Lacticaseibacillus rhamnosus MG4706 Suppresses Periodontitis in Osteoclasts, Inflammation-Inducing Cells, and Ligature-Induced Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experiment Sample
2.2. Cell Culture and Differentiation
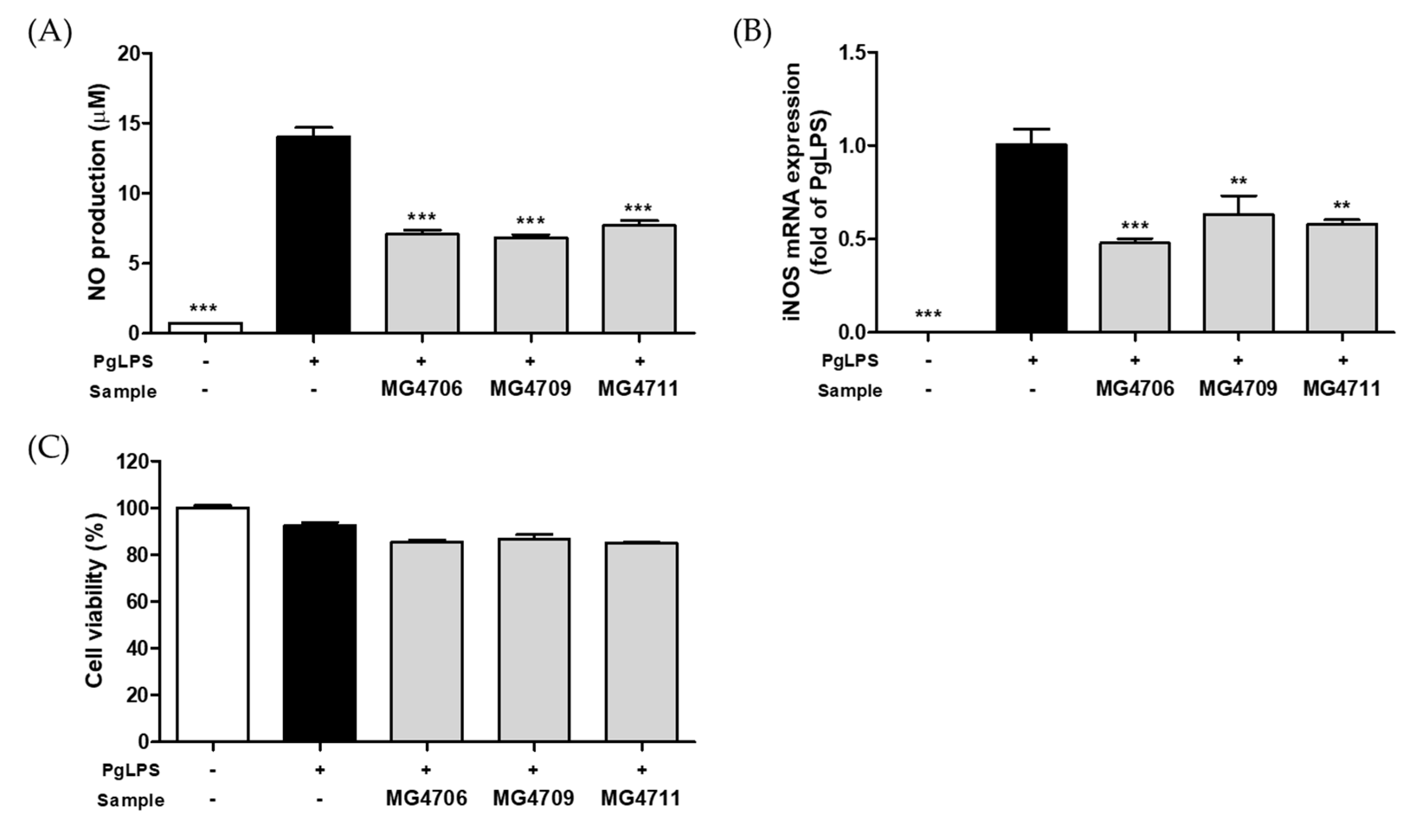
2.3. Nitric Oxide (NO) Production
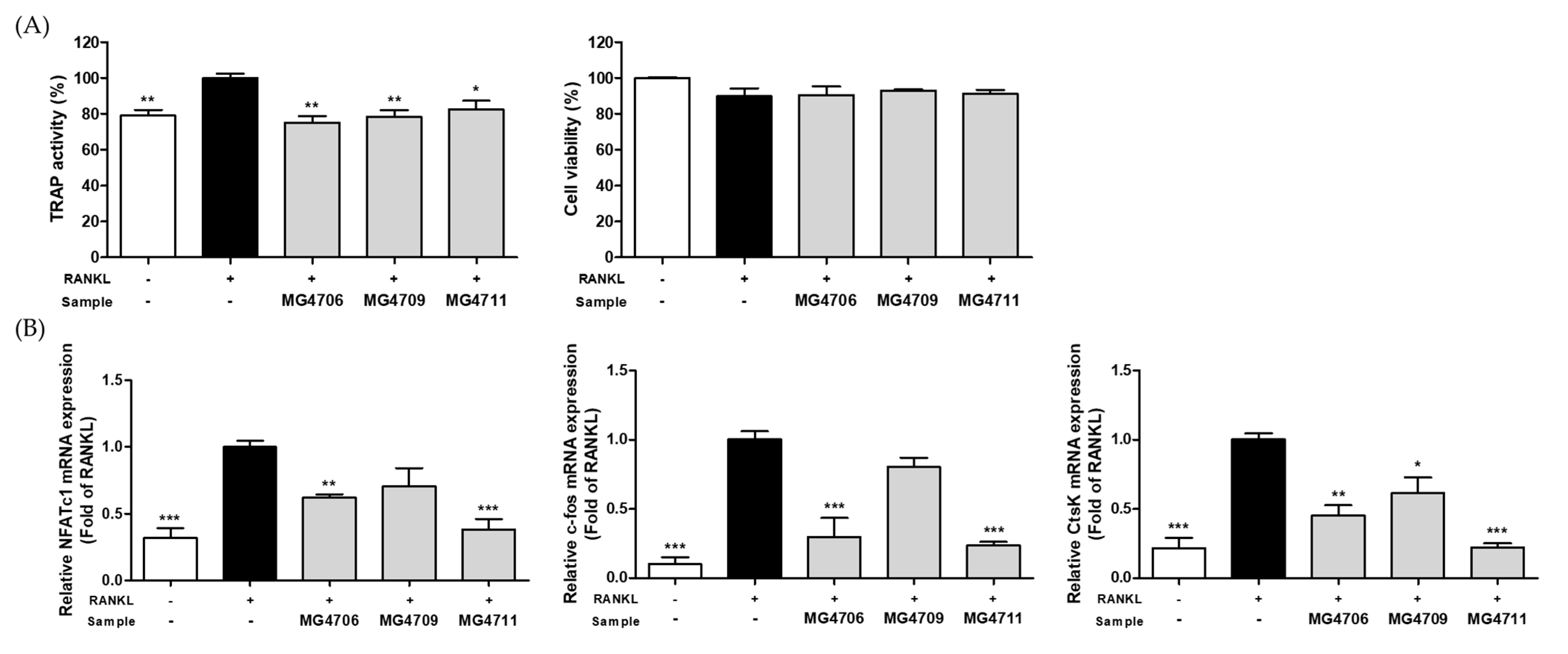
2.4. Tartrate-Resistant Acid Phosphatase (TRAP) Activity
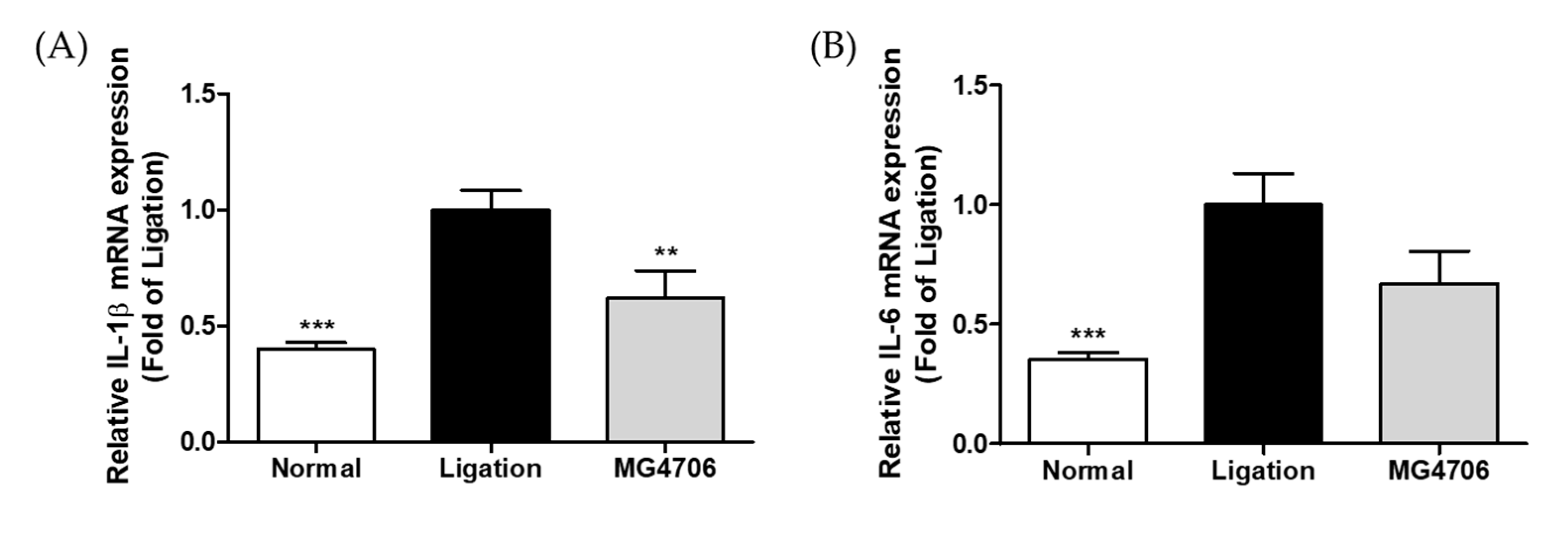
2.5. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
2.6. Aninal Experiment Design
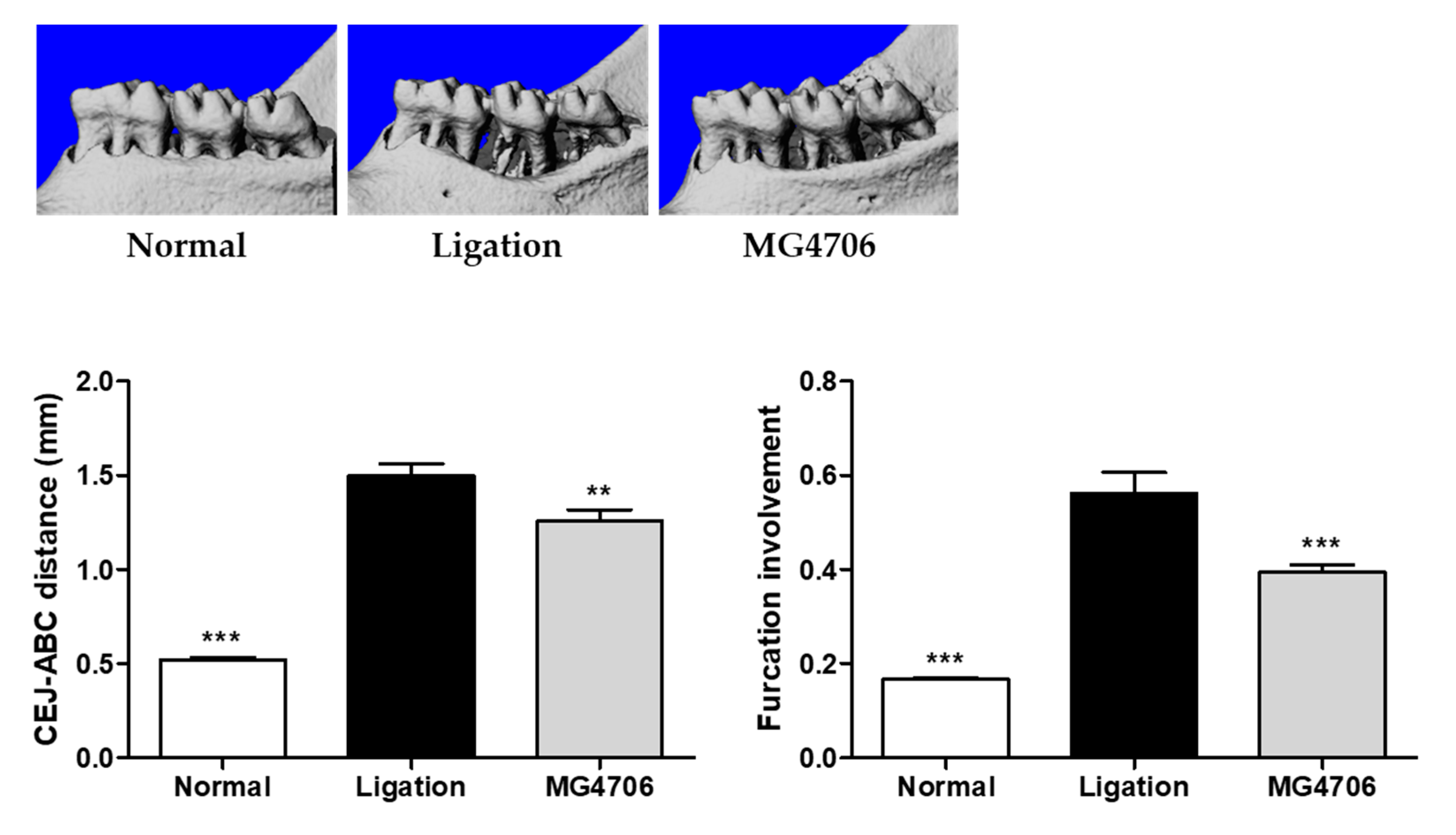
2.7. Micro-Computerized Tomography (Micro-CT)
2.8. Statistical Analysis
3. Results
3.1. Effect of LAB on the NO Production and Gene Expression of iNOS in PgLPS-Activated RAW264.7 Cells
3.2. Effect of LAB on TRAP Activity and Osteoclastogenesis Specific Gene Expression in RANKL-Induced RAW264.7 Cells
3.3. Effect of LAB on Pro-Inflammatory Cytokines and MMP Gene Expression in PgLPS-Stimulated HGF-1 Cells
3.4. Effect of L. rhamnosus MG4706 on Alveolar Bone Loss in Ligatured-induced Periodontal Rats
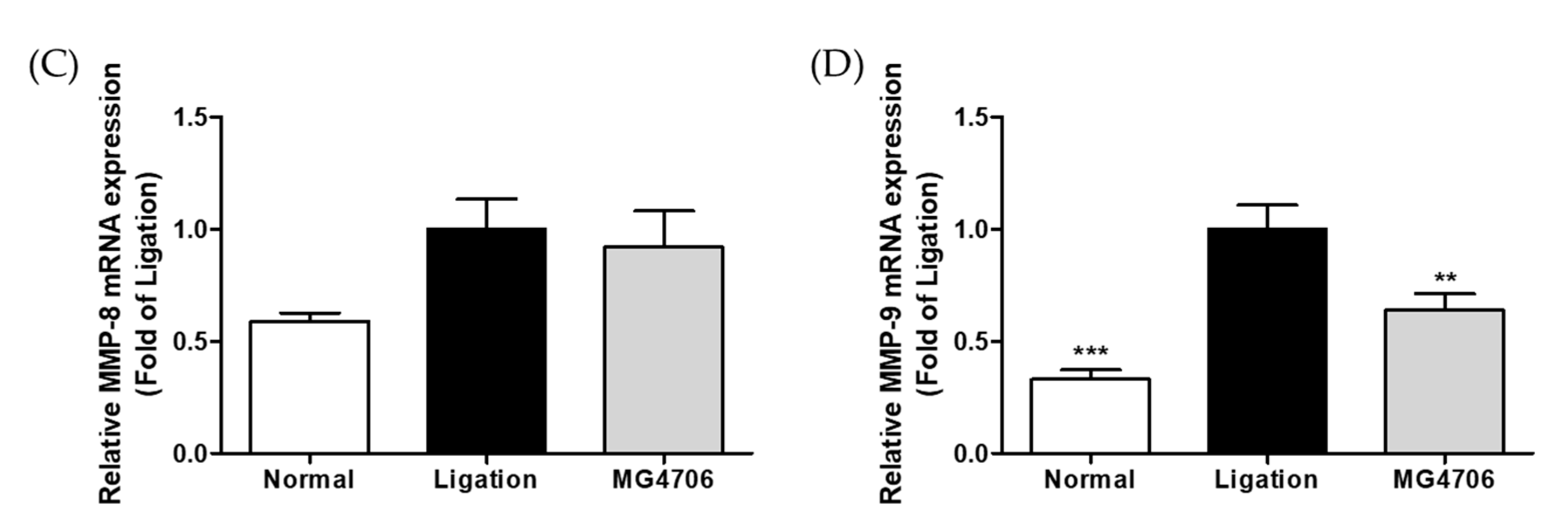
3.5. Effect of L. rhamnosus MG4706 on Pro-Inflammatory Cytokines and MMP Gene Expression in Gingival Tissue
3.6. Effect of L. rhamnosus MG4706 on Alveolar Bone Loss Related Gene Expression in Gingival Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Nie, M.; Fan, M.-W.; Bian, Z. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology 2008, 82, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, P.; Lin, H.; Gao, H.; Yin, J. ETC-1002 attenuates Porphyromonas gingivalis lipopolysaccharide-induced inflammation in RAW264. 7 cells via the AMPK/NF-κB pathway and exerts ameliorative effects in experimental periodontitis in mice. Dis. Markers 2022, 2022, 8583674. [Google Scholar] [PubMed]
- Hayata, M.; Watanabe, N.; Kamio, N.; Tamura, M.; Nodomi, K.; Tanaka, K.; Iddamalgoda, A.; Tsuda, H.; Ogata, Y.; Sato, S. Cynaropicrin from Cynara scolymus L. suppresses Porphyromonas gingivalis LPS-induced production of inflammatory cytokines in human gingival fibroblasts and RANKL-induced osteoclast differentiation in RAW264. 7 cells. J. Nat. Med. 2019, 73, 114–123. [Google Scholar] [CrossRef]
