Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
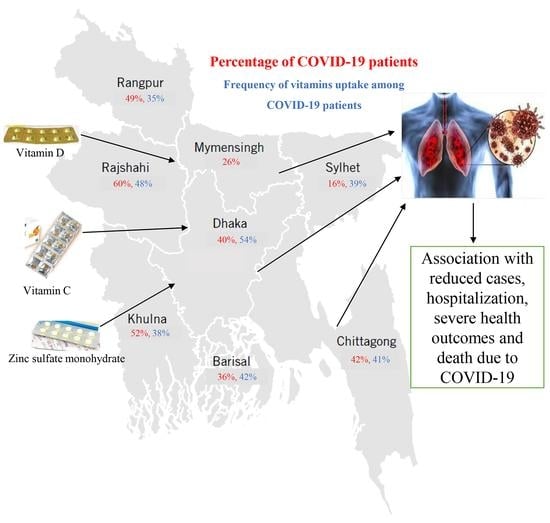
2.2. Study Area and Population
2.3. Data collection Tools and Variables
2.4. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics and Distribution of the Participants
3.2. Frequency Distribution of Vitamins and Supplements among COVID-19-Positive Participants
3.3. Association of Medicine and Vitamin Uptake with Socio-Demographic Factors
3.4. Impact of Vitamins and Supplementation with COVID-19 Infection
3.5. Impact of Vitamins and Supplementation on the Outcome of COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Map-Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html/2022 (accessed on 13 August 2022).
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int//2022 (accessed on 13 August 2022).
- Sharif, N.; Sarkar, M.K.; Ahmed, S.N.; Ferdous, R.N.; Nobel, N.U.; Parvez, A.K.; Talukder, A.A.; Dey, S.K. Environmental correlation and epidemiologic analysis of COVID-19 pandemic in ten regions in five continents. Heliyon 2021, 7, e06576. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of coronavirus and SARS-CoV-2. Malays J Pathol. 2020, 42, 3–11. [Google Scholar] [PubMed]
- Sharif, N.; Dey, S.K. Phylogenetic and whole genome analysis of first seven SARS-CoV-2 isolates in Bangladesh. Future Virol. 2020, 15, 735–746. [Google Scholar] [CrossRef]
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Ahmed, S.N.; Opu, R.R.; Tani, M.R.; Dewan, D.; Daullah, M.U.; Shanto, R.I.; Parvez, A.K.; Talukder, A.A.; Dey, S.K. Prevalence and impact of diabetes and cardiovascular disease on clinical outcome among patients with COVID-19 in Bangladesh. Diabetes Metab Syndr. 2021, 15, 1009–1016. [Google Scholar] [CrossRef]
- Sharif, N.; Opu, R.R.; Ahmed, S.N.; Sarkar, M.K.; Jaheen, R.; Daullah, M.U.; Khan, S.; Mubin, M.; Rahman, H.; Islam, F.; et al. Prevalence and impact of comorbidities on disease prognosis among patients with COVID-19 in Bangladesh: A nationwide study amid the second wave. Diabetes Metab Syndr. 2021, 15, 102148. [Google Scholar] [CrossRef]
- Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Peana, M.; Menzel, A.; Dadar, M.; Benahmed, A.G.; Bjørklund, G. Micronutrients as immunomodulatory tools for COVID-19 management. J. Clin. Immunol. 2020, 220, 108545. [Google Scholar] [CrossRef]
- Wong, Y.; Chan, C.H.; Venkatakrishnan, K.; Chiu, H.F.; Shen, Y.C.; Glovinskaia, O.; Han, Y.C.; Wang, C.K. Impact of dietary nutrients (functional foods/nutraceuticals) and micronutrients on COVID-19: A review. J. Food Bioact. 2021, 15, 29–38. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Abobaker, A.; Alzwi, A.; Alraied, A.H. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol Rep. 2020, 72, 1517–1528. [Google Scholar] [CrossRef]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Pereira, C.P.M. Zinc, Vitamin D and Vitamin C: Perspectives for COVID-19 with a focus on physical tissue barrier integrity. Front. Nutr. 2020, 7, 295. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Li, Y.; Zhang, Y.; Zhong, M.; Meng, X. Evaluation of the nutritional status in patients with COVID-19. J. Clin. Biochem. Nutr. 2020, 67, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Fath, M.K.; Naderi, M.; Hamzavi, H.; Ganji, M.; Shabani, S.; Khalesi, B.; Pourzardosht, N.; Hashemi, Z.S.; Khalili, S. Molecular Mechanisms and therapeutic effects of different vitamins and minerals in COVID-19 patients. J. Trace. Elem. Med. Biol. 2022, 73, 127044. [Google Scholar] [CrossRef]
