Hepatoprotective Effects of Radish (Raphanus sativus L.) on Acetaminophen-Induced Liver Damage via Inhibiting Oxidative Stress and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Sample Extract
2.3. Animal Experiments
2.4. Measurement of Plasma Aminotransferase Levels
2.5. Measurement of Antioxidant Enzymes and Lipid Peroxidation in Plasma
2.6. Histopathologic Examination
2.7. RNA Isolation and Real-Time Polymerase Chain Reaction Analysis
2.8. Western Blot Analysis
2.9. Statistical Analyses
3. Results
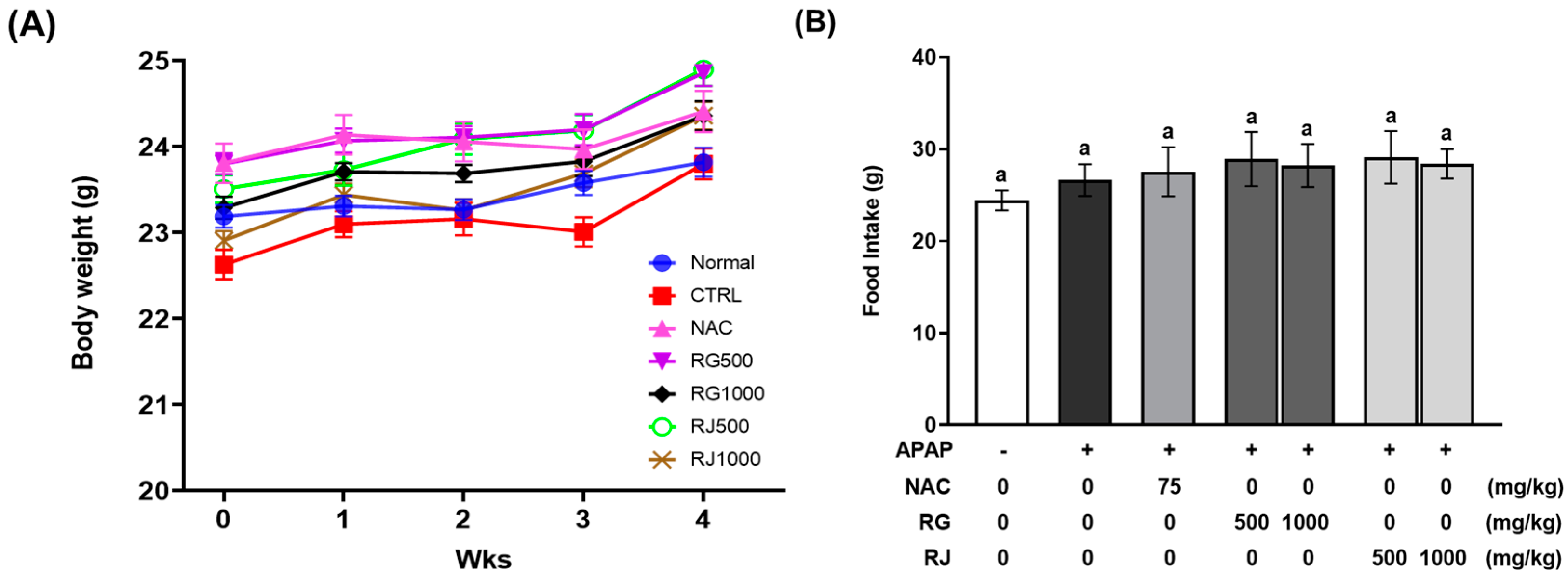
3.1. Effects of Radish Extracts on Body Weight, Tissue Weight, and Food Intake of APAP-Induced Liver-Damaged Mice
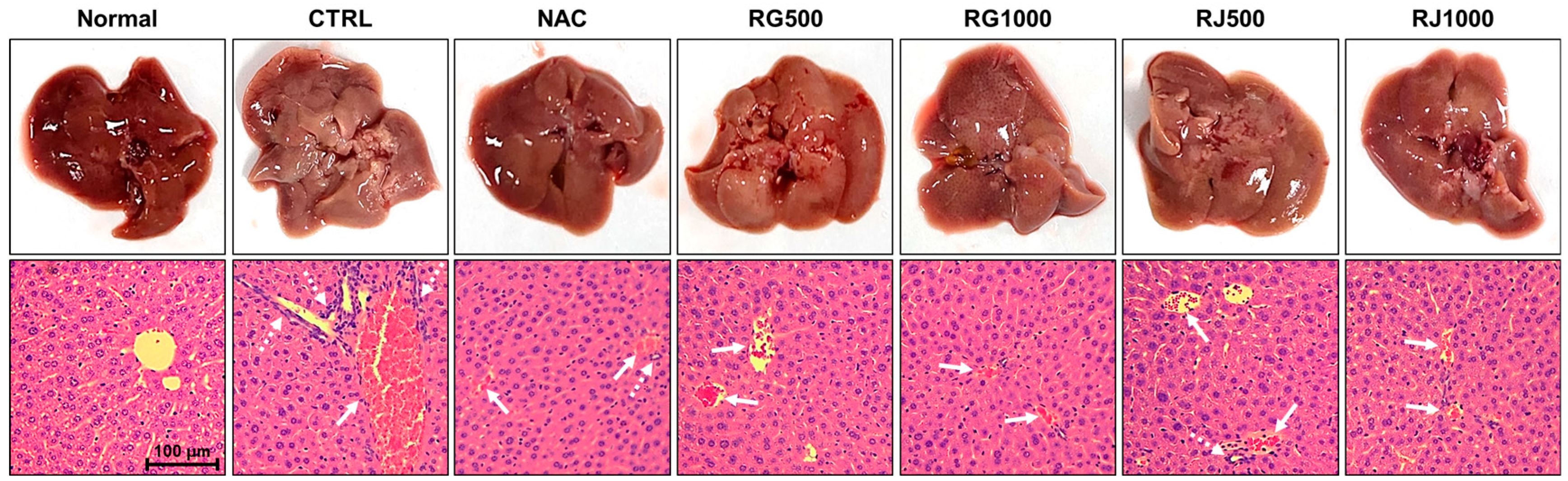
3.2. Radish Extracts Attenuated the APAP-Induced Liver Damage in Mice
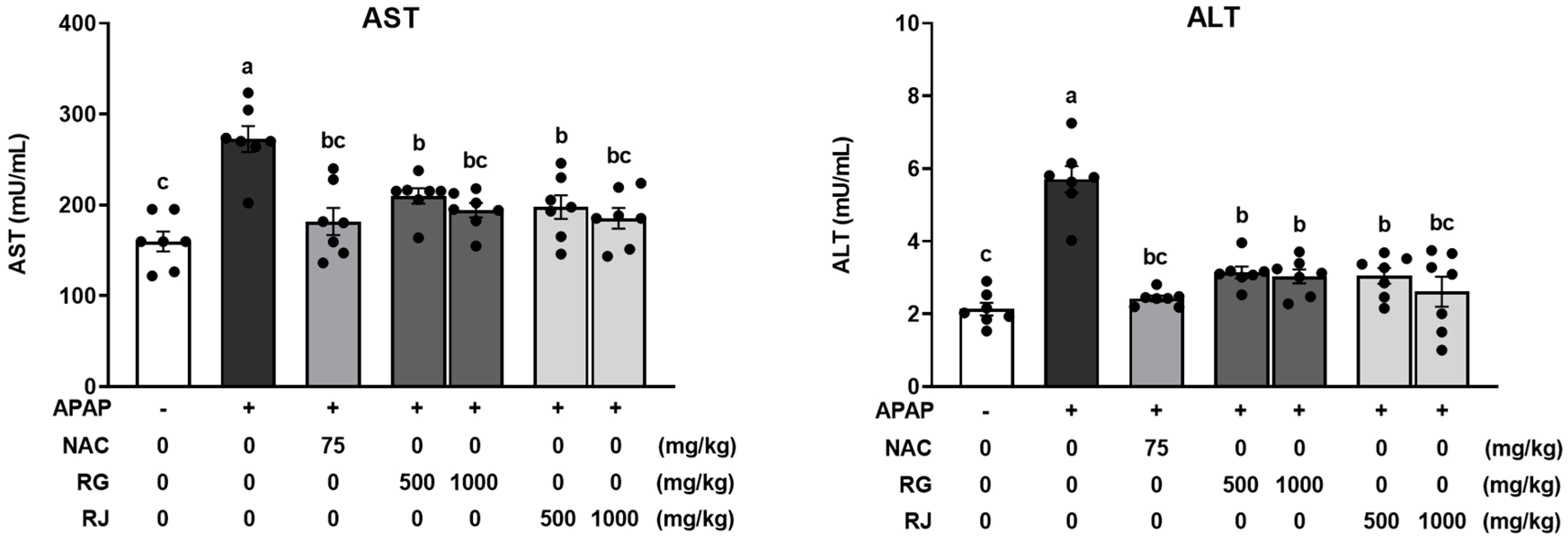
3.3. Effect of Radish Extracts on Hepatotoxicity in APAP-Induced Liver-Damaged Mice
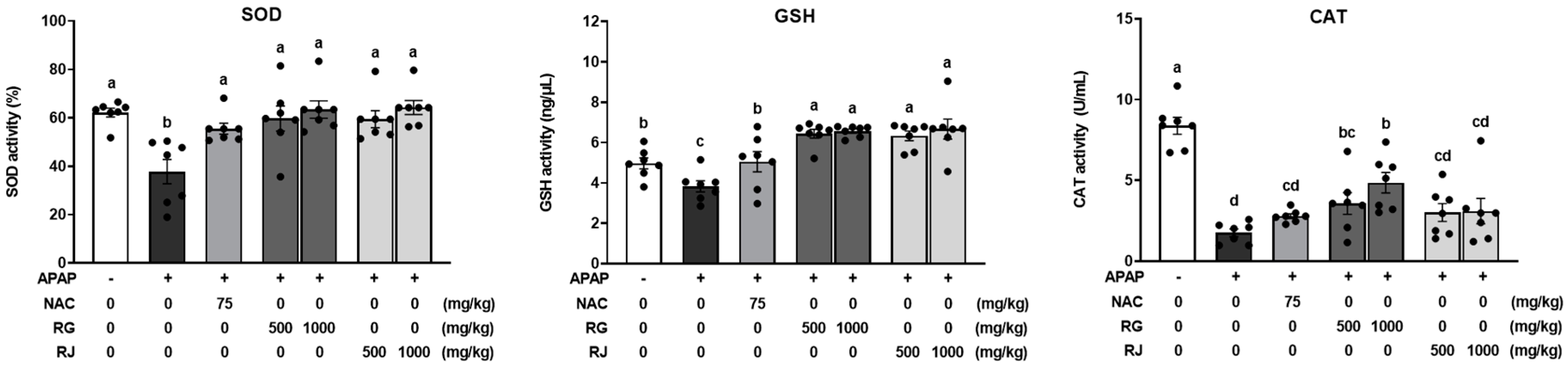
3.4. Effects of Radish Extracts on Antioxidant Enzymes and Glutathione Production in APAP-Induced Liver-Damaged Mice
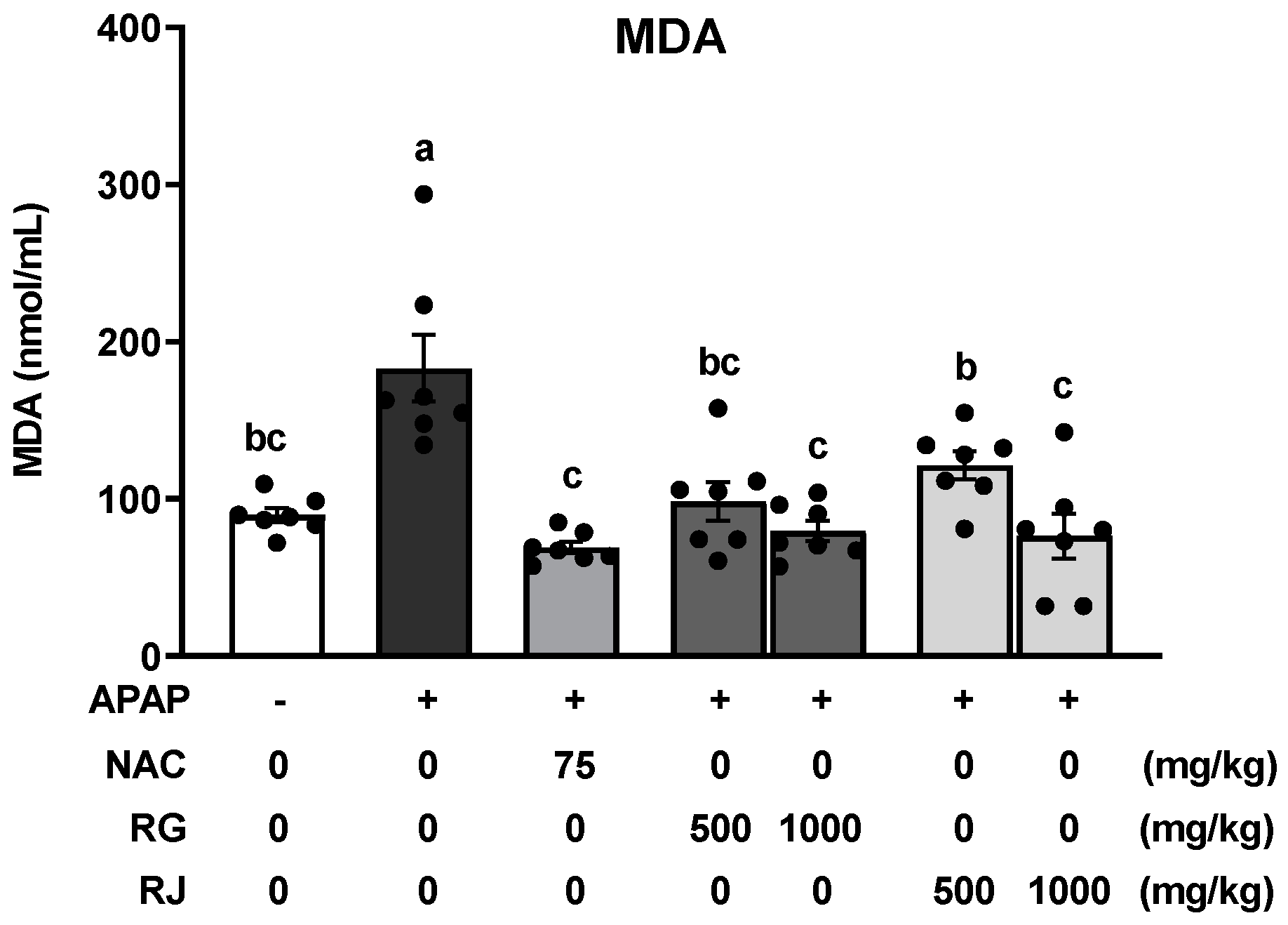
3.5. Effects of Radish Extracts on Lipid Peroxidation in APAP-Induced Liver-Damaged Mice
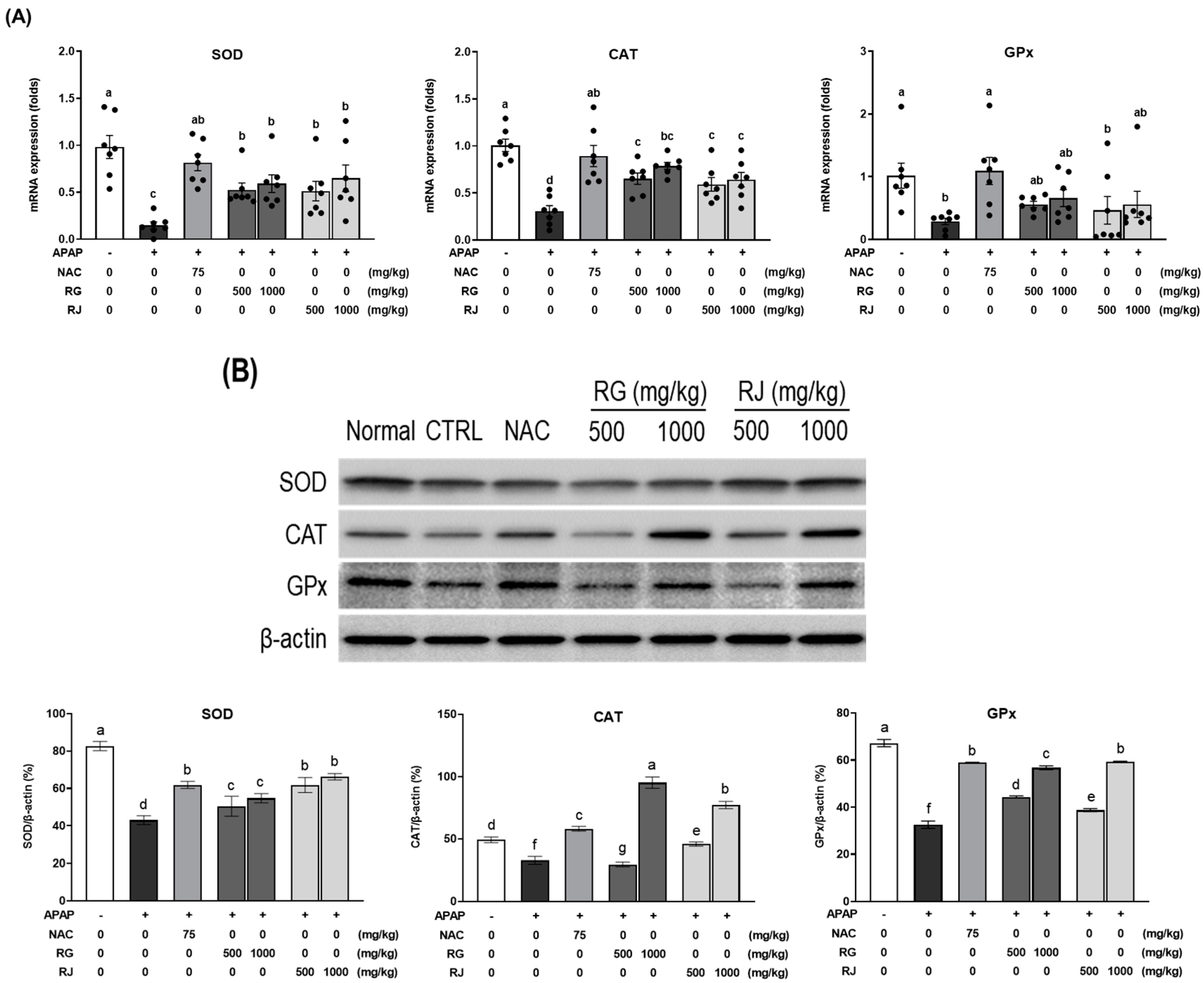
3.6. Effects of Radish Extracts on the mRNA Expression of Antioxidant Enzymes in APAP-Induced Liver-Damaged Mice
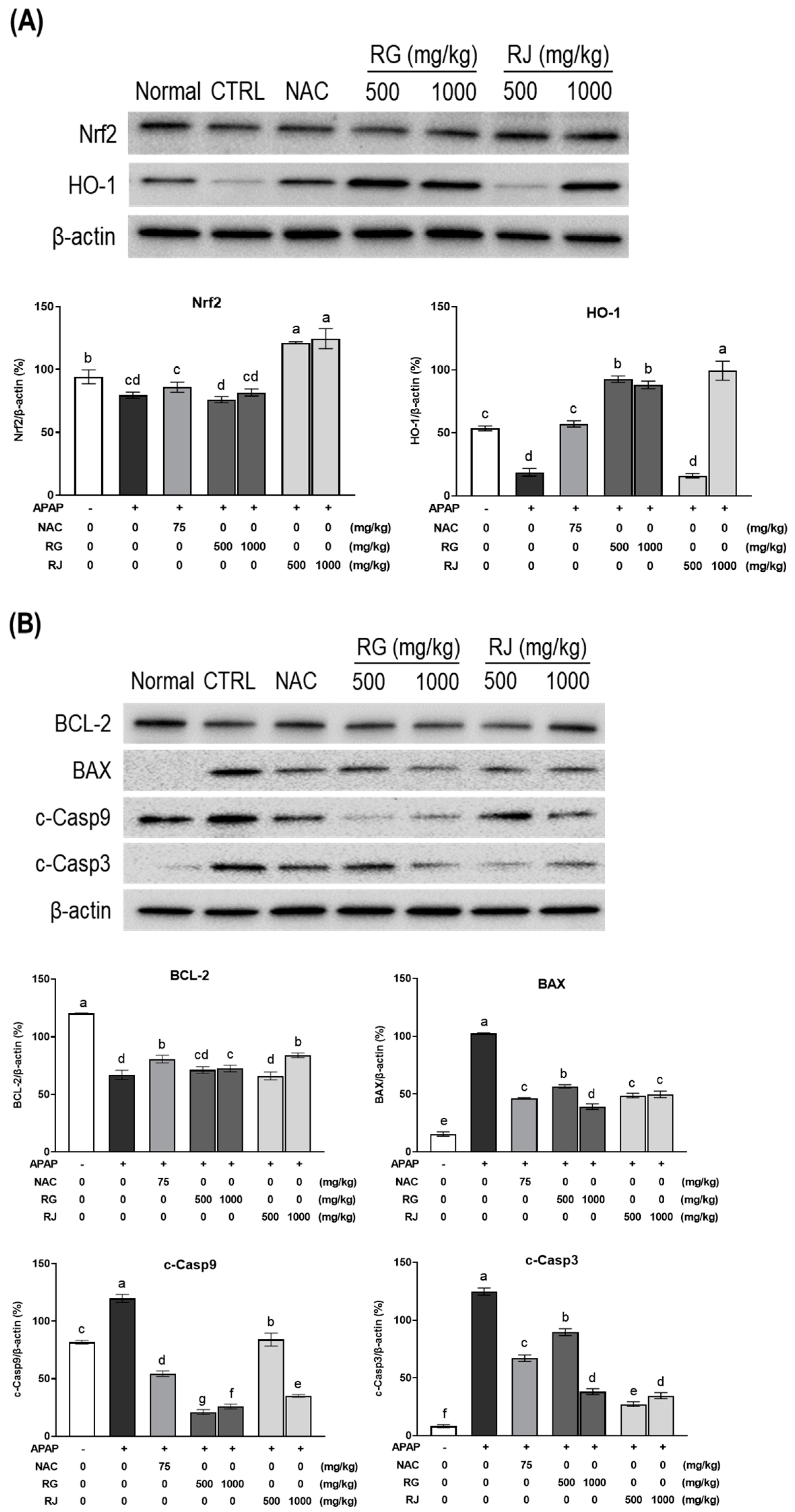
3.7. Effects of Radishes Extract Regulated Nrf2/HO-1 Signaling Pathway in APAP-Induced Liver-Damaged Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamora Nava, L.E.; Aguirre Valadez, J.; Chavez-Tapia, N.C.; Torre, A. Acute-on-chronic liver failure: A review. Ther. Clin. Risk. Manag. 2014, 10, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.Y.; Chen, L.; Zhou, W.; Hu, L.; Li, L.; Tu, Q.; Chang, Y.; Liu, Q.; Sun, X.; Wu, M.; et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J. Hepatol. 