- Buhlin, K.; Gustafsson, A.; Pockley, A.G.; Frostegård, J.; Klinge, B. Risk factors for cardiovascular disease in patients with periodontitis. Eur. Heart J. 2003, 24, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Vernal, R.; Díaz-Zúñiga, J.; Melgar-Rodríguez, S.; Pujol, M.; Diaz-Guerra, E.; Silva, A.; Sanz, M.; Garcia-Sanz, J.A. Activation of RANKL-induced osteoclasts and memory T lymphocytes by Porphyromonas gingivalis is serotype dependant. J. Clin. Periodontol. 2014, 41, 451–459. [Google Scholar] [CrossRef]
- Romero-Castro, N.S.; Vázquez-Villamar, M.; Muñoz-Valle, J.F.; Reyes-Fernández, S.; Serna-Radilla, V.O.; García-Arellano, S.; Castro-Alarcón, N. Relationship between TNF-α, MMP-8, and MMP-9 levels in gingival crevicular fluid and the subgingival microbiota in periodontal disease. Odontology 2020, 108, 25–33. [Google Scholar] [CrossRef]
- Park, Y.-D.; Kim, Y.-S.; Jung, Y.-M.; Lee, S.-I.; Lee, Y.-M.; Bang, J.-B.; Kim, E.-C. Porphyromonas gingivalis lipopolysaccharide regulates interleukin (IL)-17 and IL-23 expression via SIRT1 modulation in human periodontal ligament cells. Cytokine 2012, 60, 284–293. [Google Scholar] [CrossRef]
- Akkaoui, J.; Yamada, C.; Duarte, C.; Ho, A.; Vardar-Sengul, S.; Kawai, T.; Movila, A. Contribution of Porphyromonas gingivalis lipopolysaccharide to experimental periodontitis in relation to aging. GeroScience 2021, 43, 367–376. [Google Scholar] [CrossRef]
- Herrera, B.S.; Martines-Porto, R.; Maia-Dantas, A.; Campi, P.; Spolidorio, L.C.; Costa, S.K.; Van Dyke, T.E.; Gyurko, R.; Muscara, M.N. iNOS-derived nitric oxide stimulates osteoclast activity and alveolar bone loss in ligature-induced periodontitis in rats. J. Periodontol. 2011, 82, 1608–1615. [Google Scholar] [CrossRef]
- Baek, K.J.; Choi, Y.; Ji, S. Gingival fibroblasts from periodontitis patients exhibit inflammatory characteristics in vitro. Arch. Oral Biol. 2013, 58, 1282–1292. [Google Scholar] [CrossRef]
