Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Quantitation of Blood Hormones and Metabolic Parameters
2.3. Tissue Histology
2.4. Glucose and Insulin Tolerance Tests
2.5. Real-Time Reverse Transcription Quantitative Polymerase Chain Reaction
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
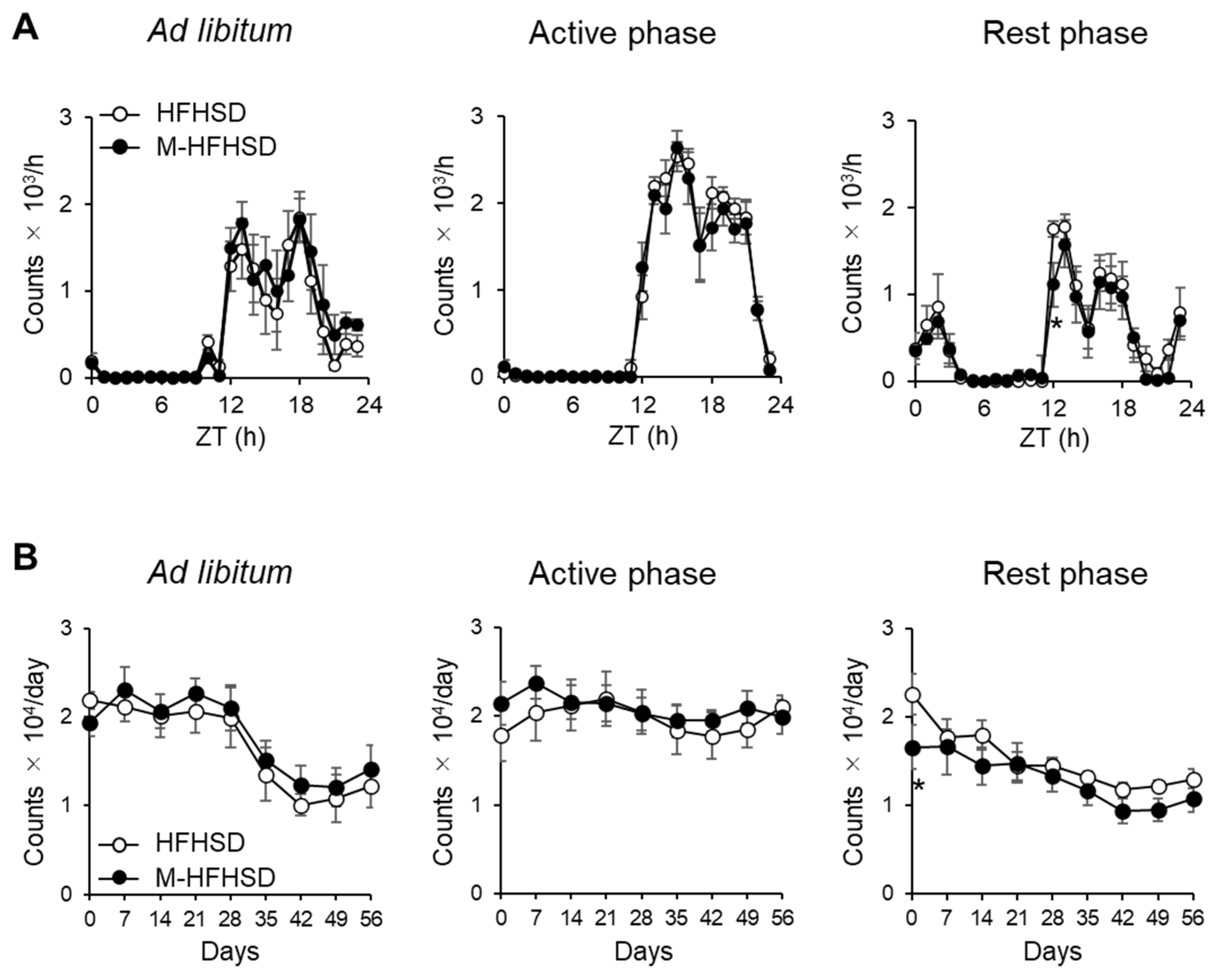
3.1. Dietary MCT Did Not Affect Voluntary Wheel-Running
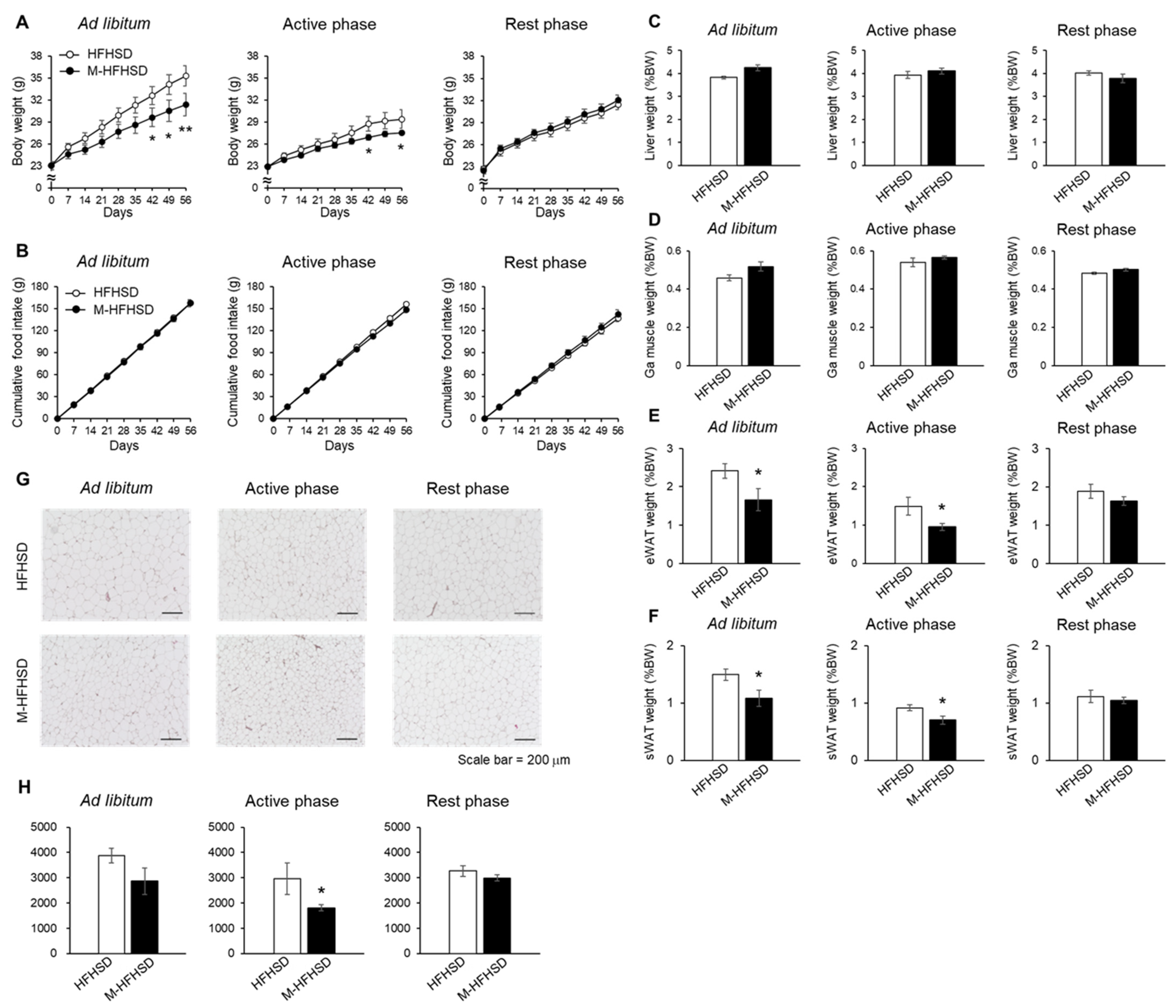