2011, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Formica, D.; Sultana, J.; Cutroneo, P.M.; Lucchesi, S.; Angelica, R.; Crisafulli, S.; Trifirò, G. The economic burden of preventable adverse drug reactions: A systematic review of observational studies. Expert. Opin. Drug. Saf. 2018, 17, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Berson, A.; Renault, S.; Letteron, P.; Fromenty, B.; Fau, D.; Le Bot, M.L.; Riche, C.; Durand-Schneider, A.; Feldmann, G.; Pessayre, D. Uncoupling of rat and human mitochondria: A possible explanation for tacrine-induced liver dysfunction. Gastroenterology 1996, 110, 1878–1890. [Google Scholar] [CrossRef]
- Fromenty, B.; Pessayre, D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol. Ther. 1995, 67, 101–154. [Google Scholar] [CrossRef]
- Helieh, S.O.; Theresa, S.C. Green-tea polyphenols downregulate cyclooxygenase and Bcl-2 activity in acetaminophen-induced hepatotoxicity. Dig. Dis. Sci. 2008, 53, 2980–2988. [Google Scholar] [CrossRef]
- Stefan, D.; Hamilton, J.P. Drug-induced Liver Injury. US Gastroenterol. Hepatol. Rev. 2010, 6, 73–80. [Google Scholar]
- Wu, H.; Xie, Y.; Xu, Y.; Hu, Z.; Wan, X.; Huang, H.; Huang, D. Protective Effect of epicatechin on APAP-induced Acute Liver injury of mice through anti-inflammation and apoptosis inhibition. Nat. Prod. Res. 2020, 34, 855–858. [Google Scholar] [CrossRef]
- Tujios, S.; Fontana, R.J. Mechanisms of drug-induced liver injury: From bedside to bench. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 202–211. [Google Scholar] [CrossRef]
- Abdeen, A.; Abdelkader, A.; Abdo, M.; Wareth, G.; Aboubakr, M.; Aleya, L.; Abdel-Daim, M. Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ. Sci. Pollut. Res. Int. 2019, 26, 240–249. [Google Scholar] [CrossRef]
- Shahripour, R.B.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Bebarta, V.S.; Kao, L.; Froberg, B.; Clark, R.F.; Laconas, E.; Qi, M.; Delgado, J.; McDonagh, J.; Arnold, T.; Odujebe, O.; et al. A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin. Toxicol. 2010, 48, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Yarema, M.; Chopra, P.; Sivilotti, M.L.A.; Johnson, D.; Nettel-Aguirre, A.; Bailey, B.; Victorino, C.; Gosselin, S.; Purssell, R.; Thompson, M.; et al. Anaphylactoid reactions to intravenous n-acetylcysteine during treatment for acetaminophen poisoning. J. Med. Toxicol. 