Halting the Metabolic Complications of Antipsychotic Medication in Patients with a First Episode of Psychosis: How Far Can We Go with the Mediterranean Diet? A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Assessment of Current Dietary Intake
2.3. Nutritional Intervention Process
2.4. Anthropometric Measurements
2.5. Hematological/Biochemical Assessment
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Anthropometric Measurements
3.3. Modification in Eating Habits
3.4. Hematological and Biochemical Indices
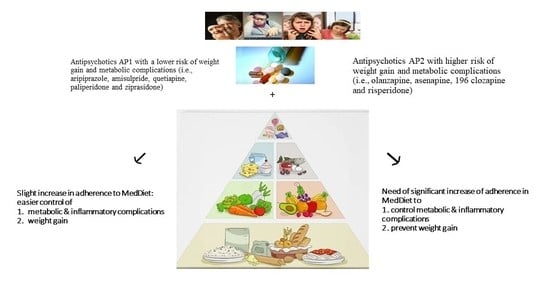
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Birth date:
- Birth age (weeks of gestation):
- Born by:
- Natural birth
- Cesarean
- Birth weight:
- Birth height:
- Siblings
- No
- Yes Sibling’s/Siblings’ age:
- Breastfed?
- No
- Yes
- Duration of exclusive breastfeeding: months
- Did your mother avoid certain foods while breastfeeding
- No
- Yes Kind(s) of food:
- Did your mother smoke during pregnancy?
- No
- Yes Number of cigarettes:
- Did your mother smoke during breastfeeding?
- No
- Yes Number of cigarettes:
- Was anyone around your mother smoking during her pregnancy?
- No
- Yes
- Was anyone smoking around you in the first 3 years of your life?
- No
- Yes
- Were you given antibiotics during the first 3 years of life?
- No
- Yes
- 15.
- Were you hospitalized during the first 3 years of life?
- No
- Yes
- 16.
- In the first 3 years of life, you lived in:
- A city
- A semi-urban environment
- A village/farm
- 17.
- Did you have pets at home during the first 3 years of life?
- No
- Yes
- 18.
- At what age did you consume milk replacer?
- 19.
- Age of solid food introduction: months
- 20.
- Sequence of food introduction (number it):
- 21.
- At what age did you start consuming sugar? months
- 22.
- When did you stop consuming puréed foods? months
- 23.
- Did you drink before the age of 3?
- 24.
- Did you eat before the age of 3?
Appendix B
| Variable | T0 | T1 | p-Value |
|---|---|---|---|
| Energy (Kcal) | 1730 (504) | 1430 (477) | 0.05 |
| Proteins (g) | 55.6 (24.1) | 64.0 (27.7) | 0.26 |
| Carbohydrates (g) | 193 (71.7) | 145 (70.0) | 0.03 |
| Fiber (g) | 19.1 (14.6) | 13.5 (6.77) | 0.21 |
| Sugars (g) | 51.8 (33.0) | 36.3 (16.3) | 0.16 |
| Fats (g) | 83.3 (31.7) | 67.2 (20.6) | 0.15 |
| Saturated fats (g) | 24.9 (12.1) | 19.2 (8.42) | 0.39 |
| Mono-unsaturated fats (g) | 41.6 (16.8) | 35.4 (13.0) | 0.69 |
| Poly-unsaturated fats (g) | 11.3 (8.94) | 7.57 (3.02) | 0.93 |
| Trans fats (g) | 1.23 (1.31) | 1.02 (0.968) | 0.77 |
| Dietary cholesterol (mg) | 210 (193) | 193 (128) | 0.45 |
| Vitamin A (RE) | 825 (1380) | 557 (799) | 0.08 |
| Vitamin B1 (mg) | 1.41 (0.471) | 1.12 (0.534) | 0.2 |
| Vitamin B2 (mg) | 1.68 (0.669) | 1.43 (0.641) | 0.86 |
| Vitamin B3 (mg) | 15.4 (6.56) | 17.9 (11.1) | 0.32 |
| Vitamin B6 (mg) | 1.47 (0.698) | 1.24 (0.612) | 0.58 |
| Vitamin B12 (mcg) | 2.51 (2.40) | 2.45 (1.60) | 0.58 |
| Vitamin C (mg) | 86.5 (79.0) | 64.0 (55.0) | 0.43 |
| Vitamin D (mcg) | 1.48 (1.56) | 16.7 (66.5) | 0.17 |
| Vitamin E (mg) | 9.35 (6.01) | 7.67 (4.15) | 0.26 |
| Folic acid (mcg) | 373 (302) | 302 (191) | 0.36 |
| Vitamin B5 (mg) | 3.32 (1.55) | 3.71 (1.68) | 0.53 |
| Ca (mg) | 689 (346) | 585 (323) | 0.31 |
| Fe (mg) | 11.7 (6.09) | 11.0 (5.85) | 0.6 |
| Mg (mg) | 213 (75.2) | 217 (116) | 0.64 |
| Phosphorus (mg) | 957 (370) | 891 (365) | 0.62 |
| K (mg) | 2050 (1030) | 1860 (706) | 0.69 |
