Diosgenin Ameliorated Type II Diabetes-Associated Nonalcoholic Fatty Liver Disease through Inhibiting De Novo Lipogenesis and Improving Fatty Acid Oxidation and Mitochondrial Function in Rats
Abstract
1. Introduction
2. Materials and Methods
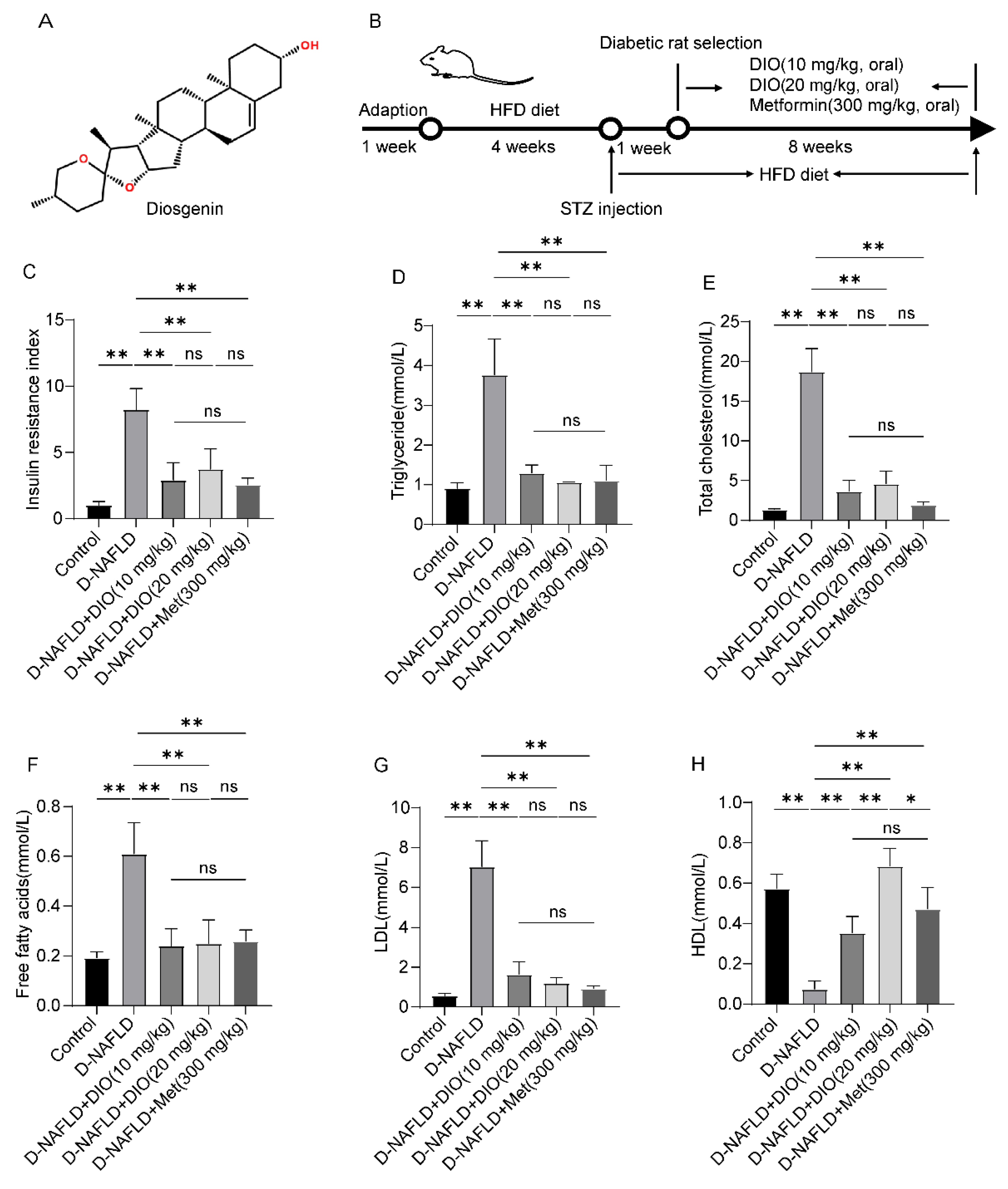
2.1. Animals and Experimental Design
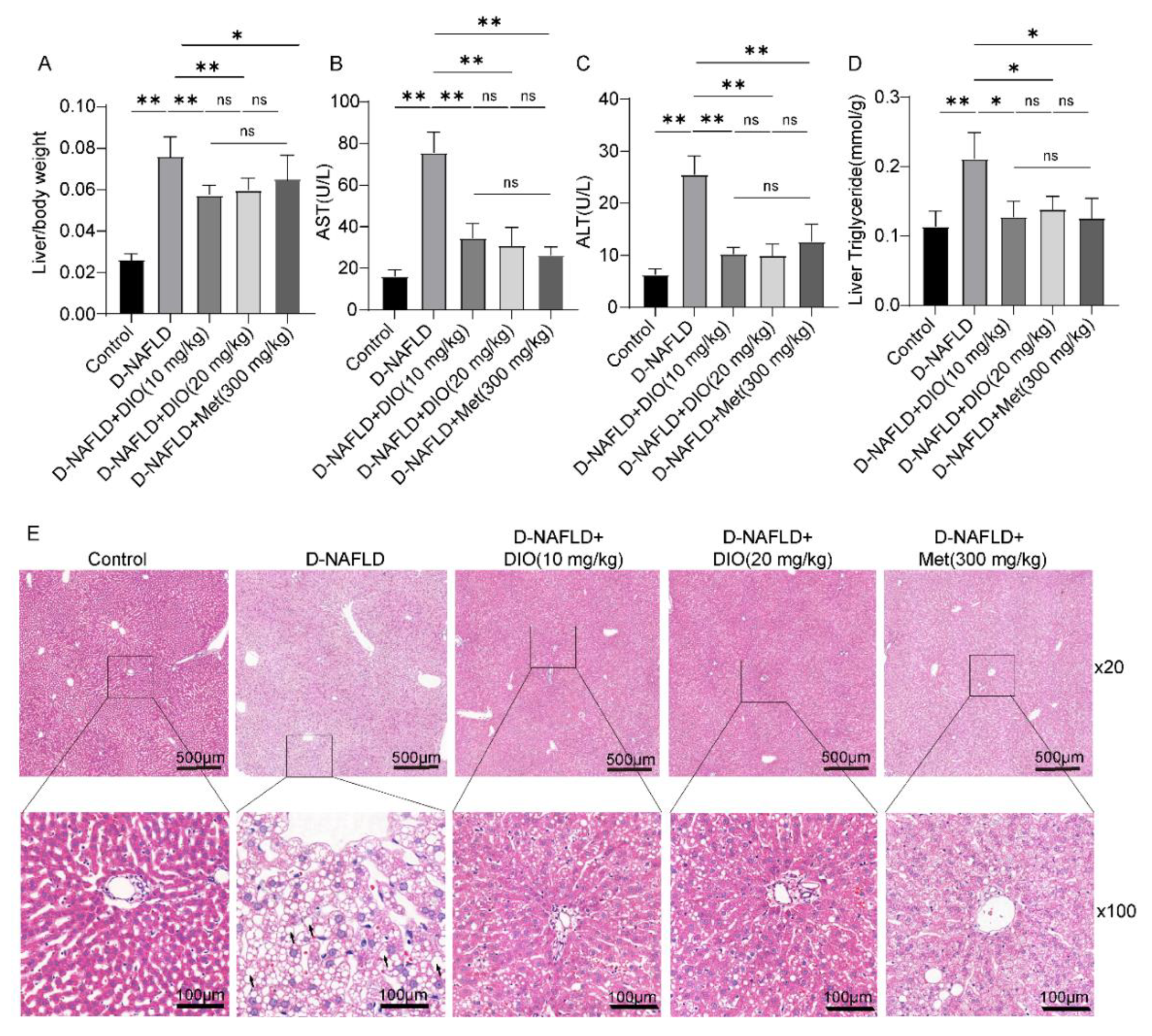
2.2. Biochemical Measurement
2.3. Measurement of Insulin Resistance Index
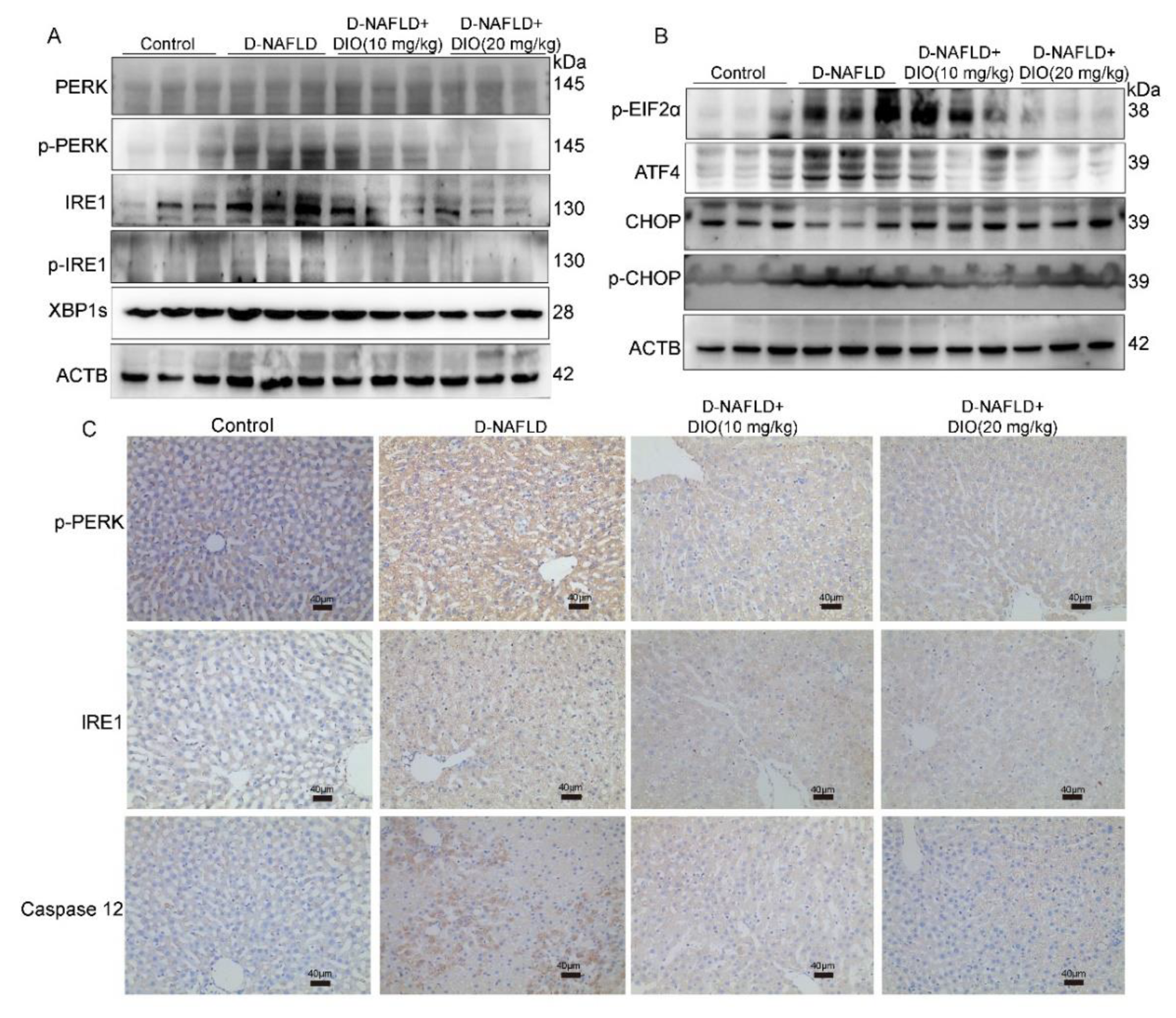
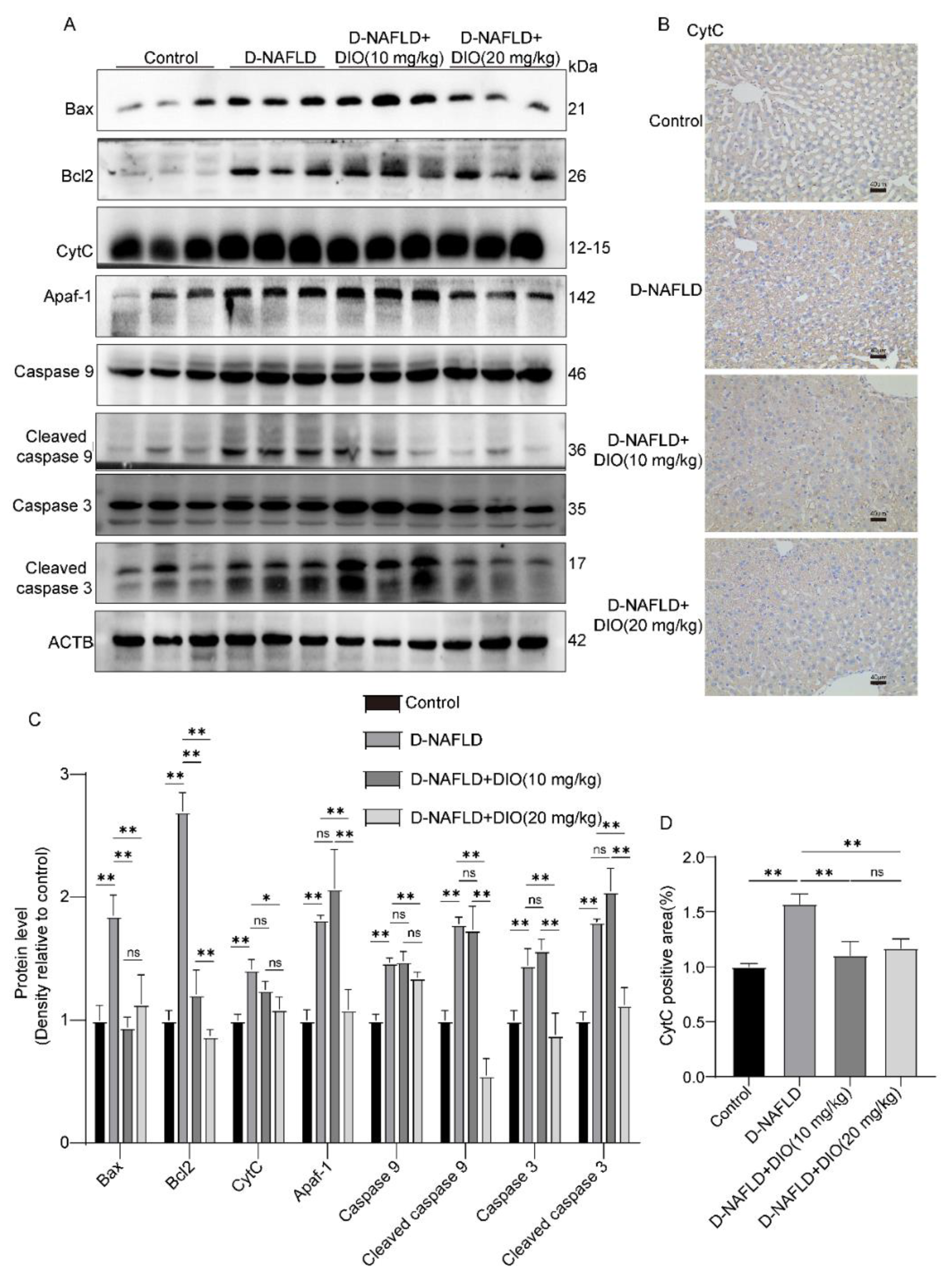
2.4. Hematoxylin and Eosin (H&E) and Immunohistochemistry Assay
2.5. Real-Time PCR Analysis
2.6. ROS Measurement
2.7. Measurement of Antioxidant Enzyme Activities
2.8. Measurement of Lipid Peroxidation
2.9. Western Blot Assay
2.10. Statistical Analysis
3. Results
3.1. DIO Reduced Insulin Resistance and Improved Dyslipidemia in D-NAFLD Rats
3.2. DIO Relieved Pancreatic Injury and Mitochondrial Apoptosis in D-NAFLD Rats
3.3. DIO Ameliorated Liver Injury and Lipid Deposition in D-NAFLD Rats
3.4. DIO Inhibited DNL and Enhanced FAO via AMPK-ACC/SREBP1 and AMPK-ACC Pathways
3.5. DIO Inhibited ER Stress through Regulating PERK and IRE1 Pathways
3.6. DIO Ameliorated Oxidative Stress and Inflammation in the Liver of D-NAFLD Rats
3.7. DIO Ameliorated Mitochondrial Apoptosis in the Liver of D-NAFLD Rats
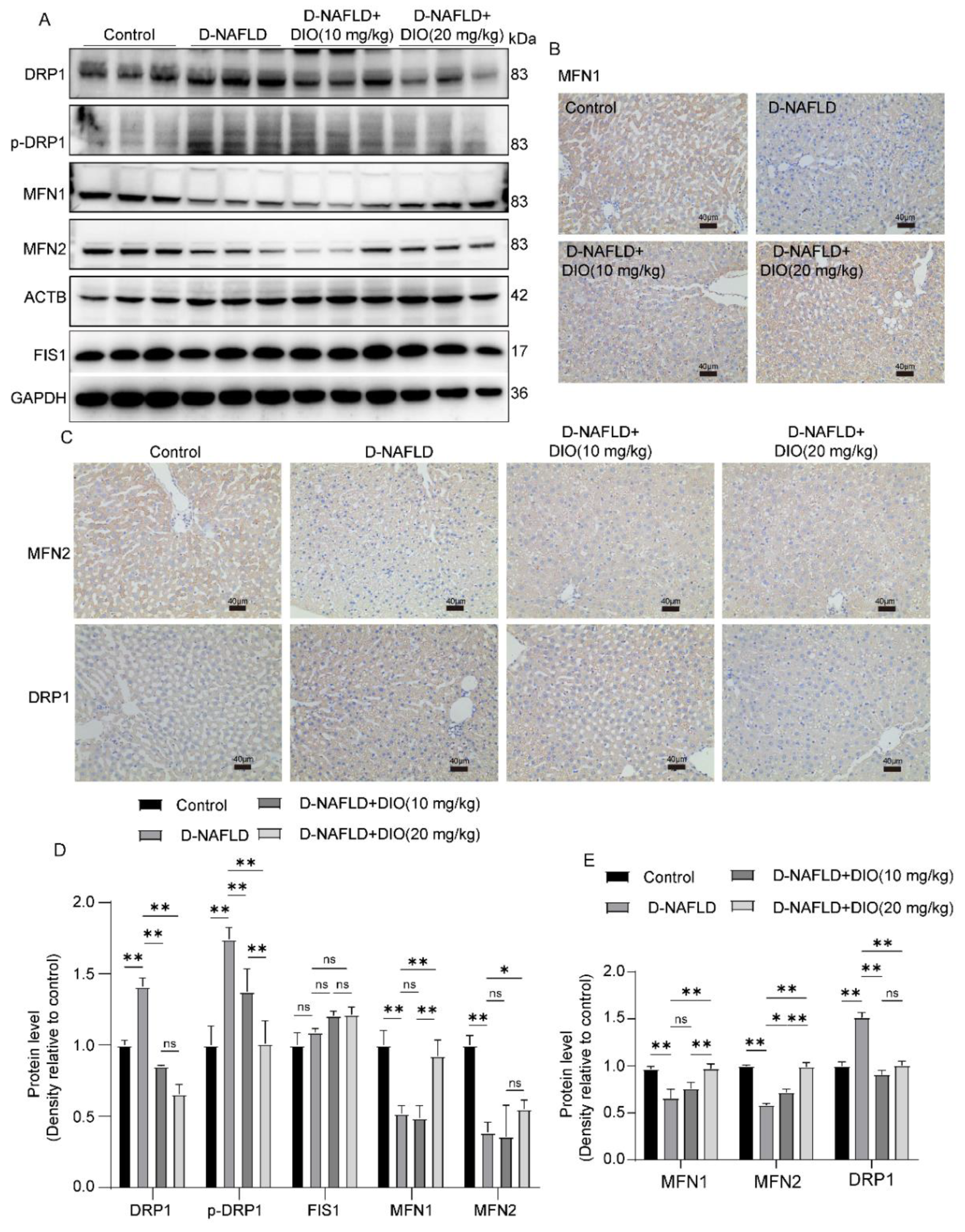