- Araújo, A.A.; Souza, T.O.; Moura, L.M.; Brito, G.A.; Aragão, K.S.; Araújo, L.S.; Medeiros, C.A.; Alves, M.S.; Araújo, R.F., Jr. Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J. Clin. Periodontol. 2013, 40, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Kuraji, R.; Taya, Y.; Ito, H.; Numabe, Y. Effects of theaflavins on tissue inflammation and bone resorption on experimental periodontitis in rats. J. Periodontal Res. 2018, 53, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Leyden, J.J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Goetze, S.; Hiernickel, C.; Elsner, P. Phototoxicity of doxycycline: A systematic review on clinical manifestations, frequency, cofactors, and prevention. Skin Pharmacol. Physiol. 2017, 30, 76–80. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Balzarini, D.; Ando-Suguimoto, E.S.; Ishikawa, K.H.; Simionato, M.R.; Holzhausen, M.; Mayer, M.P. Probiotics alter the immune response of gingival epithelial cells challenged by Porphyromonas gingivalis. J. Periodontal Res. 2019, 54, 115–127. [Google Scholar] [CrossRef]
- Maekawa, T.; Hajishengallis, G. Topical treatment with probiotic Lactobacillus brevis CD 2 inhibits experimental periodontal inflammation and bone loss. J. Periodontal Res. 2014, 49, 785–791. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Guo, L.; Su, Y.; Luo, Z.; Du, J.; Mei, S.; Liu, Y. Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res. Ther. 2020, 11, 61. [Google Scholar] [CrossRef]
- Kang, J.-H.; Yun, S.-I.; Park, M.-H.; Park, J.-H.; Jeong, S.-Y.; Park, H.-O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef]
- Park, D.-Y.; Ahn, Y.-T.; Park, S.-H.; Huh, C.-S.; Yoo, S.-R.; Yu, R.; Sung, M.-K.; McGregor, R.A.; Choi, M.-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.-I.; Jang, M.; Jeong, Y.-A.; Kang, C.-H.; Kim, G.-H. Combination of Limosilactobacillus fermentum MG4231 and MG4244 attenuates lipid accumulation in high-fat diet-fed obese mice. Benef. Microbes 2021, 12, 479–491. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017, 8, 3155–3164. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Won, G.; Choi, S.-I.; Kang, C.-H.; Kim, G.-H. Lactiplantibacillus plantarum MG4296 and Lacticaseibacillus paracasei MG5012 ameliorates insulin resistance in palmitic acid-induced HepG2 cells and high fat diet-induced mice. Microorganisms 2021, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Sawada, J.; Morita, H.; Tanaka, A.; Salminen, S.; He, F.; Matsuda, H. Ingestion of heat-treated Lactobacillus rhamnosus GG prevents development of atopic dermatitis in NC/Nga mice. Clin. Exp. Allergy 2007, 37, 296–303. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, B.K.; Park, H.J.; Park, Y.H.; Kim, B.O.; Pyo, S. Atopic dermatitis-mitigating effects of new L actobacillus strain, L actobacillus sakei probio 65 isolated from Kimchi. J. Appl. Microbiol. 2013, 115, 517–526. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.-I.; Kim, G.-H.; Imm, J.-Y. Anti-inflammatory effect of Ecklonia cava extract on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages and a periodontitis rat model. Nutrients 2019, 11, 1143. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.M. Assessment of bovine sperm viability by MTT reduction assay. Anim. Reprod. Sci. 2006, 92, 1–8. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.-S.; Choi, S.-I.; Kim, G.-H.; Imm, J.-Y. Ecklonia cava extract containing dieckol suppresses RANKL-induced osteoclastogenesis via MAP kinase/NF-κB pathway inhibition and heme oxygenase-1 induction. J. Microbiol. Biotechnol. 2019, 29, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.; Jeong, Y.; Kang, C.-H. Lactic acid bacteria exert a hepatoprotective effect against ethanol-Induced liver injury in HepG2 cells. Microorganisms 2021, 9, 1844. [Google Scholar] [CrossRef]
- Jung, J.-I.; Kim, Y.G.; Kang, C.-H.; Imm, J.-Y. Effects of Lactobacillus curvatus MG5246 on inflammatory markers in Porphyromonas gingivalis lipopolysaccharide-sensitized human gingival fibroblasts and periodontitis rat model. Food Sci. Biotechnol. 2022, 31, 111–120. [Google Scholar] [CrossRef]