| Na (mg) | 2010 (1040) | 1290 (778) | 0.01 |
| Zinc (mg) | 7.59 (3.76) | 7.45 (4.05) | 0.77 |
| Omega-3 (g) | 1.05 (0.893) | 0.840 (0.593) | 0.78 |
| Omega-6 (g) | 8.07 (6.35) | 6.44 (2.65) | 0.88 |
| Omega-3/omega-6 | 0.125 (0.0483) | 0.136 (0.0757) | 0.9 |
| Variable | T0 | T1 | p-Value |
|---|---|---|---|
| Red Blood Cells (RBC) (Μ/μL) | 7.11 (10.8) | 7.08 (10.4) | 0.85 |
| White Blood Cells (WBC) (K/μL) | 7.85 (2.61) | 7.89 (3.18) | 0.89 |
| Platelets (K/μL) | 270 (59.7) | 269 (68.3) | 0.83 |
| Ferritin (ng/mL) | 82.6 (61.5) | 83.5 (64.1) | 0.9 |
| Serum B12 (pg/mL) | 360 (127) | 349 (110) | 0.9 |
| Creatinine (mg/dL) | 0.830 (0.177) | 0.861 (0.189) | 0.61 |
| Oxaloacetate Transaminase (SGOT) (IU/L) | 17.7 (5.95) | 20.4 (13.2) | 0.67 |
| Pyruvate Transaminase (SGPT) (IU/L) | 22.5 (15.8) | 28.2 (31.6) | 0.95 |
| Lactate Dehydrogenase (LDH) (IU/L) | 131 (75.4) | 133 (69.4) | 0.53 |
| γ-Glutamyltransferase (γ-GT) (IU/L) | 21.9 (17.4) | 28.7 (39.2) | 0.98 |
| C-Reactive Protein (CRP) (mg/dL) | 0.413 (0.727) | 0.214 (0.230) | 0.53 |
| High Density Lipoprotein (HDL) (mg/dL) | 51.1 (14.5) | 49.6 (16.5) | 0.67 |
| Triglycerides (mg/dL) | 103 (51.1) | 124 (73.4) | 0.4 |
| Serum K+ (mEq/L) | 4.30 (0.335) | 4.30 (0.340) | 0.96 |
References
- Breitborde, N.J.; Srihari, V.H.; Woods, S.W. Review of the operational definition for first-episode psychosis. Early Interv. Psychiatry 2009, 3, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dipasquale, S.; Pariante, C.M.; Dazzan, P.; Aguglia, E.; Mcguire, P.; Mondelli, V. The dietary pattern of patients with schizophrenia: A systematic review. J. Psychiatr. Res. 2013, 47, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, K.; Kilner, K.; Clibbens, N. A comparison of the nutrient intake of a community-dwelling first-episode psychosis cohort, aged 19–64 years, with data from the UK population. J. Nutr. Sci. 2015, 4, e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teasdale, S.B.; Ward, P.B.; Samaras, K.; Firth, J.; Stubbs, B.; Tripodi, E.; Burrows, T.L. Dietary intake of people with severe mental illness: Systematic review and meta-analysis. Br. J. Psychiatr. 2019, 214, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Aucoin, M.; La Chance, L.; Cooley, K.; Kidd, S. Diet and Psychosis: A Scoping Review. Neuropsychobiology 2020, 79, 20–42. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Efthymiou, D.; Tsironis, V.; Athanassis, P.; Chatzioannidis, S.; Kesoglou, T.; Severin, A.; Bozikas, V. The benefits of the Mediterranean diet in first episode psychosis patients taking antipsychotics. Toxicol. Rep. 2022, 9, 120–125. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Kale, A.; Naphade, N.; Sapkale, S.; Kamaraju, M.; Pillai, A.; Joshi, S.; Mahadik, S. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: Implications for altered one-carbon metabolism. Psychiatry Res. 2010, 175, 47–53. [Google Scholar] [CrossRef]
- Wang, D.; Zhai, J.-X.; Liu, D.-W. Serum folate levels in schizophrenia: A meta-analysis. Psychiatry Res. 2016, 235, 83–89. [Google Scholar] [CrossRef]
- Cao, B.; Wang, D.F.; Xu, M.Y.; Liu, Y.Q.; Yan, L.L.; Wang, J.Y.; Lu, Q.B. Lower folate levels in schizophrenia: A meta-analysis. Psychiatry Res. 2016, 245, 1–7. [Google Scholar] [CrossRef]