2018, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Kim, D.J.; Han, B.H.; Park, S.E.; Paek, H.S. Supply and Demand Trend and Outlook of root vegetables. In Agriculture Outlook; 2021: Changes and Future of Agriculture and Rural Communities after COVID-19; Korea Rural Economic Institute: Naju-si, Korea, 2021; pp. 353–411. [Google Scholar]
- Oh, S.; Moon, K.H.; Song, E.Y.; Wi, S.H.; Koh, S.C. Photosynthesis, productivity, and mineral content of winter radishes by soil type on Jeju Island. Korean. J. Hortic. Sci. Technol. 2019, 37, 167–177. [Google Scholar] [CrossRef]
- Yi, G.; Lim, S.; Chae, W.B.; Park, J.E.; Park, H.R.; Lee, E.J.; Huh, J.H. Root glucosinolate profiles for screening of radish (Raphanus sativus L.) genetic resources. J. Agric. Food Chem. 2016, 64, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Hwang, H.J.; Shin, M.S.; Kang, M.; Lee, J.; Bang, G.; Kim, Y.J.; Hwang, Y.J.; Hwang, K.A.; Park, Y.H. Extract of radish (R. sativus Linn) promotes anti-atherosclerotic effect using urine metabolomics in ApoE−/− mice. J. Funct. Foods 2021, 78, 104368. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, H.M.; Park, M.W.; Kim, S.R.; Choi, I.W. Physicochemical and functional properties of turnip. J. Korean Soc. Food Sci. Nutr. 1999, 28, 333–341. [Google Scholar]
- Tena, N.; Martin, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Parikh, H.; Pandita, N.; Khanna, A. Phytoextract of Indian mustard seeds acts by suppressing the generation of ROS against acetaminophen-induced hepatotoxicity in HepG2 cells. Pharm. Biol. 2015, 53, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Saba, E.; Lee, Y.Y.; Kim, M.; Kim, H.K.; Rhee, M.H. Comparison of the Antioxidant Activities of Various Processed Fruits and Vegetables in APAP-induced Oxidative Stress in BALB/c Mice. Biomed. Sci. Lett. 2019, 25, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, J.; Zhang, Y.; Dai, B.; An, Y.; Yu, L.L. Structural, thermal, and anti-inflammatory properties of a novel pectic polysaccharide from alfalfa (Medicago sativa L.) stem. J. Agric. Food Chem. 2015, 63, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Pigeolet, E.; Corbisier, P.; Houbion, A.; Lambert, D.; Michiels, C.; Raes, M.; Zachary, M.D.; Remacle, J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing. Dev. 1990, 51, 283–297. [Google Scholar] [CrossRef]
- Zubkova, E.V.; Robaire, B. Effect of Glutathione Depletion on Antioxidant Enzymes in the Epididymis, Seminal Vesicles, and Liver and on Spermatozoa Motility in the Aging Brown Norway Rat. Biol. Reprod. 2004, 71, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Moshaie-Nezhad, P.; Faed Maleki, F.; Hosseini, S.M.; Yahyapour, M.; Iman, M.; Khamesipour, A. Hepatoprotective and antioxidant effects of Hedera helix extract on acetaminophen induced oxidative stress and hepatotoxicity in mice. Biotech. Histochem. 2019, 94, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pu, C.; Zhou, P.; Wang, P.; Liang, D.; Wang, Q.; Hu, Y.; Li, B.; Hao, X. Cinnamaldehyde Prevents Endothelial DysFunction Induced by High Glucose by Activating Nrf2. Cell. Physiol. Biochem. 2015, 36, 315–324. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, Q.; Hua, W.; Liu, Y.; Liu, X.; Zhu, Y. Hepatoprotective effects of berberine on acetaminophen-induced hepatotoxicity in mice. Biomed. Pharmacother. 2018, 103, 1319–1326. [Google Scholar] [CrossRef]
- Chorley, B.N.; Campbell, M.R.; Wang, X.; Karaca, M.; Sambandan, D.; Bangura, F.; Xue, P.; Pi, J.; Kleeberger, S.R.; Bell, D.A. Identification of Novel NRF2-regulated Genes by ChIP-Seq: Influence on Retinoid X Receptor Alpha. Nucleic Acids Res. 2012, 40, 7416–7429. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Katsuoka, F.; Miyoshi, C.; Uchimura, Y.; Saitoh, H.; Francastel, C.; Engel, J.D.; Yamamoto, M. MafG Sumoylation Is Required for Active Transcriptional Repression. Mol. Cell. Biol. 2006, 26, 4652–4663. [Google Scholar] [CrossRef] [Green Version]
- Chao, D.T.; Korsmeyer, S.J. BCL-2 FAMILY: Regulators of cell death. Annu. Rev. Immunol. 1998, 16, 395–419. [Google Scholar] [CrossRef]
