T.; Yang, H.J.; Park, J.H.; Sohn, C.I.; Ryu, S.; Park, D.I. Weight loss and waist reduction is associated with im-provement in gastroesophageal disease reflux symptoms: A longitudinal study of 15 subjects undergoing health checkups. Neuro. Gastroenterol. Motil. 2016, 29, e13009. [Google Scholar] [CrossRef]
- De Groot, N.L.; Burgerhart, J.S.; Van De Meeberg, P.C.; De Vries, D.R.; Smout, A.J.P.M.; Siersema, P.D. Systematic review: The effects of conservative and surgical treatment for obesity on gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2009, 30, 1091–1102. [Google Scholar] [CrossRef]
- Djärv, T. Physical activity, obesity and gastroesophageal reflux disease in the general population. World J. Gastroenterol. 2012, 18, 3710–3714. [Google Scholar] [CrossRef]
- De Bortoli, N.; Guidi, G.; Martinucci, I.; Savarino, E.; Imam, H.; Bertani, L.; Russo, S.; Franchi, R.; Macchia, L.; Furnari, M.; et al. Voluntary and controlled weight loss can reduce symptoms and proton pump inhibitor use and dosage in patients with gastroesophageal reflux disease: A comparative study. Dis. Esophagus 2014, 29, 197–204. [Google Scholar] [CrossRef]
- Ness-Jensen, E.; Lindam, A.; Lagergren, J.; Hveem, K. Weight loss and reduction in gastroesophageal reflux. a prospective population-based cohort study: The HUNT study. Am. J. Gastroenterol. 2013, 108, 376–382. [Google Scholar] [CrossRef]
- Singh, M.; Lee, J.; Gupta, N.; Gaddam, S.; Smith, B.K.; Wani, S.B.; Sullivan, D.K.; Rastogi, A.; Bansal, A.; Donnelly, J.E.; et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: A prospective intervention trial. Obesity 2013, 21, 284–290. [Google Scholar] [CrossRef]
- Madalosso, C.; Gurski, R.R.; Jacques, S.M.C.; Navarini, D.; Mazzini, G.; Pereira, M.D.S. The impact of gastric bypass on gastroesophageal reflux disease in morbidly obese patients. Ann. Surg. 2016, 263, 110–116. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Y.; Wang, H.; Cao, L.; Zhao, Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int. J. Surg. 2020, 76, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Dobszai, D.; Mátrai, P.; Gyöngyi, Z.; Csupor, D.; Bajor, J.; Erőss, B.; Mikó, A.; Szakó, L.; Meczker, A.; Hágendorn, R.; et al. Body-mass index correlates with severity and mortality in acute pancreatitis: A meta-analysis. World J. Gastroenterol. 2019, 25, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Qumseya, B.; Qumsiyeh, Y.; Sarheed, A.; Rosasco, R.; Qumseya, A. Barrett’s Esophagus in Obese Patient Post-Roux-en-Y Gastric Bypass: A Systematic Review. Obes. Surg. 2022, 32, 3513–3522. [Google Scholar] [CrossRef] [PubMed]
- Duroux, P.; Bauerfeind, P.; Emde, C.; Koelz, H.R.; Blum, A.L. Early dinner reduces nocturnal gastric acidity. Gut 1989, 30, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Lanzon-Miller, S.; Pounder, R.E.; McIsaac, R.L.; Wood, J.R. The timing of the evening meal affects the pattern of 24-hour intragastric acidity. Aliment. Pharmacol. Ther. 1990, 4, 547–553. [Google Scholar] [CrossRef]
- Orr, W.C.; Harnish, M.J. Sleep-related gastro-oesophageal reflux: Provocation with a late evening meal and treatment with acid suppression. Aliment. Pharmacol. Ther. 1998, 12, 1033–1038. [Google Scholar] [CrossRef]
- Savarino, V.; Mela, G.S.; Celle, G. Influence of time of dinner on nocturnal gastric pH. Gut 1990, 31, 364. [Google Scholar] [CrossRef][Green Version]
- Ness-Jensen, E.; Hveem, K.; El-Serag, H.; Lagergren, J. Lifestyle Intervention in Gastroesophageal Reflux Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 175–182.e3. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, Z.-K.; Huang, Z.-B.; Chen, X.-L.; Liu, F.-B. Dietary and Lifestyle Factors Related to Gastroesophageal Reflux Disease: A Systematic Review. Ther. Clin. Risk Manag. 2021, 17, 305–323. [Google Scholar] [CrossRef]