- Tomioka, Y.; Numata, S.; Kinoshita, M.; Umehara, H.; Watanabe, S.Y.; Nakataki, M.; Iwayama, Y.; Toyota, T.; Ikeda, M.; Yamamori, H.; et al. Decreased serum pyridoxal levels in schizophrenia: Meta-analysis and Mendelian randomization analysis. J. Psychiatry Neurosci. 2018, 43, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valipour, G.; Saneei, P.; Esmaillzadeh, A. Serum vitamin D levels in relation to schizophrenia: A systematic review and meta-analysis of observational studies. J. Clin. Endocrinol. Metab. 2014, 99, 3863–3872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firth, J.; Carney, R.; Stubbs, B.; Teasdale, S.B.; Vancampfort, D.; Ward, P.B.; Berk, M.; Sarris, J. Nutritional deficiencies and clinical correlates in first-episode psychosis: A systematic review and meta-analysis. Schizophr. Bull. 2018, 44, 1275–1292. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, W.W.; Cetkovich-Bakmas, M.; De Hert, M.; Hennekens, C.H.; Lambert, M.; Leucht, S.; Maj, M.; McIntyre, R.S.; Naber, D.; Newcomer, J.W.; et al. Comorbid somatic illnesses in patients with severe mental disorders: Clinical, policy and research challenges. J. Clin. Psychiatry 2008, 64, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Hennekens, A.R.; Hollar, D. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 2005, 150, 1115–1121. [Google Scholar] [CrossRef]
- De Hert, M.; Schreurs, V.; Sweers, K.; Van Eyck, D.; Hanssens, L.; Šinko, S.; Wampers, M.; Scheen, A.; Peuskens, J.; van Winkel, R. Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: A retrospective chart review. Schizophr. Res. 2008, 101, 295–303. [Google Scholar] [CrossRef]
- Papatriantafyllou, E.; Efthymiou, D.; Markopoulou, M.; Sakellariou, E.-M.; Vassilopoulou, E. The Effects of Use of Long-Term Second-Generation Antipsychotics on Liver and Kidney Function: A Prospective Study. Diseases 2022, 10, 48. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Efthymiou, D.; Papatriantafyllou, E.; Markopoulou, M.; Sakellariou, E.-M.; Popescu, A.C. Long Term Metabolic and Inflammatory Effects of Second-Generation Antipsychotics: A Study in Mentally Disordered Offenders. J. Pers. Med. 2021, 11, 1189. [Google Scholar] [CrossRef]
- De Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.A.N.; Asai, I.; Detraux, J.; Gautam, S.; Möller, H.J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.; Griffiths, L.A.; Band, Μ.; Horne, D. Cardiometabolic Risk in First Episode Psychosis Patients. Front. Endocrinol. 2020, 11, 1664–2392. [Google Scholar] [CrossRef]
- Pillinger, T.; Beck, K.; Stubbs, B.; Howes, O. Cholesterol and triglyceride levels in first-episode psychosis: Systematic review and meta-analysis. Br. J. Psychiatry 2017, 211, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolenic, M.; Franke, K.; Hlinka, J.; Matejka, M.; Capkova, J.; Pausova, Z.; Uher, R.; Alda, M.; Spaniel, F.; Hajek, T. Obesity, dyslipidemia and brain age in first-episode psychosis. J. Psychiatr. Res. 2018, 99, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Morgan, V.A.; Waterreus, A.; Jablensky, A.; Mackinnon, A.; McGrath, J.J.; Carr, V.; Bush, R.; Castle, D.; Cohen, M.; Harvey, C.; et al. People living with psychotic illness in 2010: The second Australian national survey of psychosis. Aust. N. Z. J. Psychiatry 2012, 46, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Fransen, A.; Janssen, J.; van Os, J.; Drukker, M. Almost All Antipsychotics Result in Weight Gain: A Meta-Analysis. PLoS ONE 2014, 9, e94112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spertus, J.; Horvitz-Lennon, M.; Abing, H.; Normand, S.-L. Risk of weight gain for specific antipsychotic drugs: A meta-analysis. NPJ Schizophr. 2018, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Barton, B.B.; Segger, F.; Fischer, K.; Obermeier, M.; Musil, R. Update on weight-gain caused by antipsychotics: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2020, 19, 295–314. [Google Scholar] [CrossRef]