- Ahn, D.S.; Lee, H.J.; Hwang, J.; Han, H.; Kim, B.; Shim, B.; Kim, S.H. Lambertianic acid sensitizes non-small cell lung cancers to trail-induced apoptosis via inhibition of xiap/nf-κb and activation of caspases and death receptor 4. Int. J. Mol. Sci. 2018, 19, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yuan, X.; Chen, Y.; Zheng, Q.; Xu, L.; Wu, Y. Endoplasmic reticulum stress mediated mdrv p10.8 protein-induced cell cycle arrest and apoptosis through the perk/eIF2α pathway. Front. Microbiol. 2018, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, F.; Wang, K.; Liu, G.; Yang, M.; Luan, Y.; Zhao, Z. Protective effect of allyl methyl disulfide on acetaminophen-induced hepatotoxicity in mice. Chem. Biol. Interact. 2016, 249, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mitka, M. FDA asks physicians to stop prescribing high-dose acetaminophen products. JAMA 2014, 311, 563. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepatol. 2016, 66, 836–848. [Google Scholar] [CrossRef] [Green Version]
- McGill, M.R.; Williams, C.D.; Xie, Y.; Ramachandran, A.; Jaeschke, H. Acetaminophen-induced liver injury in rats and mice: Comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012, 264, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.D.; Antoine, D.J.; Shaw, P.J.; Benson, C.; Farhood, A.; Williams, D.P.; Jaeschke, H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol. Appl. Pharmacol. 2011, 252, 2892–2897. [Google Scholar] [CrossRef] [Green Version]
- Chao, X.; Wang, H.; Jaeschke, H.; Ding, W.X. Role and mechanisms of autophagy in acetaminophen-induced liver injury. Liver Int. 2018, 38, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Abuzalat, O.; El-Mehalmey, W.A.; Tantawy, H.; Baraka, A.; Alkordi, M.H. Heterogeneous catalytic decomposition of hydrogen peroxide utilizing a Fe(iii)-based metal–organic framework as an efficient and persistent nanozyme. Mater. Adv. 2022, 3, 4262–4267. [Google Scholar] [CrossRef]
- Fisher, L.; Green, M.D.; Harman, A.W. Levels of acetaminophen and its metabolites in mouse tissues after a toxic does. J. Pharmacol. Exp. Ther. 1982, 221, 407–413. [Google Scholar]