- Kim, S.; Kook, K.E.; Kim, C.; Hwang, J.-K. Inhibitory effects of Curcuma xanthorrhiza supercritical extract and xanthorrhizol on LPS-induced inflammation in HGF-1 cells and RANKL-induced osteoclastogenesis in RAW264. 7 cells. J. Microbiol. Biotechnol. 2018, 28, 1270–1281. [Google Scholar] [CrossRef]
- Seymour, G.; Ford, P.; Cullinan, M.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G. Probiotics and periodontal health. J. Med. Life 2011, 4, 387. [Google Scholar] [PubMed]
- Minty, M.; Canceil, T.; Serino, M.; Burcelin, R.; Tercé, F.; Blasco-Baque, V. Oral microbiota-induced periodontitis: A new risk factor of metabolic diseases. Rev. Endocr. Metab. Disord. 2019, 20, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.B.; Kang, S.S.; Park, O.J.; Kim, T.I. Irradiation by gallium–aluminum–arsenate diode laser enhances the induction of nitric oxide by Porphyromonas gingivalis in RAW 264.7 cells. J. Periodontol. 2014, 85, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Yan, J.; Jiang, N.; Zhang, C.; Geng, X.; Li, Z.; Li, C. Leuconostoc mesenteroides LVBH107 antibacterial activity against Porphyromonas gingivalis and anti-inflammatory activity against P. gingivalis lipopolysaccharide-stimulated RAW 264.7 cells. Nutrients 2022, 14, 2584. [Google Scholar] [CrossRef]
- Yiemwattana, I.; Kaomongkolgit, R.; Wirojchanasak, S.; Chaisomboon, N. Morus alba stem extract suppresses matrix metalloproteinases (MMP)-1, MMP-9, and tissue inhibitors of metalloproteinase (TIMP)-1 expression via Inhibition of IκBα degradation induced by Porphyromonas gingivalis LPS signal in THP-1 cells. Eur. J. Dent. 2019, 13, 229–234. [Google Scholar] [CrossRef][Green Version]
- Meschiari, C.A.; Marcaccini, A.M.; Moura, B.C.S.; Zuardi, L.R.; Tanus-Santos, J.E.; Gerlach, R.F. Salivary MMPs, TIMPs, and MPO levels in periodontal disease patients and controls. Clin. Chim. Acta 2013, 421, 140–146. [Google Scholar] [CrossRef]