- Allison, D.; Mentore, J.; Heo, M.; Chandler, L.; Cappelleri, J.; Infante, M.; Weiden, P. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am. J. Psychiatry 1999, 156, 1686–1696. [Google Scholar] [CrossRef]
- Samele, C. Factors leading to poor physical health in people with psychosis. Epidemiol. Psichiatr. Soc. 2004, 13, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Mazza, E.; Ferro, Y.; Pujia, R.; Mare, R.; Maurotti, S.; Montalcini, T.; Pujia, A. Mediterranean Diet in Healthy Aging. J. Nutr. Health Aging 2021, 25, 1076–1083. [Google Scholar] [CrossRef]
- Teasdale, S.B.; Samaras, K.; Wade, T.; Jarman, R.; Ward, P.B. A review of the nutritional challenges experienced by people living with severe mental illness: A role for dietitians in addressing physical health gaps. J. Hum. Nutr. Diet. 2017, 30, 545–553. [Google Scholar] [CrossRef]
- Fulton, E.; Peet, M.; Williamson, K. More Harm than Good? A Pilot of a Motivational Interviewing Based Intervention for Increasing Readiness to Improve Nutrition in Young People Experiencing a First Episode of Psychosis. Health Psychol. Bull. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Perkins, D.; Belger, A.; Chakos, M.; Jarskog, F.; Boteva, K.; Gilmore, J. The early stages of schizophrenia: Speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol. Psychiatry 2001, 50, 884–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, R.; Mandiberg, J.M. Social networks, support and early psychosis: Mutual support within service-user communities. Epidemiol. Psychiatr. Sci. 2013, 22, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.H.; Satterthwaite, T.D.; Kantrowitz, J.J.; Katchmar, N.; Vandekar, L.; Elliott, M.A.; Ruparel, K. Amotivation in schizophrenia: Integrated assessment with behavioral, clinical, and imaging measures. Schizophr. Bull. 2014, 40, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Bonfioli, E.; Berti, L.; Goss, C.; Muraro, F.; Burti, L. Health promotion lifestyle interventions for weight management in psychosis: A systematic review and meta-analysis of randomized controlled trials. BMC Psychiatry 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruins, J.; Jörg, F.; Bruggeman, R.; Slooff, C.; Corpeleijn, E.; Pijnenborg, M. The Effects of Lifestyle Interventions on (Long-Term) Weight Management, Cardiometabolic Risk and Depressive Symptoms in People with Psychotic Disorders: A Meta-Analysis. PLoS ONE 2014, 9, e112276. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, S.B.; Ward, P.B.; Rosenbaum, S.; Samaras, K.; Stubbs, B. Solving a weighty problem: Systematic review and meta-analysis of nutrition interventions in severe mental illness. Br. J. Psychiatry 2017, 210, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peet, M.; Stokes, C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 2005, 65, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Sarris, J.; Rosenbaum, S.; Teasdale, S.; Berk, M.; Yung, A.R. The effects of vitamin and mineral supplementation