Mechanistic Study of Coffee Effects on Gut Microbiota and Motility in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Coffee Solution
2.2. Preparations of Intraluminal Fecal Contents and In Vitro Bacteria Growth Assay
2.3. Oral Gavage Treatment of Rats with Coffee Solution and Fecal and Tissue Preparations
2.4. Total (Anaerobic and Aerobic) Bacterial Culture from the Colon and Ileum Contents
2.5. Genomic DNA Extraction and Quantitative RT-PCR Study of Gut Microbiota Abundance
2.6. In Vivo Gut Motility Study
2.7. Intestinal and Colonic Muscle Contractility Study
2.8. Statistical Analysis
3. Results
3.1. In Vitro effects of Regular and Decaffeinated Coffee on Gut Microbiota
3.2. In Vivo Effects of Regular and Decaffeinated Coffee on Gut Microbiota
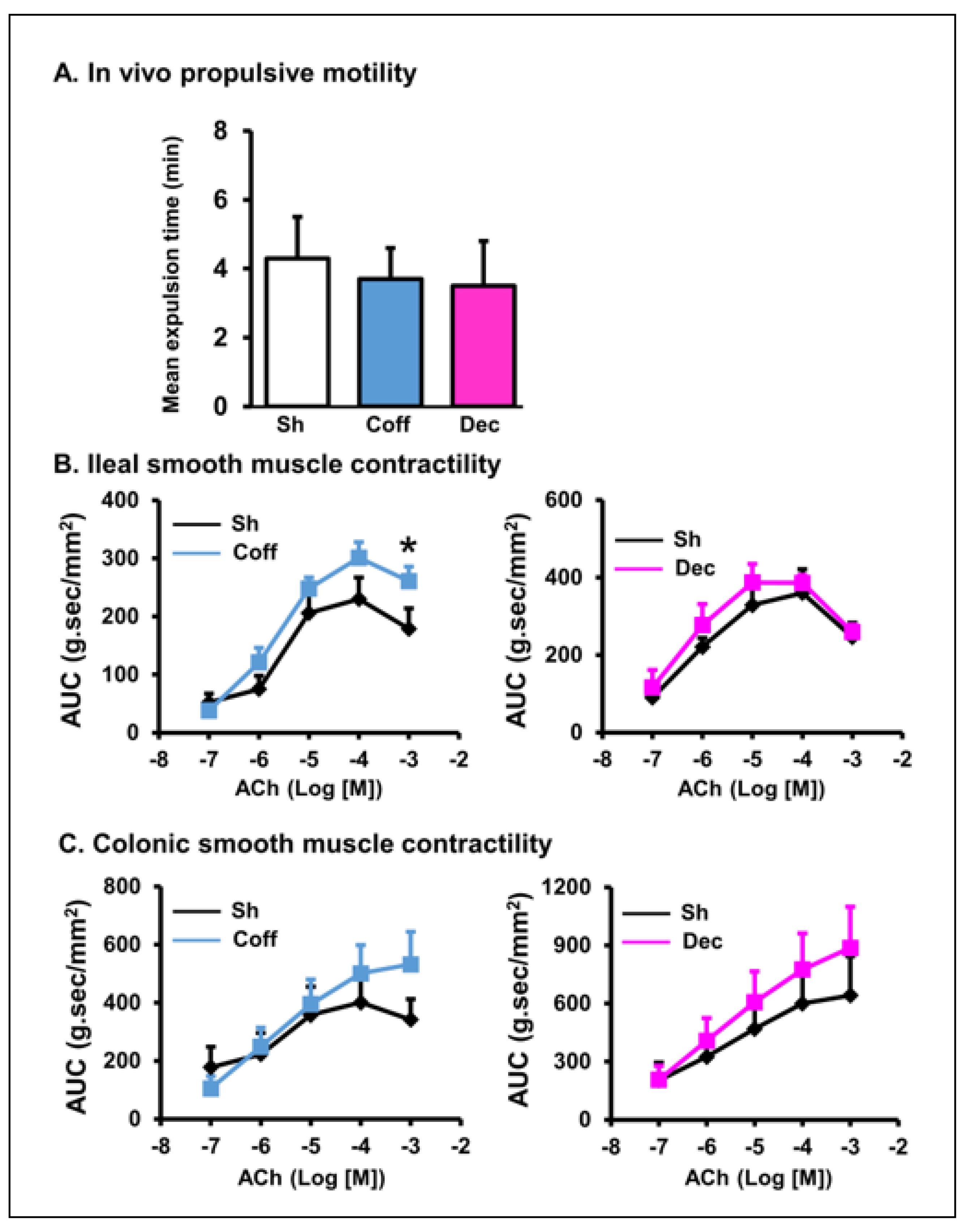
3.3. Effects of Consumption of Regular and Decaffeinated Coffee on Gut Motility and Smooth Muscle Contractility of the Small Intestine and Colon
3.4. Direct Effect of Coffee on Gut Smooth Muscle Contractility
3.5. Neuromuscular Mechanisms of Coffee Effect on Gut Smooth Muscle Contractions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Rexrode, K.M.; Logroscino, G.; Hu, F.B.; van Dam, R.M. Coffee consumption and risk of stroke in women. Circulation 2009, 119, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Morens, D.M.; Grandinetti, A.; Tung, K.-H.; Tanner, C.M.; Masaki, K.H.; Blanchette, P.L.; Curb, J.D.; et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 2000, 283, 2674–2679. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S.; Kempf, K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef]
- Wadhawan, M.; Anand, A.C. Coffee and Liver Disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Okubo, H.; Sasaki, S. Dietary intake in relation to self-reported constipation among Japanese women aged 18–20 years. Eur. J. Clin. Nutr. 2006, 60, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lissner, S.A.; Kaatz, V.; Brandt, W.; Keller, J.; Layer, P. The perceived effect of various foods and beverages on stool consistency. Eur. J. Gastroenterol. Hepatol. 2005, 17, 109–112. [Google Scholar] [CrossRef]
- Müller, S.A.; Rahbari, N.N.; Schneider, F.; Warschkow, R.; Simon, T.; von Frankenberg, M.; Bork, U.; Weitz, J.; Schmied, B.M.; Büchler, M.W. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br. J. Surg. 2012, 99, 1530–1538. [Google Scholar] [CrossRef]
- Müller, S.A.; Rahbari, N.N.; Schmied, B.M.; Büchler, M.W. Can postoperative coffee perk up recovery time after colon surgery? Expert Rev. Gastroenterol. Hepatol. 2013, 7, 91–93. [Google Scholar] [CrossRef]
- Güngördük, K.; Özdemir, İ.A.; Güngördük, Ö.; Gülseren, V.; Gokçü, M.; Sancı, M. Effects of coffee consumption on gut recovery after surgery of gynecological cancer patients: A randomized controlled trial. Am. J. Obstet. Gynecol. 2017, 216, 145.e1–145.e7. [Google Scholar] [CrossRef]