3.8. DIO Ameliorated the Disorder of Mitochondrial Fission and Fusion in the Liver of D-NAFLD Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lemieux, I. Reversing type 2 diabetes: The time for lifestyle medicine has come! Nutrients 2020, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Bjorkstrom, K.; Franzen, S.; Eliasson, B.; Miftaraj, M.; Gudbjornsdottir, S.; Trolle-Lagerros, Y.; Svensson, A.M.; Hagstrom, H. Risk factors for severe liver disease in patients with type 2 diabetes. Clin. Gastroenterol. Hepatol. 2019, 17, 2769–2775.e2764. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Aubin, A.; Loomba, R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr. Diab. Rep. 2021, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Bedi, O.; Aggarwal, S.; Trehanpati, N.; Ramakrishna, G.; Krishan, P. Molecular and pathological events involved in the pathogenesis of diabetes-associated nonalcoholic fatty liver disease. J. Clin. Exp. Gastroenterol. 2019, 9, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Cho, Y.; Lee, B.W.; Park, C.Y.; Lee, D.H.; Cha, B.S.; Rhee, E.J. Nonalcoholic fatty liver disease in diabetes. Part I: Epidemiology and diagnosis. Diabetes Metab. J. 2019, 43, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J. Nonalcoholic fatty liver disease and diabetes: An epidemiological perspective. Endocrinol. Metab. 2019, 34, 226–233. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: Is universal screening appropriate? Diabetologia 2016, 59, 1141–1144. [Google Scholar] [CrossRef]
- Kanchan, D.M.; Somani, G.S.; Peshattiwar, V.V.; Kaikini, A.A.; Sathaye, S. Renoprotective effect of diosgenin in streptozotocin induced diabetic rats. Pharmacol. Rep. 2016, 68, 370–377. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Fernandes-da-Silva, A.; Miranda, C.S.; Santana-Oliveira, D.A.; Oliveira-Cordeiro, B.; Rangel-Azevedo, C.; Silva-Veiga, F.M.; Martins, F.F.; Souza-Mello, V. Endoplasmic reticulum stress as the basis of obesity and metabolic diseases: Focus on adipose tissue, liver, and pancreas. Eur. J. Nutr. 2021, 60, 2949–2960. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Chen, Y.; Zhang, Y.; Shen, M.; Jia, P.; Ji, S.; Wang, T. Resveratrol alleviates endoplasmic reticulum stress-associated hepatic steatosis and injury in mice challenged with tunicamycin. Mol. Nutr. Food Res. 2020, 64, e2000105. [Google Scholar] [CrossRef]
- Ziolkowska, S.; Binienda, A.; Jablkowski, M.; Szemraj, J.; Czarny, P. The interplay between insulin resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2021, 22, 11128. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Li, R.B.; Toan, S.; Zhou, H. Role of mitochondrial quality control in the pathogenesis of nonalcoholic fatty liver disease. Aging 2020, 12, 6467–6485. [Google Scholar] [CrossRef]
- Galloway, C.A.; Lee, H.; Brookes, P.S.; Yoon, Y. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G632–G641. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, J.; Hu, J.; Lou, G.; Xiong, H.; Peng, C.; Zheng, S.; Huang, Q. The role of diosgenin in diabetes and diabetic complications. J. Steroid Biochem. Mol. Biol. 2019, 198, 105575. [Google Scholar] [CrossRef] [PubMed]
- Parama, D.; Boruah, M.; Yachna, K.; Rana, V.; Banik, K.; Harsha, C.; Thakur, K.K.; Dutta, U.; Arya, A.; Mao, X.; et al. Diosgenin, a steroidal saponin, and its analogs: Effective therapies against different chronic diseases. Life Sci. 2020, 260, 118182. [Google Scholar] [CrossRef]