on symptoms of schizophrenia: A systematic review and meta-analysis. Psychol. Med. 2017, 47, 1515–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyne, J.; O’Donoghue, B.; Owens, E.; Renwick, L.; Madigan, K.; Kinsella, A.; Clarke, M.; Turner, N.; O’Callaghan, E. Prevalence of item level negative symptoms in first episode psychosis diagnoses. Schizophr. Res. 2012, 135, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Fouhy, F.; Cullen, W.; O’Connor, K. Physical health interventions for patients who have experienced a first episode of psychosis: A narrative review. Ir. J. Psychol. Med. 2021, 38, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, S.; Harris, S.; Rosenbaum, S.; Watkins, A.; Samaras, K.; Curtis, J.; Ward, P.B. Individual Dietetic Consultations in First Episode Psychosis: A Novel Intervention to Reduce Cardiometabolic Risk. Community Ment. Health J. 2015, 51, 211–214. [Google Scholar] [CrossRef]
- Teasdale, S.B.; Ward, P.B.; Rosenbaum, S.; Watkins, A.; Curtis, J.; Kalucy, M.; Samaras, K. A nutrition intervention is effective in improving dietary components linked to cardiometabolic risk in youth with first-episode psychosis. Br. J. Nutr. 2016, 115, 1987–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, J.; Watkins, A.; Rosenbaum, S.; Teasdale, S.; Kalucy, M.; Samaras, K.; Ward, P.B. Evaluating an individualized lifestyle and life skills intervention to prevent antipsychotic-induced weight gain in first-episode psychosis. Early Interv. Psychiatry 2016, 10, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Hina, F. Integrated Review of Lifestyle Interventions Targeting Diet and Exercise in Early or First-Episode Psychosis. Open J. Psychiatry 2021, 11, 265–278. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems; 10th Revision; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Ahuja, J.K.C.; Wu, X.; Somanchi, M.; Nickle, M.; Nguyen, Q.A.; Roseland, J.M.; Williams, J.R.; Patterson, K.Y.; Li, Y.; et al. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA. 2019. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 31 May 2022).
- FÚart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef]
- Harvard University, Copyright © 2011. For More Information about the Healthy Eating Plate, Please See the Nutrition Source, Department of Nutrition, Harvard T.H. Chan School of Public Health. Available online: http://www.thenutritionsource.org and Harvard Health Publications, harvard.edu (accessed on 22 December 2020).
- MedNutrition, How Much Is a Portion? medNutrition Publications. 2015. Available online: https://www.mednutrition.gr/e-shop/ekdoseis/poster (accessed on 22 December 2020).
- Firth, J.; Veronese, N.; Cotter, J.; Shivappa, N.; Hebert, J.R.; Ee, C.; Smith, L.; Stubbs, B.; Jackson, S.E.; Sarris, J. What is the role of dietary inflammation in severe mental illness? A review of observational and experimental findings. Front. Psychiatry 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Whicher, C.A.; Price, H.C.; Holt, R.I.G. Mechanisms in endocrinology: Antipsychotic medication and type 2 diabetes and impaired glucose regulation. Eur. J. Endocrinol. 2018, 178, R245–R258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA. Acceptable Macronutrient Distribution Ranges. 2021. Available online: https://www.fns.usda.gov/resource/dietary-guidelines-americans-2010-reports-publications (accessed on 9 June 2021).