- Watanabe, J.; Miki, A.; Koizumi, M.; Kotani, K.; Sata, N. Effect of Postoperative Coffee Consumption on Postoperative Ileus after Abdominal Surgery: An Updated Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4394. [Google Scholar] [CrossRef]
- Sartini, M.; Bragazzi, N.L.; Spagnolo, A.M.; Schinca, E.; Ottria, G.; Dupont, C.; Cristina, M.L. Coffee Consumption and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 694. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Palmieri, B.; Aponte, M.; Morales-Medina, J.C.; Iannitti, T. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016, 69, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Davin-Regli, A.; Pagès, J.M. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef]
- Nakayama, T.; Oishi, K. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol. Lett. 2013, 343, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gniechwitz, D.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Dietary fiber from coffee beverage: Degradation by human fecal microbiota. J. Agric. Food Chem. 2007, 55, 6989–6996. [Google Scholar] [CrossRef] [PubMed]
- Sarna, S.K.; Shi, X.Z. Function and Regulation of Colonic Contractions in Health and Disease. In Physiology of the Gastrointestinal Tract, 4th ed.; Johnson, L.R., Barrett, K.E., Grisham, F.K., Merchant, J.L., Said, H.M., Ward, J.D., Eds.; Elsevier: San Diego, CA, USA, 2006; Chapter 39; pp. 965–993. [Google Scholar]
- Lin, Y.M.; Sarna, S.K.; Shi, X.Z. Prophylactic and therapeutic benefits of COX-2 inhibitor on motility dysfunction in bowel obstruction: Roles of PGE₂ and EP receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G267–G275. [Google Scholar] [CrossRef]
- Brown, S.R.; Cann, P.A.; Read, N.W. Effect of coffee on distal colon function. Gut 1990, 31, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Welcher, K.; Zimmerman, B.; Stumbo, P. Is coffee a colonic stimulant? Eur. J. Gastroenterol. Hepatol. 1998, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Uranga, J.A.; Del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain-Gut Axis. Nutrients 2020, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Effects of Coffee on the Gastro-Intestinal Tract: A Narrative Review and Literature Update. Nutrients 2022, 14, 399. [Google Scholar] [CrossRef]
- Hegde, S.; Shi, D.; Lin, Y.M.; Shi, X.Z. In vivo and in vitro effects of coffee on gut microbiota and smooth muscle contractility in rats (Abstract). Gastroenterology 2019, 156, S-587. [Google Scholar] [CrossRef]
- Hegde, S.; Lin, Y.M.; Golovko, G.; Khanipov, K.; Cong, Y.; Savidge, T.; Fofanov, Y.; Shi, X.Z. Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Sci. Rep. 2018, 8, 13044. [Google Scholar] [CrossRef]
- Hegde, S.; Lin, Y.M.; Fu, Y.; Savidge, T.; Shi, X.Z. Precision Lactobacillus reuteri therapy attenuates luminal distension-associated visceral hypersensitivity by inducing peripheral opioid receptors in the colon. Pain 2020, 161, 2737–2749. [Google Scholar] [CrossRef]