- Sorsa, T.; Tervahartiala, T.; Leppilahti, J.; Hernandez, M.; Gamonal, J.; Tuomainen, A.M.; Lauhio, A.; Pussinen, P.J.; Mäntylä, P. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol. Res. 2011, 63, 108–113. [Google Scholar] [CrossRef]
- Grenier, D.; Cazalis, J.; Gagnon, G. Response of periodontitis and healthy patients in a Porphyromonas gingivalis-stimulated whole-blood model. J. Investig. Clin. Dent. 2011, 2, 38–42. [Google Scholar] [CrossRef]
- Restaino, C.; Chaparro, A.; Valenzuela, M.A.; Kettlun, A.M.; Vernal, R.; Silva, A.; Puente, J.; Jaque, M.; León, R.; Gamonal, J. Stimulatory response of neutrophils from periodontitis patients with periodontal pathogens. Oral Dis. 2007, 13, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; An, H.-J.; Kim, J.-Y.; Kim, W.-H.; Gwon, M.-G.; Kim, H.-J.; Han, S.M.; Park, I.; Park, S.C.; Leem, J. Bee venom attenuates Porphyromonas gingivalis and RANKL-induced bone resorption with osteoclastogenic differentiation. Food Chem. Toxicol. 2019, 129, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Omori, K.; Nakayama, M.; Mandai, H.; Yamamoto, S.; Kobayashi, H.; Sako, H.; Sakaida, K.; Yoshimura, H.; Ishii, S. The fungal metabolite (+)-terrein abrogates osteoclast differentiation via suppression of the RANKL signaling pathway through NFATc1. Int. Immunopharmacol. 2020, 83, 106429. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, X.; Wang, Y.; Fan, B.; Guo, K.; Yang, C.; Yu, S.; Pang, Y.; Zhang, S. Chitooligosaccharide inhibits RANKL-induced osteoclastogenesis and ligation-induced periodontitis by suppressing MAPK/c-fos/NFATC1 signaling. J. Cell. Physiol. 2020, 235, 3022–3032. [Google Scholar] [CrossRef]
- Heo, S.C.; Kim, Y.N.; Choi, Y.; Joo, J.-Y.; Hwang, J.J.; Bae, M.-K.; Kim, H.J. Elevated expression of cathepsin K in periodontal ligament fibroblast by inflammatory cytokines accelerates osteoclastogenesis via paracrine mechanism in periodontal disease. Int. J. Mol. Sci. 2021, 22, 695. [Google Scholar] [CrossRef]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T. Application of ligature-induced periodontitis in mice to explore the molecular mechanism of periodontal disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef]