- Teasdale, S.B.; Burrows, T.L.; Hayes, T.; Hsia, C.Y.; Watkins, A.; Curtis, J.; Ward, P.B. Dietary intake, food addiction and nutrition knowledge in young people with mental illness. Nutr. Diet. 2020, 77, 315–322. [Google Scholar] [CrossRef]
- Aaron, K.J.; Sanders, P.W. Role of Dietary Salt and Potassium Intake in Cardiovascular Health and Disease: A Review of the Evidence. Mayo Clin. Proc. 2013, 88, 987–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Bourgon, J.; Pérez-Iglesias, R.; de la Foz, V.O.G.; Pinilla, P.S.; Martínez, D.; Crespo-Facorro, B. Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: A pragmatic clinical trial in drug-naïve patients with a first-episode of non-affective psychosis. Psychopharmacology 2017, 235, 245–255. [Google Scholar] [CrossRef]
- Atmaca, M.; Kuloglu, M.; Tezcan, E.; Ustundag, B. Serum Leptin and Triglyceride Levels in Patients on Treatment with Atypical Antipsychotics. J. Clin. Psychiatry 2003, 64, 598–604. [Google Scholar] [CrossRef]
- Melkersson, K.I.; Hulting, A.-L.; Brismar, K.E. Elevated Levels of Insulin, Leptin, and Blood Lipids in Olanzapine-Treated Patients with Schizophrenia or Related Psychoses. J. Clin. Psychiatry 2000, 61, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Usher, C.; Thompson, A.; Griebeler, M.; Senders, A.; Seibel, C.; Ly, R.; Murchison, C.; Hagen, K.; Afong, K.; Bourdette, D.; et al. Meals, mindfulness, & moving forward: A feasibility study to a multi-modal lifestyle approach in early psychosis. Early Interv. Psychiatry 2019, 13, 147–150. [Google Scholar]
- Teasdale, S.; Morkl, S.; Müller-Stierlin, A.S. Nutritional psychiatry in the treatment of psychotic disorders: Current hypotheses and research challenges. Brain Behav. Immun. Health 2020, 5, 100070. [Google Scholar] [CrossRef]
| Variable | AP1 * | AP2 * | Total | p-Value |
|---|---|---|---|---|
| 11 (52.4%) | 10 (47.6%) | 21 (100%) | >0.05 | |
| Gender | ||||
| Female | 4 (36.4%) | 4 (40.0%) | 8 (38.1%) | >0.05 |
| Male | 7 (63.6%) | 6 (60.0%) | 13 (61.9%) | >0.05 |
| Age | ||||
| Mean (SD) | 30.3 (7.44) | 42.1 (9.43) | 35.9 (10.2) | >0.05 |
| Diagnosis | ||||
| Schizophrenia (F20) Delusional disorder (F22) Unspecified non-organic psychosis (F29) Bipolar disorder with psychotic features (F31.2) | 7 (63.6%) 0 (0%) 3 (27.3%) 1 (9.1%) | 3 (30%) 1 (10%) 6 (60%) 0 (0%) | 10 (47.6%) 1 (4.8%) 9 (42.9%) 1 (4.8%) | >0.05 >0.05 >0.05 >0.05 |
| Other chronic disease | ||||
| No | 10 (90.9%) | 8 (80.0%) | 18 (85.7%) | >0.05 |
| Yes | 1 (9.1%) | 2 (20.0%) | 3 (14.3%) | >0.05 |
| Family history | ||||
| No | 7 (63.6%) | 7 (70.0%) | 14 (66.7%) | >0.05 |
| Yes | 4 (36.4%) | 3 (30.0%) | 7 (33.3%) | >0.05 |