- Thomas, W.R.; Holt, P.G. Vitamin C and immunity: An assessment of the evidence. Clin. Exp. Immunol. 1978, 32, 370. [Google Scholar]
- Hewison, M. An update on vitamin D and human immunity. Clin. Endocrinol. 2012, 76, 315–325. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Bartley, J. Vitamin D, innate immunity and upper respiratory tract infection. J. Laryngol. Otol. 2010, 124, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Pierce, G.N.; Rupp, H.; Izumi, T.; Grynberg, A. (Eds.) Molecular and Cellular Effects of Nutrition on Disease Processes; Springer Science & Business Media: Winnipeg, MB, Canada, 2013; Volume 26. [Google Scholar]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BourBour, F.; Mirzaei Dahka, S.; Gholamalizadeh, M.; Akbari, M.E.; Shadnoush, M.; Haghighi, M.; Taghvaye-Masoumi, H.; Ashoori, N.; Doaei, S. Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Arch. Physiol. Biochem. 2020, 1791188. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13, 7020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care. 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Pioggia, G.; Negrini, S. Vitamin D and Covid-19: An update on evidence and potential therapeutic implications. Clin. Mol. Allergy. 2020, 18, 23. [Google Scholar] [CrossRef]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a North American community hospital intensive care unit in May 2020: A pilot study. Med. Drug Discov. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of Covid-19—Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1836. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: The GERIA-COVID quasi-experimental study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Mahjoub, Y.; Rodenstein, D.O.; Jounieaux, V. Severe Covid-19 disease: Rather AVDS than ARDS? Crit. Care. 2020, 24, 327. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C can shorten the length of stay in the ICU: A meta-analysis. Nutrients 2019, 11, 708. [Google Scholar] [CrossRef] [Green Version]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.; Birdi, A.; Tomo, S.; Charan, J.; Bhardwaj, P.; Sharma, P. Association of vitamin D status with COVID-19 infection and mortality in the Asia Pacific region: A cross-sectional study. Indian J. Clin. Biochem. 2021, 36, 492–497. [Google Scholar] [CrossRef]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Alzaben, A.S. The Potential Influence of Vitamin A, C, and D and Zinc Supplements on the Severity of COVID-19 Symptoms and Clinical Outcomes: An Updated Review of Literature. Curr. Res. Nutr. Food Sci. 2020, 8, 703–714. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, A.; Marin, L.; Febbi, M.; Tovani-Palone, M.R. Current Evidence on Vitamin C, D, and Zinc Supplementation for COVID-19 Prevention and/or Treatment. Electron. J. Gen. Med. 2021, 18, em311. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, T.; Heianza, Y.; Qi, L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: A prospective study in UK Biobank. Am. J. Clin. Nutr. 2021, 113, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: The COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021, 4, e210369. [Google Scholar] [CrossRef]
- Ahmed, I.; Hasan, M.; Akter, R.; Sarkar, B.K.; Rahman, M.; Sarker, M.S.; Samad, M.A. Behavioral preventive measures and the use of medicines and herbal products among the public in response to Covid-19 in Bangladesh: A cross-sectional study. PLoS ONE 2020, 15, e0243706. [Google Scholar] [CrossRef] [PubMed]
- Nimer, R.M.; Khabour, O.F.; Swedan, S.F.; Kofahi, H.M. The impact of vitamin and mineral supplements usage prior to COVID-19 infection on disease severity and hospitalization. Bosn. J. Basic Med. Sci. 2022, 22, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Margolin, L.; Luchins, J.; Margolin, D.; Margolin, M.; Lefkowitz, S. 20-week study of clinical outcomes of over-the-counter COVID-19 prophylaxis and treatment. J. Evid.-Based Integr. Med. 2021, 26, 2515690X211026193. [Google Scholar] [CrossRef]
- Beigmohammadi, M.T.; Bitarafan, S.; Hoseindokht, A.; Abdollahi, A.; Amoozadeh, L.; Soltani, D. The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: A randomized clinical trial. Trials 2021, 22, 802. [Google Scholar] [CrossRef]
- Jaun, F.; Boesing, M.; Lüthi-Corridori, G.; Abig, K.; Makhdoomi, A.; Bloch, N.; Lins, C.; Raess, A.; Grillmayr, V.; Haas, P.; et al. High-dose vitamin D substitution in patients with COVID-19: Study protocol for a randomized, double-blind, placebo-controlled, multi-center study—VitCov Trial. Trials 2022, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Speakman, L.L.; Michienzi, S.M.; Badowski, M.E. Vitamins, supplements and COVID-19: A review of currently available evidence. Drugs Context 2021, 10, 2021-6-2. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.R. Vitamin C for COVID-19 Treatment: Have We Got Enough Evidence? Front. Nutr. 2022, 9, 892561. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, M.A.; Jarrar, Y. Effects of vitamin C and D on the mRNA expression of angiotensin converting enzyme 2 receptor, cathepsin L, and transmembrane serine protease in the mouse lungs. Libyan. J. Med. 2022, 17, 2054111. [Google Scholar] [CrossRef]

| Variables | Male | Female | Total |
|---|---|---|---|
| Study population | 67.6% (650/962) | 32.4% (312/962) | 100.0% (962/962) |
| Age | |||
| 5–9 | 25.0% (1/4) | 75.0% (3/4) | 0.4% (4/962) |
| 10–19 | 47.8% (22/46) | 52.2% (24/46) | 4.8% (46/962) |
| 20–29 | 61.4% (239/389) | 38.6% (150/389) | 40.4% (389/962) |
| 30–39 | 77.8% (144/185) | 22.2% (41/185) | 19.2% (185/962) |
| 40–49 | 78.7% (122/155) | 21.3% (33/155) | 16.1% (155/962) |
| 50–59 | 67.0% (71/106) | 33.0% (35/106) | 11.0% (106/962) |
| 60–69 | 60.7% (37/61) | 39.3% (24/61) | 6.3% (61/962) |
