Serum Phosphate Levels Modify the Impact of FGF23 Levels on Hemoglobin in Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data and Laboratory Determinations
2.3. Statistical Analysis
3. Results
3.1. Description of the Study Cohort
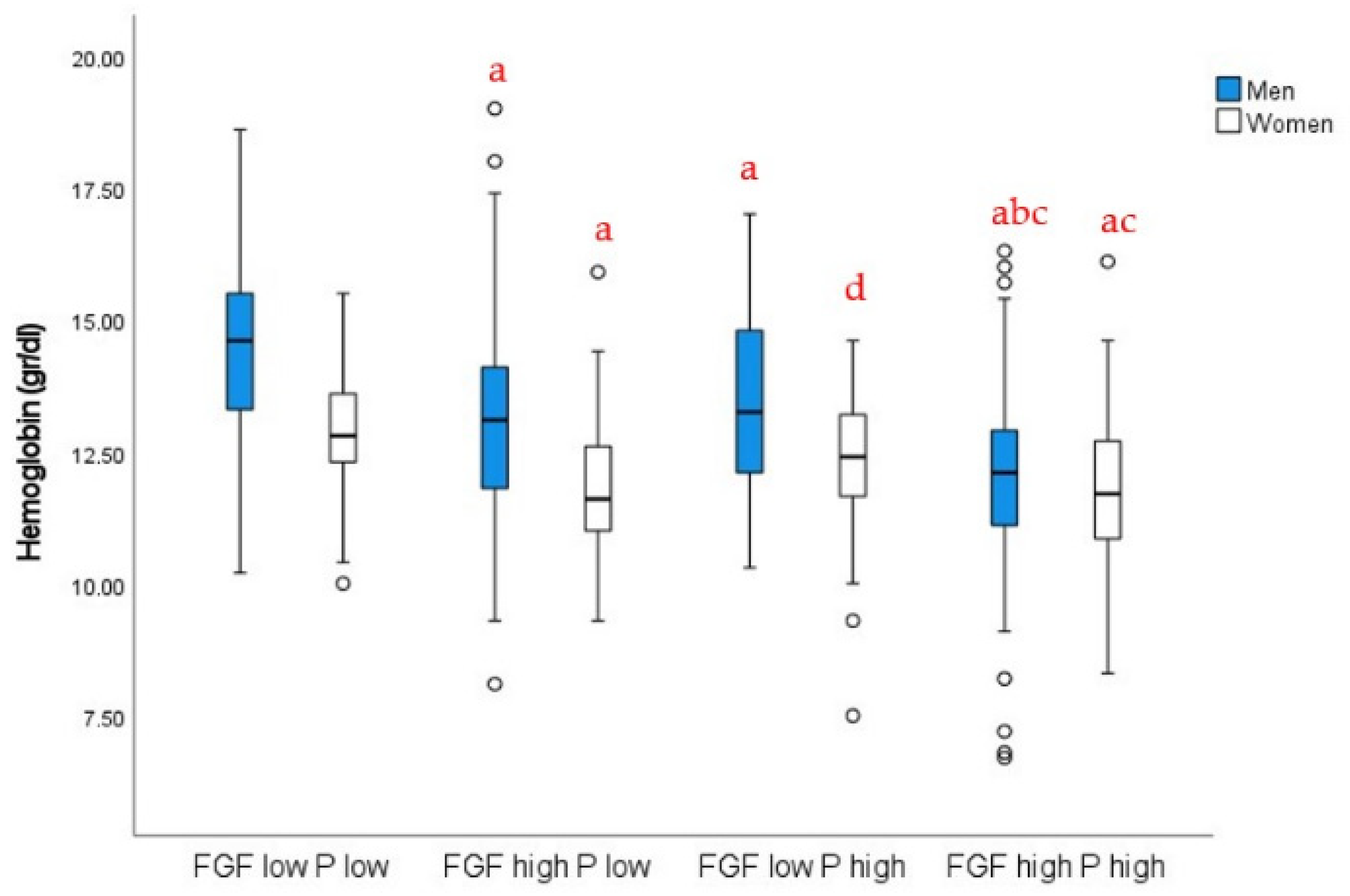
3.2. Relationships of Serum P and FGF23 with Anemia
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levin, A.; Thompson, C.R.; Ethier, J.; Carlisle, E.J.; Tobe, S.; Mendelssohn, D.; Burgess, E.; Jindal, K.; Barrett, B.; Singer, J.; et al. Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am. J. Kidney Dis. 1999, 34, 125–134. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barre, P.E. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am. J. Kidney Dis. 1996, 28, 53–61. [Google Scholar] [CrossRef]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. (Lausanne) 2021, 8, 642296. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.O.; Goldwasser, E.; Fried, W.; Plzak, L. Role of the kidney in erythropoiesis. Nature 1957, 179, 633–634. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, R.J.; Wallin, J.D.; Shadduck, R.K.; Fisher, J.W. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int. 1984, 25, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Besarab, A.; Ayyoub, F. Anemia in renal disease. In Diseases of the Kidney and Urinary Tract, 8th ed.; Schrier, R.W., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 2406–2430. [Google Scholar]
- Amnuay, K.; Srisawat, N.; Wudhikarn, K.; Assanasen, T.; Polprasert, C. Factors associated with erythropoiesis-stimulating agent hyporesponsiveness anemia in chronic kidney disease patients. Hematol. Rep. 2019, 11, 8183. [Google Scholar] [CrossRef] [Green Version]
- Diskin, C.J.; Stokes, T.J.; Dansby, L.M.; Radcliff, L.; Carter, T.B. Can acidosis and hyperphosphataemia result in increased erythropoietin dosing in haemodialysis patients? Nephrology (Carlton) 2006, 11, 394–399. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Mucsi, I.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Rosivall, L.; Kim, S.J.; Wolf, M.; Molnar, M.Z. Association of serum phosphorus level with anemia in kidney transplant recipients. Transplantation 2011, 91, 875–882. [Google Scholar] [CrossRef]
- Agoro, R.; Montagna, A.; Goetz, R.; Aligbe, O.; Singh, G.; Coe, L.M.; Mohammadi, M.; Rivella, S.; Sitara, D. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 2018, 32, 3752–3764. [Google Scholar] [CrossRef] [Green Version]
- Coe, L.M.; Madathil, S.V.; Casu, C.; Lanske, B.; Rivella, S.; Sitara, D. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J. Biol. Chem. 2014, 289, 9795–9810. [Google Scholar] [CrossRef]