- Lin, Y.M.; Li, F.; Shi, X.Z. Mechanical stress is a pro-inflammatory stimulus in the gut: In vitro, in vivo and ex vivo evidence. PLoS ONE 2014, 9, e106242. [Google Scholar] [CrossRef]
- Lin, Y.M.; Fu, Y.; Winston, J.; Radhakrishnan, R.; Sarna, S.K.; Huang, L.M.; Shi, X.Z. Pathogenesis of abdominal pain in bowel obstruction: Role of mechanical stress-induced upregulation of nerve growth factor in gut smooth muscle cells. Pain 2017, 158, 583–592. [Google Scholar] [CrossRef]
- Rettedal, E.A.; Gumpert, H.; Sommer, M.O. Cultivation-based multiplex phenotyping of human gut microbiota allows targeted recovery of previously uncultured bacteria. Nat. Commun. 2014, 5, 4714. [Google Scholar] [CrossRef]
- Walker, A.W.; Martin, J.C.; Scott, P.; Parkhill, J.; Flint, H.J.; Scott, K.P. 16S rRNA gene-based profiling of the human infant gut microbiota is strongly influenced by sample processing and PCR primer choice. Microbiome 2015, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3578. [Google Scholar] [CrossRef] [PubMed]
- Mühling, M.; Woolven-Allen, J.; Murrell, J.C.; Joint, I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J. 2008, 2, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Haarman, M.; Knol, J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Pastar, M.; Mitter, B.; Lippert, K.; Hackl, E.; Lojan, P.; Oswald, A.; Sessitsch, A. Improved group-specific primers based on the full SILVA 16S rRNA gene reference database. Environ. Microbiol. 2014, 16, 2389–2407. [Google Scholar] [CrossRef]
- Broccardo, M.; Agostini, S.; Petrella, C.; Guerrini, R.; Improta, G. Central and peripheral role of the nociceptin/orphaninFQ system on normal and disturbed colonic motor function and faecal pellet output in the rat. Neurogastroenterol. Motil. 2008, 20, 939–948. [Google Scholar] [CrossRef]
- Raffa, R.B.; Mathiasen, J.R.; Jacoby, H.I. Colonic bead expulsion time in normal and mu-opioid receptor deficient (CXBK) mice following central (ICV) administration of mu- and delta-opioid agonists. Life Sci. 1987, 41, 2229–2234. [Google Scholar] [CrossRef]
- Shi, X.Z.; Choudhury, B.K.; Pasricha, P.J.; Sarna, S.K. A novel role of VIP in colonic motility function: Induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology 2007, 132, 1388–1400. [Google Scholar] [CrossRef]
- Shi, X.Z.; Sarna, S.K. Gene therapy of Cav1.2 channel with VIP and VIP receptor agonists and antagonists: A novel approach to designing promotility and antimotility agents. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G187–G196. [Google Scholar] [CrossRef][Green Version]
- Li, F.; Lin, Y.M.; Sarna, S.K.; Shi, X.Z. Cellular mechanism of mechano-transcription in colonic smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G670–G679. [Google Scholar] [CrossRef]