- Balci Yuce, H.; Lektemur Alpan, A.; Gevrek, F.; Toker, H. Investigation of the effect of astaxanthin on alveolar bone loss in experimental periodontitis. J. Periodontal Res. 2018, 53, 131–138. [Google Scholar] [CrossRef]
- Lohinai, Z.; Benedek, P.; Fehér, E.; Györfi, A.; Rosivall, L.; Fazekas, Á.; Salzman, A.L.; Szabó, C. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br. J. Pharmacol. 1998, 123, 353–360. [Google Scholar] [CrossRef]
- Nibali, L.; Zavattini, A.; Nagata, K.; Di Iorio, A.; Lin, G.H.; Needleman, I.; Donos, N. Tooth loss in molars with and without furcation involvement–a systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 156–166. [Google Scholar] [CrossRef]
- Parihar, A.S.; Katoch, V. Furcation involvement & its treatment: A review. J. Adv. Med. Dent. Sci. Res. 2015, 3, 81. [Google Scholar]
- Lee, H.J.; Lee, D.-R.; Choi, B.-K.; Yang, S.H. Antiperiodontitis effects of magnolia biondii extract on ligature-induced periodontitis in Rats. Nutrients 2019, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y. Myricetin suppresses LPS-induced MMP expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW 264.7 cells. Arch. Oral Biol. 2012, 57, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Katagiri, S.; Takeuchi, Y.; Komazaki, R.; Ohtsu, A.; Udagawa, S.; Izumi, Y. Bone metabolic microarray analysis of ligature-induced periodontitis in streptozotocin-induced diabetic mice. J. Periodontal Res. 2017, 52, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef] [PubMed]


| Gene * | Origin | Sequence (5′→3′) | ||
|---|---|---|---|---|
| Forward | Reverse | |||
| Inflammatory | iNOS | Mouse | CCTCACGCTTGGGTCTTGTT | GCACAAGGGGTTTTCTTCACG |
| IL-1β | Human | AGAAGTACCTGAGCTCGCCA | CCTGAAGCCCTTGCTGTAGT | |
| Rat | TGAAGCAGCTATGGCAACTG | GGGTCCGTCAACTTCAAAGA | ||