| Variable | Period | AP1 * (n = 11) | AP2 * (n = 10) | Total (n = 21) | p-Value |
|---|---|---|---|---|---|
| Body Weight (kg) | Τ0 | 82.7 (20.8) | 78.9 (15.6) | 80.9 (18.2) | >0.05 |
| Τ1 | 80.3 (19.8) | 80.4 (17.1) | 80.3 (18.1) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.85 | ||
| Body mass index (BMI) (kg/m2) | Τ0 | 27.1 (4.44) | 26.1 (5.53) | 26.6 (4.89) | >0.05 |
| Τ1 | 26.4 (4.30) | 26.6 (6.14) | 26.5 (5.12) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.72 | ||
| Body Fat (%) | Τ0 | 29.0 (7.54) | 29.2 (8.02) | 29.1 (7.57) | >0.05 |
| Τ1 | 28.7 (6.86) | 29.8 (9.35) | 29.2 (7.95) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.99 | ||
| Muscle Mass (kg) | Τ0 | 55.5 (13.9) | 52.4 (7.44) | 54.0 (11.1) | >0.05 |
| Τ1 | 54.3 (13.7) | 52.7 (7.97) | 53.5 (11.1) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.85 | ||
| Muscle Quality (MQ) | Τ0 | 48.1 (8.81) | 52.6 (8.47) | 50.2 (8.75) | >0.05 |
| Τ1 | 51.1 (8.32) | 53.0 (7.41) | 52.0 (7.77) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.59 | ||
| Bone Mass (kg) | Τ0 | 2.92 (1.92) | 2.8 (0.38) | 2.86 (0.56) | >0.05 |
| Τ1 | 2.86 (0.676) | 2.82 (0.346) | 2.84 (0.532) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.89 | ||
| Visceral Fat (LV) | Τ0 | 7.77 (4.42) | 7.3 (2.74) | 7.55 (3.36) | >0.05 |
| Τ1 | 7.18 (4.19) | 7.55 (3.31) | 7.36 (3.71) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.83 | ||
| Basal Metabolic Rhythm (Kcal/day) | Τ0 | 1770 (423) | 1640 (239) | 1710 (346) | >0.05 |
| Τ1 | 1740 (415) | 1660 (251) | 1700 (341) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.89 | ||
| Body Water (%) | Τ0 | 53.5 (6.84) | 51.0 (6.42) | 52.3 (6.60) | >0.05 |
| Τ1 | 52.8 (5.21) | 50.5 (7.37) | 51.7 (6.27) | >0.05 | |
| p-value | >0.05 | >0.05 | 0.93 |
| Variable | Group | T0 | T1 | p-Value |
|---|---|---|---|---|
| Vegetables | AΡ1 * | 1.00 (1.00, 4.00) | 3.00 (1.00, 4.00) | 0.02 |
| AΡ2 * | 2.00 (1.00, 3.00) | 4.00 (3.00, 5.00) | <0.001 | |
| p-value | 0.05 | 0.05 | ||
| Total | 1.00 (1.00, 4.00) | 3.00 (1.00, 5.00) | <0.001 | |
| Fruits | AΡ2 * | 2.00 (0.00, 5.00) | 4.00 (1.00, 5.00) | 0.02 |
| Total | 2.00 (0.00, 5.00) | 3.00 (1.00, 5.00) | <0.001 | |
| Red Meat | AΡ2 * | 3.00 (1.00, 5.00) | 4.00 (3.00, 5.00) | 0.04 |
| Total | 4.00 (1.00, 5.00) | 4.00 (3.00, 5.00) | <0.001 | |
| Poultry | AΡ2 * | 5.00 (3.00, 5.00) | 5.00 (5.00, 5.00) | 0.03 |
| Total | 5.00 (3.00, 5.00) | 5.00 (4.00, 5.00) | 0.05 | |
| MedDiet Score | AΡ1 * | 33.0 (26.0, 37.0) | 37.0 (29.0, 43.0) | <0.01 |
| AΡ2 * | 31.0 (22.0, 35.0) | 39.0 (34.0, 49.0) | <0.001 | |
| Total | 32.0 (22.0, 37.0) | 39.0 (29.0, 49.0) | <0.001 |
| Variable | Group | T0 | T1 | p-Value |