| Above 70 | 87.5% (14/16) | 12.5% (2/16) | 1.7% (16/962) |
| Monthly income (Thousands in taka) | |||
| Less than 10 | 54.0% (102/189) | 46.0% (87/189) | 19.6% (189/962) |
| 10–29 | 73.2% (101/138) | 26.8% (37/138) | 14.3% (138/962) |
| 30–49 | 80.6% (100/124) | 19.4% (24/124) | 12.9% (124/962) |
| 50–79 | 76.0% (38/50) | 24.0% (12/50) | 5.2% (50/962) |
| More than 80 | 79.2% (19/24) | 20.8% (5/24) | 2.5% (24/962) |
| Not applicable | 66.4% (290/437) | 33.6% (147/437) | 45.4% (437/962) |
| Residence | |||
| Village | 73.6% (39/53) | 26.4% (14/53) | 5.5% (53/962) |
| District town | 70.4% (107/152) | 29.6% (45/152) | 15.8% (152/962) |
| Divisional city | 66.6% (504/757) | 33.4% (253/757) | 78.7% (757/962) |
| Access to health services | |||
| Less | 73.6% (39/53) | 26.4% (14/53) | 5.5% (53/962) |
| Moderate | 70.4% (107/152) | 29.6% (45/152) | 15.8% (152/962) |
| Better | 66.6% (504/757) | 33.4% (253/757) | 78.7% (757/962) |
| Occupation | |||
| Physician | 57.9% (11/19) | 42.1% (8/19) | 2.0% (19/962) |
| Teacher | 48.6% (18/37) | 51.4% (19/37) | 3.8% (37/962) |
| Researcher | 100.0% (5/5) | 0.0% (0/5) | 0.5% (5/962) |
| Farmer | 100.0% (2/2) | 0.0% (0/2) | 0.2% (2/962) |
| Nurse | 24.1% (7/29) | 75.9% (22/29) | 3.0% (29/962) |
| Student | 54.8% (176/321) | 45.2% (145/321) | 33.4% (321/962) |
| Journalist | 100.0% (3/3) | 0.0% (0/3) | 0.3% (3/962) |
| Lawyer | 0.0% (0/1) | 100.0% (1/1) | 0.1% (1/962) |
| Police | 79.2% (19/24) | 20.8% (5/24) | 2.5% (24/962) |
| Banker | 81.3% (26/32) | 18.8% (6/32) | 3.3% (32/962) |
| Administrative Officer | 100.0% (7/7) | 0.0% (0/7) | 0.7% (7/962) |
| Private employee | 96.1% (195/203) | 3.9% (8/203) | 21.1% (203/962) |
| Rickshaw driver/Van driver/Car driver | 100.0% (24/24) | 0.0% (0/24) | 2.5% (24/962) |
| Businessman | 94.2% (49/52) | 5.8% (3/52) | 5.4% (52/962) |
| Government employee | 65.7% (23/35) | 34.3% (12/35) | 3.6% (35/962) |
| Others | 50.6% (85/168) | 49.4% (83/168) | 17.5% (168/962) |
| Variables | Vitamin C | Vitamin D | Zinc | Medicines Taken for Treatment of COVID-19 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Paracetamol 500 mg | Fexofenadine Hydrochloride 120 mg | Antibiotics | Montelukast Sodium 10 mg | Remdesivir 200 mg | None | |
| Sex | ||||||||||||
| Male | 52.7% (168/319) | 47.3% (151/319) | 52.7% (168/319) | 47.3% (151/319) | 48.6% (155/319) | 51.4% (164/319) | 74.6% (238/319) | 48.3% (154/319) | 59.9% (191/319) | 37.0% (118/319) | 11.3% (36/319) | 9.1% (29/319) |
| Female | 59.8% (110/184) | 40.2% (74/184) | 59.8% (110/184) | 40.2% (74/184) | 45.7% (84/184) | 54.3% (100/184) | 66.8% (123/184) | 40.2% (74/184) | 54.3% (100/184) | 36.4% (67/184) | 7.6% (14/184) | 10.3% (19/184) |
| Age in years | ||||||||||||
| 5–9 | 75.0% (3/4) | 25.0% (1/4) | 75.0% (3/4) | 25.0% (1/4) | 25.0% (1/4) | 75.0% (3/4) | 75.0% (3/4) | 0.0% (0/4) | 25.0% (1/4) | 0.0% (0/4) | 0.0% (0/4) | 25.0% (1/4) |
| 10–19 | 72.1% (31/43) | 27.9% (12/43) | 72.1% (31/43) | 27.9% (12/43) | 46.5% (20/43) | 53.5% (23/43) | 39.5% (17/43) | 18.6% (8/43) | 16.3% (7/43) | 9.3% (4/43) | 2.3% (1/43) | 4.7% (2/43) |