- Yung, J.H.; Chiu, L.C.; Ooi-Clin, V. Effect of polysaccharide peptide on glutathione and protection against paracetamol-induced hepatotoxity in the rat. Clin. Pharmacol. 1994, 16, 723–729. [Google Scholar]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Lin, C.C. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J. Ethnopharmacol. 2007, 111, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell. 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitazumi, I.; Tsukahara, M. Regulation of DNA fragmentation: The role of caspases and phosphorylation. FEBS J. 2011, 278, 427–441. [Google Scholar] [CrossRef]
- Li, P.; Su, L.; Li, X.; Di, W.; Zhang, X.; Zhang, C.; He, T.; Zhu, X.; Zhang, Y.; Li, Y. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion injury via upregulating expression of Nrf2, HO-1 and NQO-1 in mice. Int. J. Neurosci. 2016, 126, 552–559. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, M.; Xiong, Y. BTB Protein Keap1 Targets Antioxidant Transcription Factor Nrf2 for Ubiquitination by the Cullin 3-Roc1 Ligase. Mol. Cell. Biol. 2005, 25, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.M.; Wang, Y.; Tan, H.S.; Yu, T.; Fan, X.M.; Chen, P.; Zeng, H.; Huang, M.; Bi, H.C. Schisandrol B protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the NRF2/ARE signaling pathway. Acta Pharmacol. Sin. 2016, 37, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, Y.; Xu, Y.; et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Vojnovic, D.; Kochevar, I.E.; Jurkunas, U.V. UV-A irradiation activates Nrf2-regulated antioxidant defense and induces p53/Caspase3-dependent apoptosis in corneal endothelial Cells UV-A activates Nrf2 and induces p53 in corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2319–2327. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Zhou, J.; Tan, G.; Deng, X.; Ci, X. Daphnetin-mediated Nrf2 antioxidant signaling pathways ameliorate tert-butyl hydroperoxide (t-BHP)-induced mitochondrial dysfunction and cell death. Free Radic. Biol. Med. 2017, 106, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xiao, Q.; Zhou, J.; Feng, H.; Liu, G.; Ci, X. Licochalcone A Upregulates Nrf2 Antioxidant Pathway and Thereby Alleviates Acetaminophen-Induced Hepatotoxicity. Front. Pharmacol. 