- Mehta, R.; Cai, X.; Hodakowski, A.; Lee, J.; Leonard, M.; Ricardo, A.; Chen, J.; Hamm, L.; Sondheimer, J.; Dobre, M.; et al. Fibroblast Growth Factor 23 and Anemia in the Chronic Renal Insufficiency Cohort Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, K.H.; Kim, H.; An, S.Y.; Lee, M.; Cha, M.-U.; Park, J.T.; Yoo, T.-H.; Lee, K.-B.; Kim, Y.-H.; Sung, S.-A.; et al. Circulating Fibroblast Growth Factor-23 Levels are Associated with an Increased Risk of Anemia Development in Patients with Nondialysis Chronic Kidney Disease. Sci. Rep. 2018, 8, 7294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.H.; Leu, J.-G.; Fang, Y.-W.; Liou, H.-H. High Fibroblast Growth Factor 23 Levels Associated With Low Hemoglobin Levels in Patients With Chronic Kidney Disease Stages 3 and 4. Medicine (Baltimore) 2016, 95, e3049. [Google Scholar] [CrossRef] [PubMed]
- Akalin, N.; Okuturlar, Y.; Harmankaya, Ö.; Gedıkbaşi, A.; Sezıklı, S.; Yucel, S.K. Prognostic importance of fibroblast growth factor-23 in dialysis patients. Int. J. Nephrol. 2014, 2014, 602034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, H.; Michihata, T.; Shishido, K.; Takahashi, K.; Takahashi, G.; Hosaka, N.; Ikeda, M.; Sanada, D.; Shibata, T. High fibroblast growth factor 23 levels are associated with decreased ferritin levels and increased intravenous iron doses in hemodialysis patients. PLoS ONE 2017, 12, e0176984. [Google Scholar] [CrossRef]
- Musgrove, J.; Wolf, M. Regulation and Effects of FGF23 in Chronic Kidney Disease. Annu. Rev. Physiol. 2020, 82, 365–390. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, B.; Tobiasch, M.; Wagner, S.; Glodny, B.; Tilg, H.; Wolf, M.; Zoller, H. Hypophosphatemia after intravenous iron therapy: Comprehensive review of clinical findings and recommendations for management. Bone 2022, 154, 116202. [Google Scholar] [CrossRef]
- Junyent, M.; Martínez, M.; Borrás, M.; Bertriu, A.; Coll, B.; Craver, L.; Marco, M.P.; Sarró, F.; Valdivielso, J.M.; Fernández, E. Usefulness of imaging techniques and novel biomarkers in the prediction of cardiovascular risk in patients with chronic kidney disease in Spain: The NEFRONA project. Nefrologia 2010, 30, 119–126. [Google Scholar]
- Junyent, M.; Martínez, M.; Borràs, M.; Coll, B.; Valdivielso, J.M.; Vidal, T.; Sarró, F.; Roig, J.; Craver, L.; Fernández, E. Predicting cardiovascular disease morbidity and mortality in chronic kidney disease in Spain. The rationale and design of NEFRONA: A prospective, multicenter, observational cohort study. BMC Nephrol. 2010, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- De La Piedra, C.; Fernández, E.; Casaus, M.L.G.; Parra, E.G. Different biological functions in PTH molecules? What are we measuring? Nefrologia 2008, 28, 123–128. [Google Scholar]