| IL-6 | Human | AAGCCAGAGCTGTGCAGATGAGTA | TGTCCTGCAGCCACTGGTTC | |
| Rat | TGATGGATGCTTCCAAACTG | GAGCATTGGAAGTTGGGGTA | ||
| MMP-8 | Human | CCACTTTCAGAATGTTGAAGGGAAG | TCACGGAGGACAGGTAGAATGGA | |
| Rat | GGATTCCCAAGGAGTGTCCA | CTGGGAACACGCTTGCTATG | ||
| MMP-9 | Human | GCACGACGTCTTCCAGTACC | GCACTGCAGGATGTCATAGGT | |
| Rat | GCGCTGGGCTTAGATCATTC | TGGGACACATAGTGGGAGGA | ||
| Osteoclastogenesis related | NFATc1 | Mouse | GGAGAGTCCGAGAATCGAGAT | TTGCAGCTAGGAAGTACGTCT |
| c-fos | Mouse | CCAGTCAAGAGCATCAGCAA | AAGTAGTGCAGCCCGGAGTA | |
| CtsK | Mouse | GAAGAAGACTCACCAGAAGCAG | TCCAGGTTATGGGCAGAGATT | |
| Alveolar bone loss related | RANKL | Rat | CATGAAACCTCAGGGAGCGT | GTTGGACACCTGGACGCTAA |
| OPG | Rat | GTTCTTGCACAGCTTCACCA | AAACAGCCCAGTGACCATTC | |
| Housekeeping | GAPDH | Mouse | TCTCCCTCACAATTTCCATCC | GGGTGCAGCGAACTTTATTG |
| Human | ACCCACTCCTCCACCTTTG | CTCTTGTGCTCTTGCTGGG | ||
| Rat | CAAGTTCAACGGCACAGTCAAG | ACATACTCAGCACCAGCATCAC | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, J.Y.; Park, J.-Y.; Kim, Y.; Kang, C.-H. Lacticaseibacillus rhamnosus MG4706 Suppresses Periodontitis in Osteoclasts, Inflammation-Inducing Cells, and Ligature-Induced Rats. Nutrients 2022, 14, 4869. https://doi.org/10.3390/nu14224869
Kim S, Lee JY, Park J-Y, Kim Y, Kang C-H. Lacticaseibacillus rhamnosus MG4706 Suppresses Periodontitis in Osteoclasts, Inflammation-Inducing Cells, and Ligature-Induced Rats. Nutrients. 2022; 14(22):4869. https://doi.org/10.3390/nu14224869
Chicago/Turabian StyleKim, Seonyoung, Ji Yeon Lee, Jeong-Yong Park, YongGyeong Kim, and Chang-Ho Kang. 2022. "Lacticaseibacillus rhamnosus MG4706 Suppresses Periodontitis in Osteoclasts, Inflammation-Inducing Cells, and Ligature-Induced Rats" Nutrients 14, no. 22: 4869. https://doi.org/10.3390/nu14224869
APA StyleKim, S., Lee, J. Y., Park, J.-Y., Kim, Y., & Kang, C.-H. (2022). Lacticaseibacillus rhamnosus MG4706 Suppresses Periodontitis in Osteoclasts, Inflammation-Inducing Cells, and Ligature-Induced Rats. Nutrients, 14(22), 4869. https://doi.org/10.3390/nu14224869