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzuto, M.J.; Suh, N. Chemoprotection against cancer by isothiocyanates: A focus on the animal models and the protective mechanisms. In Natural Products in Cancer Prevention and Therapy; Springer: Heidelberg, Germany, 2013; pp. 179–201. [Google Scholar]
- Boddupalli, S.; Mein, J.R.; Lakkanna, S.; James, D.R. Induction of Phase 2 antioxidant enzymes by broccoli sulforaphane: Perspectives in maintaining the antioxidant activity of vitamins A, C, and E. Front. Genet. 2012, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Chen, S. Regulation of plant glucosinolate metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef]
- Mithen, R. Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica 1992, 63, 71–83. [Google Scholar] [CrossRef]
- Chung, F.L.; Conaway, C.C.; Rao, C.V.; Reddy, B.S. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis 2000, 21, 2287–2291. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Kim, B.R.; Chen, C.; Hebbar, V.; Kong, A.N. The roles of JNK and apoptosis signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1361–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdull Razis, A.F.; Noor, N.M. Cruciferous vegetables: Dietary phytochemicals for cancer prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Zhang, Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxyfication enzymes. Carcinogenesis 2001, 22, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
| Condition | Groups | Treatment (mg/kg) |
|---|---|---|
| Non | Normal | PBS |
| Acute liver injury (APAP, 500 mg/kg) | CTRL | PBS |
| NAC | N-acetylcysteine (75) | |
| RG 500 | Turnips of Ganghwa (500) | |
| RG 1000 | Turnips of Ganghwa (1000) | |
| RJ 500 | Radishes of Jeju (500) | |
| RJ 1000 | Radishes of Jeju (1000) |
| Gene | Primer | Sequences (5′→3′) |
|---|---|---|
| Sod | Forward | GGGTTGGCTTGGTTTCAATAAGGAA |