- Nangaku, M.; Eckardt, K.U. Pathogenesis of renal anemia. Semin Nephrol. 2006, 26, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.M.; Gutiérrez, O.M.; Andress, D.L.; Coyne, D.W.; Levin, A.; Wolf, M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int. 2010, 77, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eser, B.; Yayar, O.; Buyukbakkal, M.; Erdogan, B.; Ercan, Z.; Merhametsiz, O.; Haspulat, A.; Oğuz, E.G.; Dogan, İ.; Canbakan, B.; et al. Fibroblast growth factor is associated to left ventricular mass index, anemia and low values of transferrin saturation. Nefrologia 2015, 35, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Czaya, B.; Heitman, K.; Campos, I.; Yanucil, C.; Kentrup, D.; Westbrook, D.; Gutierrez, O.; Babitt, J.L.; Jung, G.; Salusky, I.B.; et al. Hyperphosphatemia increases inflammation to exacerbate anemia and skeletal muscle wasting independently of FGF23-FGFR4 signaling. Elife 2022, 11, e74782. [Google Scholar] [CrossRef]
- Arroyo, D.; Betriu, A.; Martinez-Alonso, M.; Vidal, T.; Valdivielso, J.M.; Fernández, E. Observational multicenter study to evaluate the prevalence and prognosis of subclinical atheromatosis in a Spanish chronic kidney disease cohort: Baseline data from the NEFRONA study. BMC Nephrol. 2014, 15, 168. [Google Scholar] [CrossRef] [Green Version]
- Drueke, T.B. Hyperparathyroidism in Chronic Kidney Disease; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Klotho and chronic kidney disease. Contrib. Nephrol. 2013, 180, 47–63. [Google Scholar]
- Yashiro, M.; Ohya, M.; Mima, T.; Ueda, Y.; Nakashima, Y.; Kawakami, K.; Ishizawa, Y.; Yamamoto, S.; Kobayashi, S.; Yano, T.; et al. FGF23 modulates the effects of erythropoietin on gene expression in renal epithelial cells. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 125–136. [Google Scholar] [CrossRef]
- Ribeiro Junior, H.L.; Cordeiro, J.V.A.; Oliveira, R.T.G.; Magalhães, S.M.M.; Pinheiro, R.F. Klotho Expression Predicts Poor Prognosis in Myelodysplastic Syndrome. Blood 2019, 134 (Suppl. 1), 5404. [Google Scholar] [CrossRef]
- Ishii, S.; Suzuki, T.; Wakahashi, K.; Asada, N.; Kawano, Y.; Kawano, H.; Minagawa, K.; Nakamura, Y.; Mizuno, S.; Takahashi, S.; et al. FGF-23 from erythroblasts promotes hematopoietic progenitor mobilization. Blood 2021, 137, 1457–1467. [Google Scholar] [CrossRef]
- Vadakke Madathil, S.; Coe, L.M.; Casu, C.; Sitara, D. Klotho deficiency disrupts hematopoietic stem cell development and erythropoiesis. Am. J. Pathol. 2014, 184, 827–841. [Google Scholar] [CrossRef] [PubMed]



| ALL | ANEMIA | p | ||
|---|---|---|---|---|
| NO | YES | |||
| n (%) | 896 (100) | 489 (54.6) | 407 (45.4) | |
| Age (years) | 59 (48; 68) | 62 (51; 68) | 57.0 (44; 67) | 0.003 |
| Sex (women) | 361 (40.3) | 212 (43.4) | 149 (36.6) | 0.04 |
| Diabetes | 178 (19.9) | 80 (16.4) | 98 (24.1) | 0.004 |
| CKD stage | ||||
| Control | 95 (10.6) | 89 (18.2) | 6 (1.5) | Ref. |