- Stanciu, C.; Bennett, J. Effects of posture on gastro-oesophageal reflux. Digestion 1977, 15, 104–109. [Google Scholar] [CrossRef]
- Hamilton, J.W.; Boisen, R.J.; Yamamoto, D.T.; Wagner, J.L.; Reichelderfer, M. Sleeping on a wedge diminishes exposure of the esophagus to refluxed acid. Am. J. Dig. Dis. 1988, 33, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, H.; Zillessen, E.; Pohl, J. Effect of elevated head position in bed in therapy of gastroesophageal reflux [in German]. Z. Gastroenterol. 1996, 34 (Suppl. S2), 93–99. [Google Scholar] [PubMed]
- Huang, H.C.; Fang, S.Y. A Systematic Review of the Literature Related to Elevating the Head of the Bed for Patients. With Gastroesophageal Reflux Disease: Applications in Patients After Esophageal Cancer Surgery. Hu Li Za Zhi 2016, 63, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Sodhi, J.S.; Zargar, S.A.; Javid, G.; Yattoo, G.N.; Shah, A.; Gulzar, G.M.; Khan, M.A. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J. Gastroenterol. Hepatol. 2012, 27, 1078–1082. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chang, Y.-J.; Tseng, Y.-L.; Fang, S.-Y. Effect of Head-of-Bed Elevation on Nocturnal Reflux Symptoms of Esophageal Cancer Patients With Esophagectomy and Reconstruction. Cancer Nurs. 2021, 44, 244–250. [Google Scholar] [CrossRef]
- Person, E.; Rife, C.; Freeman, J.; Clark, A.; Castell, D.O. A Novel Sleep Positioning Device Reduces Gastroesophageal Reflux. J. Clin. Gastroenterol. 2015, 49, 655–659. [Google Scholar] [CrossRef]
- Tobin, J.M.; McCloud, P.; Cameron, D.J.S. Posture and gastro-oesophageal reflux: A case for left lateral positioning. Arch. Dis. Child. 1997, 76, 254–258. [Google Scholar] [CrossRef]
- Scott, D.R.; Simon, R.A. Supraesophageal Reflux: Correlation of Position and Occurrence of Acid Reflux-Effect of Head-of-Bed Elevation on Supine Reflux. J. Allergy Clin. Immunol. Pract. 2015, 3, 356–361. [Google Scholar] [CrossRef]
- Oh, D.S.; Demeester, S.R. Pathophysiology and treatment of Barrett’s esophagus. World J. Gastroenterol. 2010, 14, 3762–3772. [Google Scholar] [CrossRef]
- Falk, G.W. Barrett’s oesophagus: Frequency and prediction of dysplasia and cancer. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 125–138. [Google Scholar] [CrossRef]
- Patti, M.G. Gastroesophageal reflux disease: From heartburn to cancer. World J. Gastroenterol. 2010, 16, 3743–3744. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P. Determination of risk for Barrett’s esophagus and esophageal adenocarcinoma. Curr. Opin. Gastroenterol. 2016, 32, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh-Esfahani, N.; Soleimani, D.; Hajiahmadi, S.; Moradi, S.; Heidarzadeh, N.; Nachvak, A.S.M. Dietary Intake in Relation to the Risk of Reflux Disease: A Systematic Review. Prev. Nutr. Food Sci. 2021, 26, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Onstad, L.; Hardikar, S.; Blount, P.L.; Reid, B.J.; Vaughan, T.L. Association between markers of obesity and progression from Barrett’s esophagus to esophageal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 934–943. [Google Scholar] [CrossRef]
- Lynch, K.L. Is Obesity Associated with Barrett’s Esophagus and Esophageal Adenocarcinoma? Gastroenterol. Clin. N. Am. 2016, 45, 615–624. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Suzuki, H.; Kobayakawa, M.; Inadomi, J.; Takayama, M.; Makino, K.; Iwao, Y.; Sugino, Y.; Kanai, T. Association of Visceral Fat Area, Smoking, and Alcohol Consumption with Reflux Esophagitis and Barrett’s Esophagus in Japan. PLoS ONE 2015, 10, e0133865. [Google Scholar] [CrossRef]