| 20–29 | 48.8% (83/170) | 51.2% (87/170) | 48.8% (83/170) | 51.2% (87/170) | 49.4% (84/170) | 50.6% (86/170) | 57.6% (98/170) | 31.8% (54/170) | 37.6% (64/170) | 20.0% (34/170) | 5.3% (9/170) | 10.6% (18/170) |
| 30–39 | 50.5% (52/103) | 49.5% (51/103) | 50.5% (52/103) | 49.5% (51/103) | 52.4% (54/103) | 47.6% (49/103) | 82.5% (85/)103 | 54.4% (56/103) | 70.9% (73/103) | 44.7% (46/103) | 7.8% (8/103) | 3.9% (4/103) |
| 40–49 | 56.4% (44/78) | 43.6% (34/78) | 56.4% (44/78) | 43.6% (34/78) | 46.2% (36/78) | 53.8% (42/78) | 82.1% (64/78) | 57.7% (45/78) | 67.9% (53/78) | 50.0% (39/78) | 15.4% (12/78) | 9.0% (7/78) |
| 50–59 | 61.8% (34/55) | 38.2% (21/55) | 61.8% (34/55) | 38.2% (21/55) | 45.5% (25/55) | 54.5% (30/55) | 92.7% (51/55) | 56.4% (31/55) | 89.1% (49/55) | 49.1% (27/55) | 7.3% (4/55) | 16.4% (9/55) |
| 60–69 | 60.5% (26/43) | 39.5% (17/43) | 60.5% (26/43) | 39.5% (17/43) | 32.6% (14/43) | 67.4% (29/43) | 86.0% (37/43) | 65.1% (28/43) | 90.7% (39/43) | 67.4% (29/43) | 25.6% (11/43) | 14.0% (6/43) |
| Above 70 | 71.4% (5/7) | 28.6% (2/7) | 71.4% (5/7) | 28.6% (2/7) | 71.4% (5/7) | 28.6% (2/7) | 85.7% (6/7) | 85.7% (6/7) | 71.4% (5/7) | 85.7% (6/7) | 71.4% (5/7) | 14.3% (1/7) |
| Duration of supplementation | ||||||||||||
| 7 days | 22.3% (62/278) | - | 22.3% (62/278) | - | 18.4% (44/239) | - | 19.9% (72/361) | 20.2% (46/228) | 17.5% (51/291) | 11.9% (22/185) | 16.0% (8/50) | 29.2% (14/48) |
| 14 days | 34.2% (95/278) | - | 34.2% (95/278) | - | 28.0% (67/239) | - | 43.2% (156/361) | 39.9% (91/228) | 39.5% (115/291) | 42.7% (79/185) | 26.0% (13/50) | 37.5% (18/48) |
| 21 days | 10.4% (29/278) | - | 10.4% (29/278) | - | 18.0% (43/239) | - | 14.4% (52/361) | 15.8% (36/228) | 15.5% (45/291) | 16.2% (30/185) | 22.0% (11/50) | 8.3% (4/48) |
| 1 month | 21.2% (59/278) | - | 21.2% (59/278) | - | 22.6% (54/239) | - | 18.0% (65/361) | 18.0% (41/228) | 19.6% (57/291) | 22.7% (42/185) | 20.0% (10/50) | 10.4% (5/48) |
| 2 months | 5.4% (15/278) | - | 5.4% (15/278) | - | 5.9% (14/239) | - | 1.9% (7/361) | 3.1% (7/228) | 3.4% (10/291) | 2.2% (4/185) | 6.0% (3/50) | 6.3% (3/48) |
| More than 2 months | 6.5% (18/278) | - | 6.5% (18/278) | - | 7.1% (17/239) | - | 2.5% (9/361) | 3.1% (7/228) | 4.5% (13/291) | 4.3% (8/185) | 10.0% (5/50) | 8.3% (4/48) |
| Times required to recover from the onset of the taking of vitamins and medicine | ||||||||||||
| 1–5 days | 45.9% (73/159) | 54.1% (86/159) | 45.9% (73/159) | 54.1% (86/159) | 42.1% (67/159) | 57.9% (92/159) | 67.3% (107/159) | 45.9% (73/159) | 51.6% (82/159) | 28.3% (45/159) | 6.9% (11/159) | 7.5% (12/159) |
| 7–14 days | 55.7% (141/253) | 44.3% (112/253) | 55.7% (141/253) | 44.3% (112/253) | 47.8% (121/253) | 52.2% (132/253) | 77.1% (195/253) | 44.3% (112/253) | 59.3% (150/253) | 38.3% (97/253) | 10.3% (26/253) | 9.1% (23/253) |
| 15–30 days | 68.1% (47/69) | 31.9% (22/69) | 68.1% (47/69) | 31.9% (22/69) | 56.5% (39/69) | 43.5% (30/69) | 63.8% (44/69) | 44.9% (31/69) | 69.6% (48/69) | 46.4% (32/69) | 10.1% (7/69) | 13.0% (9/69) |
| No decrease | 77.3% (17/22) | 22.7% (5/22) | 77.3% (17/22) | 22.7% (5/22) | 54.5% (12/22) | 45.5% (10/22) | 68.2% (15/22) | 54.5% (12/22) | 50.0% (11/22) | 50.0% (11/22) | 27.3% (6/22) | 18.2% (4/22) |