| Reverse | AGGTAGTAAGCGTGCTCCCACACAT | |
| Cat | Forward | AAGACAATGTCACTCAGGTGCGGA |
| Reverse | GGCAATGTTCTCACACAGGCGTTT | |
| Gpx | Forward | CTCGGTTTCCCGTGCAATCAG |
| Reverse | GTGCAGCCAGTAATCACCAAG | |
| Gapdh | Forward | GAGCCAAAAGGGTCATCATC |
| Reverse | TAAGCAGTTGGTGGTGCAGG |
| Treatment | Body Weight Gain (g) | Liver (g) | Spleen (g) | Kidney (g) | Heart (g) | Colon (g) | |
|---|---|---|---|---|---|---|---|
| Normal | 0.633 ± 0.479 b | 1.049 ± 0.016 a | 0.088 ± 0.001 ab | 0.357 ± 0.005 a | 0.125 ± 0.003 ab | 0.205 ± 0.003 a | |
| CTRL | 1.171 ± 0.144 ab | 0.977 ± 0.017 a | 0.089 ± 0.002 a | 0.351 ± 0.008 a | 0.124 ± 0.002 ab | 0.213 ± 0.003 a | |
| NAC | 0.600 ± 0.200 b | 0.968 ± 0.020 a | 0.083 ± 0.002 ab | 0.352 ± 0.005 a | 0.121 ± 0.001 ab | 0.214 ± 0.003 a | |
| RG | 500 | 1.057 ± 0.268 ab | 1.033 ± 0.012 a | 0.077 ± 0.002 ab | 0.367 ± 0.004 a | 0.131 ± 0.002 a | 0.223 ± 0.003 a |
| 1000 | 1.071 ± 0.203 ab | 0.971 ± 0.016 a | 0.087 ± 0.002 a | 0.379 ± 0.005 a | 0.128 ± 0.003 ab | 0.207 ± 0.003 a | |
| RJ | 500 | 1.386 ± 0.122 ab | 0.975 ± 0.009 a | 0.088 ± 0.002 a | 0.356 ± 0.006 a | 0.127 ± 0.002 ab | 0.197 ± 0.004 a |
| 1000 | 1.433 ± 0.237 b | 0.973 ± 0.005 a | 0.084 ± 0.001 ab | 0.356 ± 0.004 a | 0.115 ± 0.001 b | 0.197 ± 0.004 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, K.-A.; Hwang, Y.; Hwang, H.-J.; Park, N. Hepatoprotective Effects of Radish (Raphanus sativus L.) on Acetaminophen-Induced Liver Damage via Inhibiting Oxidative Stress and Apoptosis. Nutrients 2022, 14, 5082. https://doi.org/10.3390/nu14235082
Hwang K-A, Hwang Y, Hwang H-J, Park N. Hepatoprotective Effects of Radish (Raphanus sativus L.) on Acetaminophen-Induced Liver Damage via Inhibiting Oxidative Stress and Apoptosis. Nutrients. 2022; 14(23):5082. https://doi.org/10.3390/nu14235082
Chicago/Turabian StyleHwang, Kyung-A, YuJin Hwang, Hye-Jeong Hwang, and NaYeong Park. 2022. "Hepatoprotective Effects of Radish (Raphanus sativus L.) on Acetaminophen-Induced Liver Damage via Inhibiting Oxidative Stress and Apoptosis" Nutrients 14, no. 23: 5082. https://doi.org/10.3390/nu14235082