- Kubo, A.; Corley, D.A.; Jensen, C.D.; Kaur, R. Dietary factors and the risks of oesophageal adenocarcinoma and Barrett’s oesophagus. Nutr. Res. Rev. 2010, 23, 230–246. [Google Scholar] [CrossRef]
- Filiberti, R.A.; Fontana, V.; De Ceglie, A.; Blanchi, S.; Grossi, E.; Della Casa, D.; Lacchin, T.; De Matthaeis, M.; Ignomirelli, O.; Cappiello, R.; et al. Alcohol consumption pattern and risk of Barrett’s oesophagus and erosive oesophagitis: An Italian case–control study. Br. J. Nutr. 2017, 117, 1151–1161. [Google Scholar] [CrossRef][Green Version]
- A Filiberti, R.; Fontana, V.; De Ceglie, A.; Blanchi, S.; Grossi, E.; Della Casa, D.; Lacchin, T.; De Matthaeis, M.; Ignomirelli, O.; Cappiello, R.; et al. Association between coffee or tea drinking and Barrett’s esophagus or esophagitis: An Italian study. Eur. J. Clin. Nutr. 2017, 71, 980–986. [Google Scholar] [CrossRef][Green Version]
- Arcidiacono, D.; Zaramella, A.; Fabris, F.; Sánchez-Rodríguez, R.; Nucci, D.; Fassan, M.; Nardi, M.; Benna, C.; Cristofori, C.; Morbin, T.; et al. Insulin/IGF-1 Signaling Is Downregulated in Barrett’s Esophagus Patients Undergoing a Moderate Calorie and Protein Restriction Program: A Randomized 2-Year Trial. Nutrients 2021, 13, 3638. [Google Scholar] [CrossRef]
- Proaño-Vasco, A.; Baumeister, T.; Metwaly, A.; Reitmeier, S.; Kleigrewe, K.; Meng, C.; Gigl, M.; Engleitner, T.; Öllinger, R.; Rad, R.; et al. High-Fructose Diet Alters Intestinal Microbial Profile and Correlates with Early Tumorigenesis in a Mouse Model of Barrett’s Esophagus. Microorganisms 2021, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E.; Hodge, A.M.; Dashti, S.G.; Dixon-Suen, S.C.; Castaño-Rodríguez, N.; Thomas, R.J.; Giles, G.G.; Boussioutas, A.; Kendall, B.J.; English, D.R. Diet and risk of Barrett’s oesophagus: Melbourne collaborative cohort study. Br. J. Nutr. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Filiberti, R.A.; Fontana, V.; De Ceglie, A.; Blanchi, S.; Lacchin, T.; De Matthaeis, M.; Ignomirelli, O.; Cappiello, R.; Rosa, A.; D’Onofrio, V.; et al. Dietary Habits and Risk of Esophagitis and Barrett’s Esophagus: A Multicenter Italian Case–Control Study. Am. J. Dig. Dis. 2021, 66, 3448–3460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yin, Z.; Zhang, C. Lifestyle interventions can reduce the risk of Barrett’s esophagus: A systematic review and meta-analysis of 62 studies involving 250,157 participants. Cancer Med. 2021, 10, 5297–5320. [Google Scholar] [CrossRef]
- Lukić, M.; Segec, A.; Segeca, I.; Pinotić, L.; Pinotić, K.; Atalić, B.; Solić, K.; Vcev, A. The role of the nutrition in the pathogenesis of gastroesophageal reflux disease, Barrett’s oesophagus and oesophageal adenocarcinoma. Coll. Antropol. 2010, 34, 905–909. [Google Scholar]
- Solaymani-Dodaran, M.; Logan, R.F.A.; West, J.; Card, T.; Coupland, C. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut 2004, 53, 1070–1074. [Google Scholar] [CrossRef]
- Yamasaki, T.; Sakiani, S.; Maradey-Romero, C.; Mehta, R.; Sandhu, D.; Ganocy, S.; Hemond, C.; Eisa, M.; Fass, R. Barrett’s esophagus patients are becoming younger: Analysis of a large United States dataset. Esophagus 2020, 17, 190–196. [Google Scholar] [CrossRef]
- Yadlapati, R.; Gyawali, C.P.; Masihi, M.; Carlson, D.A.; Kahrilas, P.J.; Nix, B.D.; Jain, A.; Triggs, J.R.; Vaezi, M.F.; Kia, L.; et al. Optimal wireless reflux monitoring metrics to predict discontin-uation of proton pump inhibitor therapy: Optimal Reflux Monitoring Metrics to Predict PPI Discontinuation. Am. J. Gastroenterol. 2022, 117, 1573–1582. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Fass, R. Management of gastroesophageal reflux disease. Gastroenterology 2018, 154, 302–318. [Google Scholar] [CrossRef]