| CKD3 | 273 (30.5) | 217 (44.4) | 56 (13.8) | 0.012 |
| CKD4–5 | 246 (27.5) | 102 (20.9) | 144 (35.4) | <0.001 |
| Dialysis | 282 (31.5) | 81 (16.6) | 201 (49.4) | <0.001 |
| ESA use | 318 (35.5) | 84 (17.2) | 234 (57.5) | <0.001 |
| IV iron therapy | 407 (45.4) | 55 (11.2) | 122 (30.0) | <0.001 |
| BMI (kg/m2) | 27.4 (24.3; 30.9) | 28.1 (25.0; 31.2) | 26.7 (23.7; 30.4) | 0.092 |
| SBP (mmHg) | 143 (129; 157) | 141 (129; 153) | 145 (128; 161) | 0.067 |
| DBP (mmHg) | 82.0 (75.0; 90.0) | 83.0 (77.0; 89.0) | 81.0 (73.0; 90.0) | 0.243 |
| Hemoglobin (g/dL) | 12.6 (11.5; 13.7) | 13.7 (13.0; 14.9) | 11.5 (10.9; 12.1) | <0.001 |
| Ferritin (ng/mL) | 170 (81; 322) | 135 (71; 263) | 214 (102; 412) | <0.001 |
| Calcium (mg/dL) | 9.3 (8.9; 9.6) | 9.4(9.0; 9.7) | 9.1 (8.8; 9.5) | <0.001 |
| Phosphate (mg/dL) | 3.9 (3.3; 4.6) | 3.6 (3.1; 4.2) | 4.3 (3.7; 5.0) | <0.001 |
| Albumin (g/dL) | 4.1 (3.8; 4.4) | 4.2 (3.8; 4.5) | 4.0 (3.8; 4.4) | 0.002 |
| iPTH (pg/mL) | 137 (74; 237) | 98 (59; 170) | 184 (101; 276) | <0.001 |
| sKlotho (pg/mL) | 288 (176; 437) | 289 (169; 447) | 282 (183; 416) | 0.434 |
| Log iFGF23 (pg/mL) | 2.29 (1.90; 2.86) | 2.06 (1.76; 2.47) | 2.56 (2.12; 3.09) | <0.001 |
| HsCRP(mg/L) | 1.98 (1.00; 4.62) | 1.83 (0.94; 4.24) | 2.44 (1.17; 6.55) | 0.145 |
| 25-OH D (ng/mL) | 15.2 (11.5; 19.8) | 15.0 (11.2; 19.7) | 15.4 (11.8; 19.9) | 0.243 |
| 1,25-OH2 D (pg/mL) | 13.8 (8.4; 21.6) | 15.9 (9.8; 25.2) | 11.7 (7.3; 18.4) | <0.001 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Phosphate (mg/dL) | 7.9 (4.15–14.9) | <0.001 | 9.5 (4.93–18.3) | <0.001 | 4.33 (2.11–8.90) | <0.001 |
| Log iFGF23 (pg/mL) | 21.1 (8.43–52.9) | <0.001 | 26.6 (10.4–67.9) | <0.001 | 8.75 (3.17–24.2) | <0.001 |
| Phosphorus by Log iFGF23 | 0.57 (0.47–0.70) | <0.001 | 0.54 (0.44–0.66) | <0.001 | 0.66 (0.53–0.83) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-González, J.F.; Mora-Fernández, C.; Diaz-Tocados, J.M.; Bozic, M.; Bermúdez-López, M.; Martín, M.; Valdivielso, J.M. Serum Phosphate Levels Modify the Impact of FGF23 Levels on Hemoglobin in Chronic Kidney Disease. Nutrients 2022, 14, 4842. https://doi.org/10.3390/nu14224842
Navarro-González JF, Mora-Fernández C, Diaz-Tocados JM, Bozic M, Bermúdez-López M, Martín M, Valdivielso JM. Serum Phosphate Levels Modify the Impact of FGF23 Levels on Hemoglobin in Chronic Kidney Disease. Nutrients. 2022; 14(22):4842. https://doi.org/10.3390/nu14224842
Chicago/Turabian StyleNavarro-González, Juan F., Carmen Mora-Fernández, Juan Miguel Diaz-Tocados, Milica Bozic, Marcelino Bermúdez-López, Marisa Martín, and Jose Manuel Valdivielso. 2022. "Serum Phosphate Levels Modify the Impact of FGF23 Levels on Hemoglobin in Chronic Kidney Disease" Nutrients 14, no. 22: 4842. https://doi.org/10.3390/nu14224842