3.2. Effects of Dietary MCT during the Active or Resting Phases on HFHSD-Induced Fat Accumulation in Mice
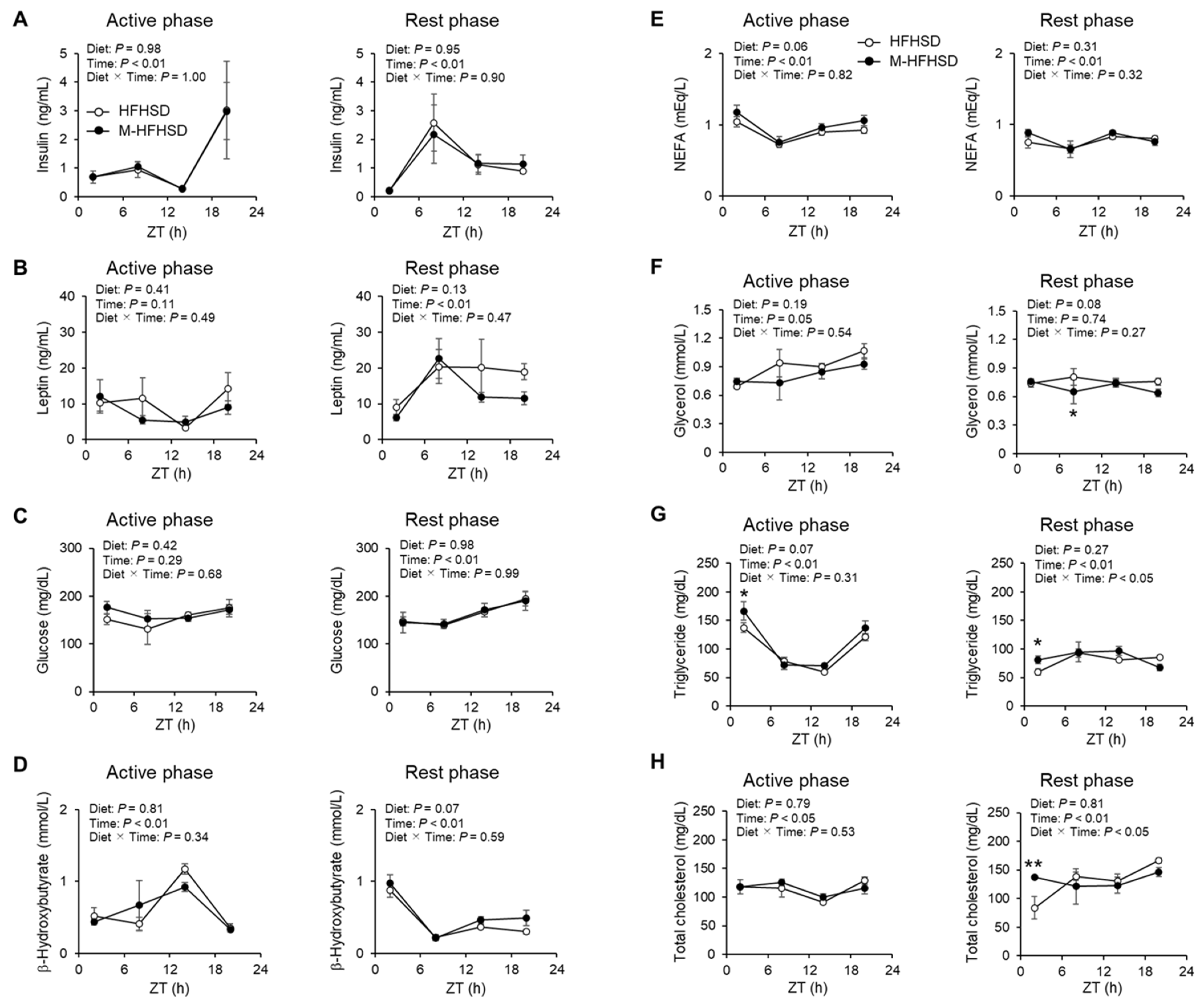
3.3. Effects of MCT Consumed during the Active and