| Diseases Caused Principally by Anatomical or Structural Damage | Diseases Caused Primordially by Alterations in Neuromuscular Control of Esophagus’ Function |
|---|---|
| Severe oropharyngeal dysphagia of neuromuscular origin (e.g., strokes, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, myotonic dystrophy, cricopharyngeal achalasia) Severe and prolonged esophageal motility disorders (e.g., achalasia, scleroderma) |
| Findings Found on Computed Tomography. | |
|---|---|
| Grade 1 | Homogenous enhancement of the esophageal wall while wall edema andmediastinal fat stranding are absent 1. |
| Grade 2a | Injuries display internal enhancement of the esophageal mucosa and hypodense aspect of the esophageal wall, which appears thickened while concomitant enhancement of the outer esophageal wall may sometimes confer a “target” aspect 2. |
| Grade 2b | Injuries present as a fine rim of external wall enhancement: the necrotic mucosa is not enhanced and fills the esophageal lumen, which indicates liquid density. Mediastinal fat stranding is uniformly present in grade 2 esophageal injuries. |
| Grade 3 | Transmural necrosis as shown by the absence of post-contrast wall enhancement 3. |
| Recommendation | Consensus | References |
|---|---|---|
| Screening and Assessment | ||
| Recommendation no. 29 Nutritional status screening should be performed for patients with GERD and overweight or obesity, encompassing basic anthropometric measurements (body weight, body height, BMI, waist circumference). Recommendation 30 Sarcopenia and sarcopenic obesity should be assessed, if there are indicators for sarcopenia, body composition analysis (DXA # or BIA *) and dynamometry (handgrip strength) should be used in GERD patients with overweight or obesity. # DXA: dual-energy X-ray absorptiometry; * BIA: bioelectrical impedance analysis | Grade of recommendation GPP—strong consensus 96% agreement Grade of recommendation GPP—strong consensus 93% agreement | [112,113,114,115,116] [117] |
| Treatment | ||
| Recommendation 31 Patients with GERD and obesity should be encouraged to lose body weight and reduce waist circumference. Recommendation 32 Patients with overweight or obesity and GERD should undergo weight reduction preferentially through lifestyle modification including dietary regimen and increased physical activity. Recommendation 33 In patients with GERD and BMI >40 kg/m2, or >35 kg/m2 when there are obesity-related comorbidities, bariatric surgery can be considered to achieve weight reduction if nonsurgical interventions failed to achieve the goals. The preferred procedure is Roux-en-Y gastric bypass (RYGB) | Grade of recommendation A—strong consensus 100% agreement Grade of recommendation B—strong consensus 100% agreement Grade of recommendation 0—strong consensus 93% agreement | [118,119,120,121,122,123,124] [109,111,116,125,126,127,128] [129,130,131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoro-Huguet, M.A. Dietary and Nutritional Support in Gastrointestinal Diseases of the Upper Gastrointestinal Tract (I): Esophagus. Nutrients 2022, 14, 4819. https://doi.org/10.3390/nu14224819
Montoro-Huguet MA. Dietary and Nutritional Support in Gastrointestinal Diseases of the Upper Gastrointestinal Tract (I): Esophagus. Nutrients. 2022; 14(22):4819. https://doi.org/10.3390/nu14224819
Chicago/Turabian StyleMontoro-Huguet, Miguel A. 2022. "Dietary and Nutritional Support in Gastrointestinal Diseases of the Upper Gastrointestinal Tract (I): Esophagus" Nutrients 14, no. 22: 4819. https://doi.org/10.3390/nu14224819
APA StyleMontoro-Huguet, M. A. (2022). Dietary and Nutritional Support in Gastrointestinal Diseases of the Upper Gastrointestinal Tract (I): Esophagus. Nutrients, 14(22), 4819. https://doi.org/10.3390/nu14224819