| Symptoms | ||||||||||||
| Fever | 52.8% (198/375) | 47.2% (177/375) | 52.8% (198/375) | 47.2% (177/375) | 38.7% (145/375) | 61.3% (230/375) | 81.9% (307/375) | 47.7% (179/375) | 63.7% (239/375) | 43.5% (163/375) | 10.4% (39/375) | 4.0% (15/375) |
| Dry Cough | 55.5% (101/182) | 44.5% (81/182) | 55.5% (101/182) | 44.5% (81/182) | 45.6% (83/182) | 54.4% (99/182) | 79.7% (145/182) | 61.5% (112/182) | 67.6% (123/182) | 56.6% (103/182) | 15.9% (29/182) | 3.8% (7/182) |
| Loss of taste or smell | 58.4% (101/173) | 41.6% (72/173) | 58.4% (101/173) | 41.6% (72/173) | 49.1% (85/173) | 50.9% (88/173) | 76.3% (132/173) | 49.7% (86/173) | 68.8% (119/173) | 41.6% (72/173) | 13.3% (23/173) | 4.0% (7/173) |
| Fatigue | 57.0% (65/114) | 43.0% (49/114) | 57.0% (65/114) | 43.0% (49/114) | 44.7% (51/114) | 55.3% (63/114) | 80.7% (92/114) | 57.9% (66/114) | 64.0% (73/114) | 48.2% (55/114) | 13.2% (15/114) | 2.6% (3/114) |
| Body aches | 54.7% (75/137) | 45.3% (62/137) | 54.7% (75/137) | 45.3% (62/137) | 40.1% (55/137) | 59.9% (82/137) | 82.5% (113/137) | 59.9% (82/137) | 63.5% (87/137) | 43.1% (59/137) | 11.7% (16/137) | 2.9% (4/137) |
| Sore throat | 68.2% (60/88) | 31.8% (28/88) | 68.2% (60/88) | 31.8% (28/88) | 60.2% (53/88) | 39.8% (35/88) | 76.1% (67/88) | 53.4% (47/88) | 63.6% (56/88) | 53.4% (47/88) | 18.2% (16/88) | 1.1% (1/88) |
| Shortness of breath | 59.2% (58/98) | 40.8% (40/98) | 59.2% (58/98) | 40.8% (40/98) | 40.8% (40/98) | 59.2% (58/98) | 80.6% (79/98) | 58.2% (57/98) | 62.2% (61/98) | 70.4% (69/98) | 26.5% (26/98) | 1.0% (1/98) |
| Chest pain or pressure | 54.1% (20/37) | 45.9% (17/37) | 54.1% (20/37) | 45.9% (17/37) | 54.1% (20/37) | 45.9% (17/37) | 78.4% (29/37) | 51.4% (19/37) | 54.1% (20/37) | 59.5% (22/37) | 29.7% (11/37) | 2.7% (1/37) |
| Diarrhea | 54.1% (20/37) | 45.9% (17/37) | 54.1% (20/37) | 45.9% (17/37) | 48.6% (18/37) | 51.4% (19/37) | 64.9% (24/37) | 43.2% (16/37) | 62.2% (23/37) | 51.4% (19/37) | 18.9% (7/37) | 8.1% (3/37) |
| Loss of speech or movement | 66.7% (16/24) | 33.3% (8/24) | 66.7% (16/24) | 33.3% (8/24) | 70.8% (17/24) | 29.2% (7/24) | 70.8% (17/24) | 62.5% (15/24) | 70.8% (17/24) | 62.5% (15/24) | 41.7% (10/24) | 4.2% (1/24) |
| Inflammation of the eye | 50.0% (2/4) | 50.0% (2/4) | 50.0% (2/4) | 50.0% (2/4) | 75.0% (3/4) | 25.0% (1/4) | 75.0% (3/4) | 50.0% (2/4) | 75.0% (3/4) | 50.0% (2/4) | 0.0% (0/4) | 25.0% (1/4) |
| Rash | 66.7% (2/3) | 33.3% (1/3) | 66.7% (2/3) | 33.3% (1/3) | 100.0% (3/3) | 0.0% (0/3) | 0.0% (0/3) | 33.3% (1/3) | 33.3% (1/3) | 33.3% (1/3) | 0.0% (0/3) | 33.3% (1/3) |
| No symptoms | 50.0% (2/4) | 50.0% (2/4) | 50.0% (2/4) | 50.0% (2/4) | 25.0% (1/4) | 75.0% (3/4) | 25.0% (1/4) | 0.0% (0/4) | 0.0% (0/4) | 0.0% (0/4) | 0.0% (0/4) | 75.0% (3/4) |
| Duration of symptoms | ||||||||||||
| 7–14 days | 54.6% (191/350) | 45.4% (159/350) | 54.6% (191/350) | 45.4% (159/350) | 45.4% (159/350) | 54.6% (191/350) | 72.3% (253/350) | 44.6% (156/350) | 55.4% (194/350) | 36.9% (129/350) | 9.7% (34/350) | 10.0% (35/350) |