- Cheng, S.; Liang, S.; Liu, Q.; Deng, Z.; Zhang, Y.; Du, J.; Zhang, Y.; Li, S.; Cheng, B.; Ling, C. Diosgenin prevents high-fat diet-induced rat non-alcoholic fatty liver disease through the AMPK and LXR signaling pathways. Int. J. Mol. Med. 2018, 41, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Luo, R.; Liu, Q.; Zhu, J.; Lei, M.; Liang, X.; Wang, X.; Peng, X. Jujuboside A ameliorates high fat diet and streptozotocin induced diabetic nephropathy via suppressing oxidative stress, apoptosis, and enhancing autophagy. Food Chem. Toxicol. 2022, 159, 112697. [Google Scholar] [CrossRef] [PubMed]
- Germoush, M.O.; Elgebaly, H.A.; Hassan, S.; Kamel, E.M.; Bin-Jumah, M.; Mahmoud, A.M. Consumption of Terpenoids-rich padina pavonia extract attenuates hyperglycemia, insulin resistance and oxidative stress, and upregulates PPAR gamma in a rat model of type 2 diabetes. Antioxidants 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cai, S.; Zhang, Y.; Ma, N.; Yi, J.; Hu, X.; Wang, T. Protective effect of Rhus chinensis Mill. fruits on 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced cholestasis in mice via ameliorating oxidative stress and inflammation. Nutrients 2022, 14, 4090. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, B.W.; Kim, Y.J.; Lee, D.H.; Cha, B.S.; Park, C.Y. Nonalcoholic fatty liver disease and diabetes: Part II: Treatment. Diabetes Metab. J. 2019, 43, 127–143. [Google Scholar] [CrossRef]
- Khateeb, S.; Albalawi, A.; Alkhedaide, A. Regulatory effect of diosgenin on lipogenic genes expression in high-fat diet-induced obesity in mice. Saudi J. Biol. Sci. 2021, 28, 1026–1032. [Google Scholar] [CrossRef]
- Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J.; Zhao, Y.; Xu, L.; Zou, X.; Lu, F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement. Altern. Med. 2019, 19, 255. [Google Scholar] [CrossRef]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Zhong, L.; Yu, S.J.; Shen, W.; Cai, C.; Yu, H.H. Inhibition of stearoyl-coenzyme A desaturase 1 ameliorates hepatic steatosis by inducing AMPK-mediated lipophagy. Aging 2020, 12, 7350–7362. [Google Scholar] [CrossRef]
- Rector, R.S.; Thyfault, J.P.; Uptergrove, G.M.; Morris, E.M.; Naples, S.P.; Borengasser, S.J.; Mikus, C.R.; Laye, M.J.; Laughlin, M.H.; Booth, F.W.; et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J. Hepatol. 2010, 52, 727–736. [Google Scholar] [CrossRef]
- Szendroedi, J.; Chmelik, M.; Schmid, A.I.; Nowotny, P.; Brehm, A.; Krssak, M.; Moser, E.; Roden, M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 2009, 50, 1079–1086. [Google Scholar] [CrossRef]
- Deguil, J.; Pineau, L.; Rowland Snyder, E.C.; Dupont, S.; Beney, L.; Gil, A.; Frapper, G.; Ferreira, T. Modulation of lipid-induced ER stress by fatty acid shape. Traffic 2011, 12, 349–362. [Google Scholar] [CrossRef]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [CrossRef]
- Lee, J.S.; Zheng, Z.; Mendez, R.; Ha, S.W.; Xie, Y.; Zhang, K. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol. Lett. 2012, 211, 29–38. [Google Scholar] [CrossRef]