|---|---|---|---|---|
| Energy (Kcal) | AP1 * | 1220 (287) | ||
| AP2 * | 1650 (553) | |||
| p-value | 0.05 | |||
| Total | 1730 (504) | 1430 (477) | 0.05 | |
| Proteins (g) | AΡ1 * | 51.6 (22.3) | ||
| AΡ2 * | 77.7 (27.6) | |||
| p-value | 0.03 | |||
| Carbohydrates (g) | AΡ1 * | 177 (80.4) | 118 (52.6) | 0.04 |
| AΡ2 * | 175 (76.6) | |||
| p-value | 0.05 | |||
| Total | 193 (71.7) | 145 (70.0) | 0.03 | |
| Vitamin E (mg) | AΡ1 * | 10.6 (7.82) | 6.56 (4.24) | 0.05 |
| Vitamin B3 (mg) | AΡ1 * | 11.8 (3.88) | ||
| AΡ2 * | 19.3 (6.76) | |||
| p-value | 0.01 | |||
| Dietary Na (mg) | AΡ2 * | 2060 (972) | 1190 (653) | 0.03 |
| Total | 2010 (1040) | 1290 (778) | 0.01 |
| Variable | Period | AP1 * (n = 11) | AP2 * (n = 10) | Total (n = 21) | p-Value |
|---|---|---|---|---|---|
| Serum Fe2+ (μg/dL) | Τ0 | 60.8 (16.1) | 86.1 (32.1) | 72.9 (27.6) | 0.02 |
| Τ1 | 53.9 (19.7) | 109 (29.8) | 80.3 (37.4) | <0.001 | |
| p-value | >0.05 | >0.05 | 0.62 | ||
| Total Cholesterol (mg/dL) | Τ0 | 179 (41.6) | 220 (41.9) | 199 (45.9) | 0.03 |
| Τ1 | 182 (49.6) | 220 (34.4) | 200 (46.3) | 0.05 | |
| p-value | >0.05 | >0.05 | 0.92 | ||
| Low Density Lipoprotein (LDL) (mg/dL) | Τ0 | 130 (102) | 132 (34.5) | 131 (75.4) | 0.05 |
| Τ1 | 124 (90.5) | 143 (37.3) | 133 (69.4) | 0.05 | |
| p-value | >0.05 | >0.05 | 0.98 | ||
| Urea (mg/dL) | Τ0 | 23.3 (6.36) | 29.6 (6.90) | 26.3 (7.21) | 0.05 |
| Τ1 | 24.8 (3.46) | 24.9 (4.30) | |||
| p-value | 0.05 | 0.57 | |||
| Glucose (mg/dL) | Τ0 | 92.0 (8.75) | |||
| Τ1 | 97.5 (7.35) | 90.8 (6.11) | 94.3 (7.47) | 0.05 | |
| p-value | 0.31 | ||||
| Serum Na+ (mEq/L) | Τ0 | 140 (1.29) | 140 (1.25) | ||
| Τ1 | 139 (1.51) | 139 (1.80) | |||
| p-value | <0.01 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntalkitsi, S.; Efthymiou, D.; Bozikas, V.; Vassilopoulou, E. Halting the Metabolic Complications of Antipsychotic Medication in Patients with a First Episode of Psychosis: How Far Can We Go with the Mediterranean Diet? A Pilot Study. Nutrients 2022, 14, 5012. https://doi.org/10.3390/nu14235012
Ntalkitsi S, Efthymiou D, Bozikas V, Vassilopoulou E. Halting the Metabolic Complications of Antipsychotic Medication in Patients with a First Episode of Psychosis: How Far Can We Go with the Mediterranean Diet? A Pilot Study. Nutrients. 2022; 14(23):5012. https://doi.org/10.3390/nu14235012
Chicago/Turabian StyleNtalkitsi, Savina, Dimitris Efthymiou, Vasilios Bozikas, and Emilia Vassilopoulou. 2022. "Halting the Metabolic Complications of Antipsychotic Medication in Patients with a First Episode of Psychosis: How Far Can We Go with the Mediterranean Diet? A Pilot Study" Nutrients 14, no. 23: 5012. https://doi.org/10.3390/nu14235012