| 15–28 days | 56.4% (75/133) | 43.6% (58/133) | 56.4% (75/133) | 43.6% (58/133) | 50.4% (67/133) | 49.6% (66/133) | 69.2% (92/133) | 44.4% (59/133) | 63.9% (85/133) | 36.8% (49/133) | 9.8% (13/133) | 7.5% (10/133) |
| 1–2 months | 58.8% (10/17) | 41.2% (7/17) | 58.8% (10/17) | 41.2% (7/17) | 64.7% (11/17) | 35.3% (6/17) | 76.5% (13/17) | 64.7% (11/17) | 58.8% (10/17) | 35.3% (6/17) | 11.8% (2/17) | 17.6% (3/17) |
| More than 2 months | 66.7% (2/3) | 33.3% (1/3) | 66.7% (2/3) | 33.3% (1/3) | 66.7% (2/3) | 33.3% (1/3) | 100.0% (3/3) | 66.7% (2/3) | 66.7% (2/3) | 33.3% (1/3) | 33.3% (1/3) | 0.0% (0/3) |
| Severity of symptoms | ||||||||||||
| No Symptoms | 50.0% (2/4) | 50.0% (2/4) | 50.0% (2/4) | 50.0% (2/4) | 25.0% (1/4) | 75.0% (3/4) | 100.0% (4/4) | 25.0% (1/4) | 25.0% (1/4) | 0.0% (0/4) | 0.0% (0/4) | 0.0% (0/4) |
| Mild Symptoms | 53.9% (241/447) | 46.1% (206/447) | 53.9% (241/447) | 46.1% (206/447) | 46.3% (207/447) | 53.7% (240/447) | 70.0% (313/447) | 44.7% (200/447) | 56.6% (253/447) | 35.6% (159/447) | 7.6% (34/447) | 10.7% (48/447) |
| Severe Symptoms | 67.3% (35/52) | 32.7% (17/52) | 67.3% (35/52) | 32.7% (17/52) | 59.6% (31/52) | 40.4% (21/52) | 84.6% (44/52) | 51.9% (27/52) | 71.2% (37/52) | 50.0% (26/52) | 30.8% (16/52) | 0.0% (0/52) |
| Outcome of the infection | ||||||||||||
| Death | 36.4% (4/11) | 36.4% (4/11) | 36.4% (4/11) | 63.6% (7/11) | 45.5% (5/11 | 54.5% (6/11) | 81.8% (9/11) | 63.6% (7/11) | 54.5% (6/11) | 45.5% (5/11) | 18.2% (2/11) | 0.0%(0/11) |
| Recovery | 55.1% (271/492) | 44.9% (221/492) | 55.1% (271/492) | 44.9% (221/492) | 52.6% (259/492) | 47.4% (233/492) | 71.5% (352/492) | 44.9% (221/492) | 57.9% (285/492) | 36.6% (180/492) | 9.8% (48/492) | 9.8% (48/492) |
| Variables | Medication | Vitamin C | Vitamin D | Zinc | Vitamin and Supplementation from Foods and Fruits |
|---|---|---|---|---|---|
| Age | 0.002 | 0.04 | 0.03 | 0.05 | 0.02 |
| Sex | 0.05 | 0.02 | 0.04 | 0.21 | 0.34 |
| Occupation | 0.04 | 0.67 | 0.002 | 0.35 | 0.04 |
| Residence | 0.001 | 0.7 | 0.04 | 0.14 | 0.05 |
| Monthly income | 0.037 | 0.05 | 0.02 | 0.04 | 0.02 |
| COVID-19 infection status | 0.001 | 0.001 | 0.05 | 0.001 | 0.005 |
| Symptoms | 0.05 | 0.005 | 0.04 | 0.03 | 0.015 |
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Age above 40 years | 3.87 (1.91–5.84) | 0.0001 |
| Male | 2.51 (1.21–4.67) | 0.03 |
| Taking vitamin C only as medication | 0.34 (0.042–0.57) | 0.003 |
| Taking vitamin D only as medication | 0.51 (0.014–0.76) | 0.001 |
| Taking zinc only as medication | 0.72 (0.16–1.85) | 0.005 |
| Taking vitamin C and D as medication | 0.04 (0.01–0.17) | 0.00001 |
| Taking vitamin C, D and zinc as medication | 0.006 (0.03–0.11) | 0.004 |
| Increased eating of vitamin C-enriched foods | 0.97 (0.51–1.85) | 0.09 |
| Increased eating of vitamin D-enriched foods | 0.84 (0.01–0.17) | 0.67 |
| Taking supplements without medicine | 0.02 (0.001–0.6) | 0.02 |
| Taking medicines without supplements | 0.07 (0.01–0.94) | 0.03 |
| Taking both medicines and supplements | 0.01 (0.006–0.09) | 0.001 |
| Taking only supplements as medication for 7 days or less | 0.05 (0.01–0.67) | 0.0007 |