- Henkel, A.S. Unfolded protein response sensors in hepatic lipid metabolism and nonalcoholic fatty liver disease. Semin. Liver Dis. 2018, 38, 320–332. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Zhu, R.M.; Zhang, Y.; Yu, T.; Wang, H.; Zhao, H.; Zhao, M.; Ji, Y.L.; Chen, Y.H.; et al. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxicol. Lett. 2012, 212, 229–240. [Google Scholar] [CrossRef]
- Cho, I.J.; Oh, D.H.; Yoo, J.; Hwang, Y.C.; Ahn, K.J.; Chung, H.Y.; Jeong, S.W.; Moon, J.Y.; Lee, S.H.; Lim, S.J.; et al. Allopurinol ameliorates high fructose diet induced hepatic steatosis in diabetic rats through modulation of lipid metabolism, inflammation, and ER stress pathway. Sci. Rep. 2021, 11, 9894. [Google Scholar] [CrossRef]
- Wu, X.; Xu, N.; Li, M.; Huang, Q.; Wu, J.; Gan, Y.; Chen, L.; Luo, H.; Li, Y.; Huang, X.; et al. Protective effect of patchouli alcohol against high-fat diet induced hepatic steatosis by alleviating endoplasmic reticulum stress and regulating VLDL metabolism in rats. Front. Pharmacol. 2019, 10, 1134. [Google Scholar] [CrossRef]
- Degli Esposti, D.; Hamelin, J.; Bosselut, N.; Saffroy, R.; Sebagh, M.; Pommier, A.; Martel, C.; Lemoine, A. Mitochondrial roles and cytoprotection in chronic liver injury. Biochem. Res. Int. 2012, 2012, 387626. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Meroni, M.; Paolini, E.; Macchi, C.; Dongiovanni, P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): New perspectives for a fairy-tale ending? Metabolism 2021, 117, 154708. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, Y.; Miyazawa, T.; Sakamoto, S.; Hanada, Y.; Wang, L.; Gotoh, K.; Uchida, K.; Katsuhara, S.; Sakamoto, R.; Ishihara, T.; et al. Non-alcoholic fatty liver disease in mice with hepatocyte-specific deletion of mitochondrial fission factor. Diabetologia 2021, 64, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Leptin-induced mitochondrial fusion mediates hepatic lipid accumulation. Int. J. Obes. 2015, 39, 1750–1756. [Google Scholar] [CrossRef]
- Sacks, J.; Mulya, A.; Fealy, C.E.; Huang, H.; Mosinski, J.D.; Pagadala, M.R.; Shimizu, H.; Batayyah, E.; Schauer, P.R.; Brethauer, S.A.; et al. Effect of Roux-en-Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity. Physiol. Rep. 2018, 6, e13600. [Google Scholar] [CrossRef]
- Hernandez, J.H.C.; Loman, W.N.R.; Gomez-Crisostomo, N.P.; De la Cruz-Hernandez, E.N.; Garcia, L.M.G.; Gomez, M.G.; Del Angel, N.A.H.; Gamas, C.F.A.; Hernandez, V.S.C.; Martinez-Abundis, E. High sugar but not high fat diet consumption induces hepatic metabolic disruption and up-regulation of mitochondrial fission-associated protein Drp1 in a model of moderate obesity. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]



| Primers | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| SREBP1 | ACCCTGCGAAGTGCTCACAA | GCGTTTCTACCACTTCAGGTTTCA |
| FASN | GAGCGTTCGTGAAACCGACA | AGGTTGGTGCACCTCCACTTG |
| CD36 | CGGCGATGAGAAAGCAGA | ACTCCAACACCAAGTAAGACCA |
| CPT1 | CTGCTGTATCGTCGCACATTAG | GTTGGATGGTGTCTGTCTCTTCC |
| PPARα | GCTCTGAACATTGGCGTTCG | TCAGTCTTGGCTCGCCTCTA |
| IL-1β | CCTTGTGCAAGTGTCTGAAGC | CCCAAGTCAAGGGCTTGGAA |
| IL-4 | AACACCACGGAGAACGAGCTCATC | AGTGAGTTCAGACCGCTGACACCT |
| TNF-α | CTCCAGCTGGAAGACTCCTCCCAG | CCCGACTACGTGCTCCTCACC |
| IL-10 | AGAAGAGGGAGGAGCCTTTG | GCCTTTGCTGGTCTTCACTC |