- Shi, X.Z.; Sarna, S.K. Inflammatory modulation of muscarinic receptor activation in canine ileal circular muscle cells. Gastroenterology 1997, 112, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Sarna, S.K. Neural regulation of in vitro giant contractions in the rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G275–G282. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Tsou, S.H.; Hu, S.W.; Yang, J.J.; Yan, M.; Lin, Y.Y. Potential Oral Health Care Agent from Coffee Against Virulence Factor of Periodontitis. Nutrients 2019, 11, 2235. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Coffee silverskin: A possible valuable cosmetic ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; de la Cueva, S.P. Antimicrobial activity of coffee melanoidins-a study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef]
- Tokutomi, Y.; Tokutomi, N.; Nishi, K. The Properties of Ryanodine-Sensitive Ca2+ Release in Mouse Gastric Smooth Muscle Cells. Br. J. Pharmacol. 2001, 133, 125–137. [Google Scholar] [CrossRef]
- Domae, K.; Hashitani, H.; Suzuki, H. Regional Differences in the Frequency of Slow Waves in Smooth Muscle of the Guinea-Pig Stomach. J. Smooth Muscle Res. 2008, 44, 231–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Argirova, M.D.; Stefanova, I.D.; Krustev, A.D.; Turiiski, V.I. Testing biological activity of model Maillard reaction products: Studies on gastric smooth muscle tissues. Amino Acids. 2010, 38, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, H.Z.; Gao, C.; Gao, N.; Xia, Y.; Wood, J.D. Actions of galanin on neurotransmission in the submucous plexus of guinea pig small intestine. Eur. J. Pharmacol. 2003, 471, 49–58. [Google Scholar] [CrossRef]
- Silva, A.D.A.; Pereira-De-Morais, L.; da Silva, R.E.R.; Dantas, D.D.M.; Milfont, C.G.B.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; de Menezes, I.R.A.; Barbosa, R. Pharmacological screening of the phenolic compound caffeic acid using rat aorta, uterus and ileum smooth muscle. Chem. Biol. Interact. 2020, 332, 109269. [Google Scholar] [CrossRef] [PubMed]
- Emmelin, N.; Feldberg, W. The smooth muscle contracting effects of various substances supposed to act on nervous structures in the intestinal wall. J. Physiol. 1947, 106, 482–502. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegde, S.; Shi, D.W.; Johnson, J.C.; Geesala, R.; Zhang, K.; Lin, Y.-M.; Shi, X.-Z. Mechanistic Study of Coffee Effects on Gut Microbiota and Motility in Rats. Nutrients 2022, 14, 4877. https://doi.org/10.3390/nu14224877
Hegde S, Shi DW, Johnson JC, Geesala R, Zhang K, Lin Y-M, Shi X-Z. Mechanistic Study of Coffee Effects on Gut Microbiota and Motility in Rats. Nutrients. 2022; 14(22):4877. https://doi.org/10.3390/nu14224877
Chicago/Turabian StyleHegde, Shrilakshmi, Daniel W. Shi, John C. Johnson, Ramasatyaveni Geesala, Ke Zhang, You-Min Lin, and Xuan-Zheng Shi. 2022. "Mechanistic Study of Coffee Effects on Gut Microbiota and Motility in Rats" Nutrients 14, no. 22: 4877. https://doi.org/10.3390/nu14224877
APA StyleHegde, S., Shi, D. W., Johnson, J. C., Geesala, R., Zhang, K., Lin, Y.-M., & Shi, X.-Z. (2022). Mechanistic Study of Coffee Effects on Gut Microbiota and Motility in Rats. Nutrients, 14(22), 4877. https://doi.org/10.3390/nu14224877