| Taking only supplements as medication for 7–14 days | 0.001 (0.03–0.09) | 0.0005 |
| Taking only supplements as medication for more than 14 days | 0.43 (0.14–0.97) | 0.00005 |
| No Symptoms | 0.6 (0.23–1.73) | 0.000001 |
| Mild Symptoms | 0.02 (0.03–0.6) | 0.0001 |
| Severe Symptoms | 0.43 (0.1–0.9) | 0.0004 |
| Better access to health facilities | 0.01 (0.001–0.2) | 0.31 |
| High income | 1.8 (0.4–3.47) | 0.0006 |
| Symptoms prevailing >14 days | 1.92 (0.57–4.28) | 0.008 |
| More than three symptoms | 0.7 (0.24–1.8) | 0.03 |
| RT-qPCR confirmed cases | .03 (0.01–0.4) | 0.00001 |
| Non-confirmed suspected cases | 0.07 (0.02–0.6) | 0.0007 |
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Age above 40 years | 5.61 (2.91–7.14) | 0.05 |
| Male | 2.51 (1.21–4.67) | 0.02 |
| Taking vitamin C only | 0.54 (0.01–0.92) | 0.001 |
| Taking vitamin D only | 0.72 (0.3–0.98) | 0.001 |
| Taking zinc only | 0.6 (0.11–1.2) | 0.0001 |
| Taking vitamin C and D | 0.01 (0.001–0.09) | 0.00001 |
| Taking vitamin C, D and zinc | 0.03 (0.01–0.22) | 0.005 |
| Increased eating of vitamin C-enriched foods | 0.95 (0.72–2.52) | 0.09 |
| Increased eating of vitamin D-enriched foods | 1.07 (0.68–2.9) | 0.87 |
| Taking supplements without medicine | 0.8 (0.3–1.9) | 0.005 |
| Taking medicines without supplements | 0.01 (0.008–0.07) | 0.001 |
| Taking both medicines and supplements | 0.002 (0.001–0.009) | 0.005 |
| Taking only supplements for 7 days or less | 0.6 (0.1–1.9) | 0.54 |
| Taking only supplements for 7–14 days | 0.1 (0.01–0.8) | 0.04 |
| Taking only supplements for more than 14 days | 0.23 (0.1–0.9) | 0.05 |
| Better access to health facilities | 0.03 (0.01–0.6) | 0.007 |
| High income | 0.4 (0.1–1.9) | 0.001 |
| More than three symptoms | 3.9 (1.01–6.8) | 0.05 |
| RT-qPCR confirmed cases | 1.9 (1.1–5.4) | 0.001 |
| Non-confirmed suspected cases | 0.4 (0.2–0.96) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, N.; Opu, R.R.; Khan, A.; Alzahrani, K.J.; Banjer, H.J.; Alzahrani, F.M.; Haque, N.; Khan, S.; Soumik, S.T.; Zhang, M.; et al. Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study. Nutrients 2022, 14, 5029. https://doi.org/10.3390/nu14235029
Sharif N, Opu RR, Khan A, Alzahrani KJ, Banjer HJ, Alzahrani FM, Haque N, Khan S, Soumik ST, Zhang M, et al. Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study. Nutrients. 2022; 14(23):5029. https://doi.org/10.3390/nu14235029
Chicago/Turabian StyleSharif, Nadim, Rubayet Rayhan Opu, Afsana Khan, Khalid J. Alzahrani, Hamsa Jameel Banjer, Fuad M. Alzahrani, Nusaira Haque, Shahriar Khan, Saimum Tahreef Soumik, Ming Zhang, and et al. 2022. "Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study" Nutrients 14, no. 23: 5029. https://doi.org/10.3390/nu14235029
APA StyleSharif, N., Opu, R. R., Khan, A., Alzahrani, K. J., Banjer, H. J., Alzahrani, F. M., Haque, N., Khan, S., Soumik, S. T., Zhang, M., Huang, H., Song, X., Parvez, A. K., & Dey, S. K. (2022). Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study. Nutrients, 14(23), 5029. https://doi.org/10.3390/nu14235029