| MCP-1 | TGCTGTCTCAGCCAGATGCAGTTA | AGAAGTGCTTGAGGTGGTTGTGGA |
| Actb | TGACAGGATGCAGAAGGAGA | TAGAGCCACCAATCCACACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Li, Z.; Jin, R.; Yao, Y.; He, S.; Lei, M.; Wang, X.; Shi, C.; Gao, L.; Peng, X. Diosgenin Ameliorated Type II Diabetes-Associated Nonalcoholic Fatty Liver Disease through Inhibiting De Novo Lipogenesis and Improving Fatty Acid Oxidation and Mitochondrial Function in Rats. Nutrients 2022, 14, 4994. https://doi.org/10.3390/nu14234994
Zhong Y, Li Z, Jin R, Yao Y, He S, Lei M, Wang X, Shi C, Gao L, Peng X. Diosgenin Ameliorated Type II Diabetes-Associated Nonalcoholic Fatty Liver Disease through Inhibiting De Novo Lipogenesis and Improving Fatty Acid Oxidation and Mitochondrial Function in Rats. Nutrients. 2022; 14(23):4994. https://doi.org/10.3390/nu14234994
Chicago/Turabian StyleZhong, Yujie, Zhiman Li, Ruyi Jin, Yanpeng Yao, Silan He, Min Lei, Xin Wang, Chao Shi, Li Gao, and Xiaoli Peng. 2022. "Diosgenin Ameliorated Type II Diabetes-Associated Nonalcoholic Fatty Liver Disease through Inhibiting De Novo Lipogenesis and Improving Fatty Acid Oxidation and Mitochondrial Function in Rats" Nutrients 14, no. 23: 4994. https://doi.org/10.3390/nu14234994
APA StyleZhong, Y., Li, Z., Jin, R., Yao, Y., He, S., Lei, M., Wang, X., Shi, C., Gao, L., & Peng, X. (2022). Diosgenin Ameliorated Type II Diabetes-Associated Nonalcoholic Fatty Liver Disease through Inhibiting De Novo Lipogenesis and Improving Fatty Acid Oxidation and Mitochondrial Function in Rats. Nutrients, 14(23), 4994. https://doi.org/10.3390/nu14234994