Rest Phases on Serum Metabolic Parameters in Mice
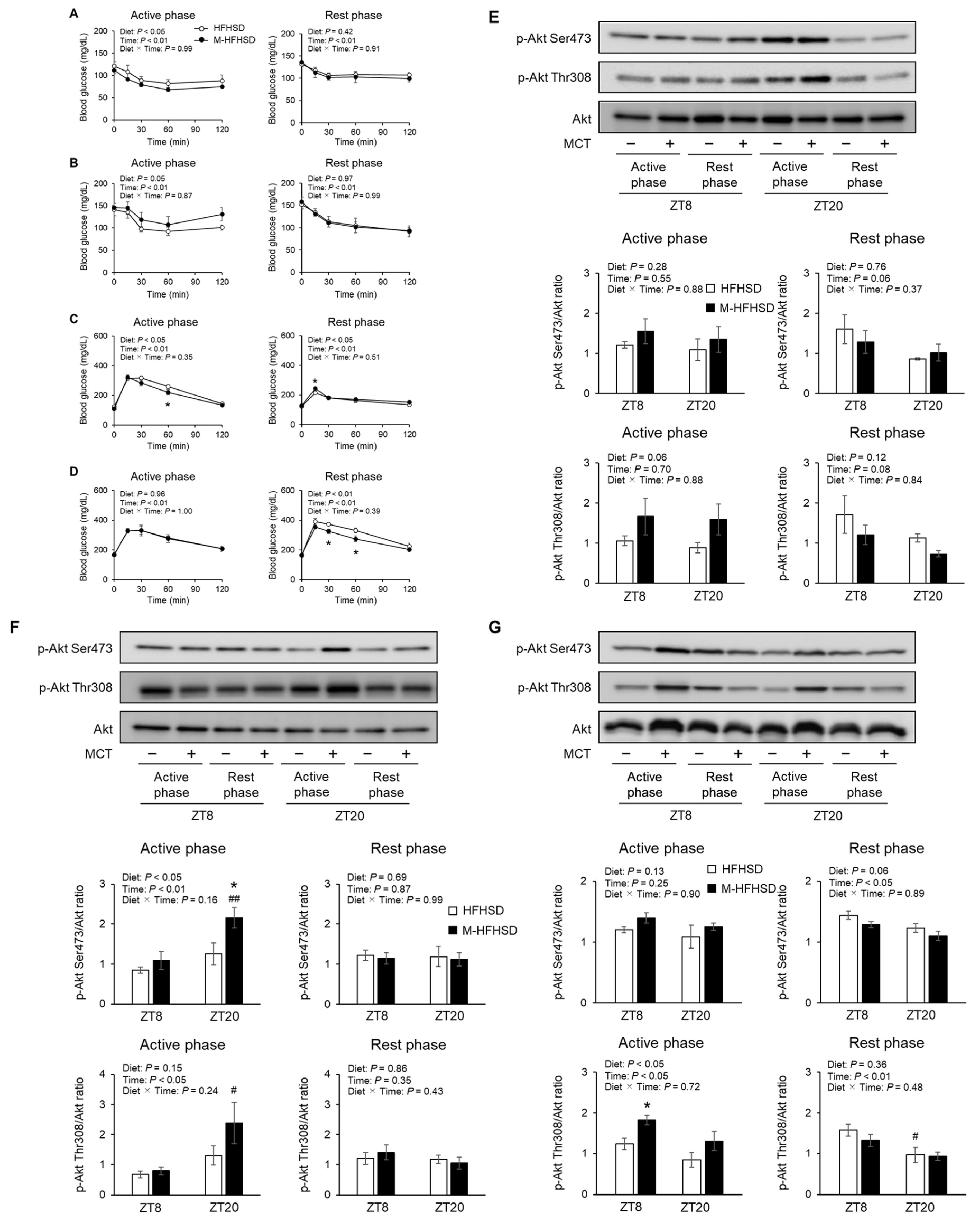
3.4. Effects of MCT Consumed during Active or Rest Phases on Glucose Tolerance and Insulin Sensitivity in Mice Fed HFHSD
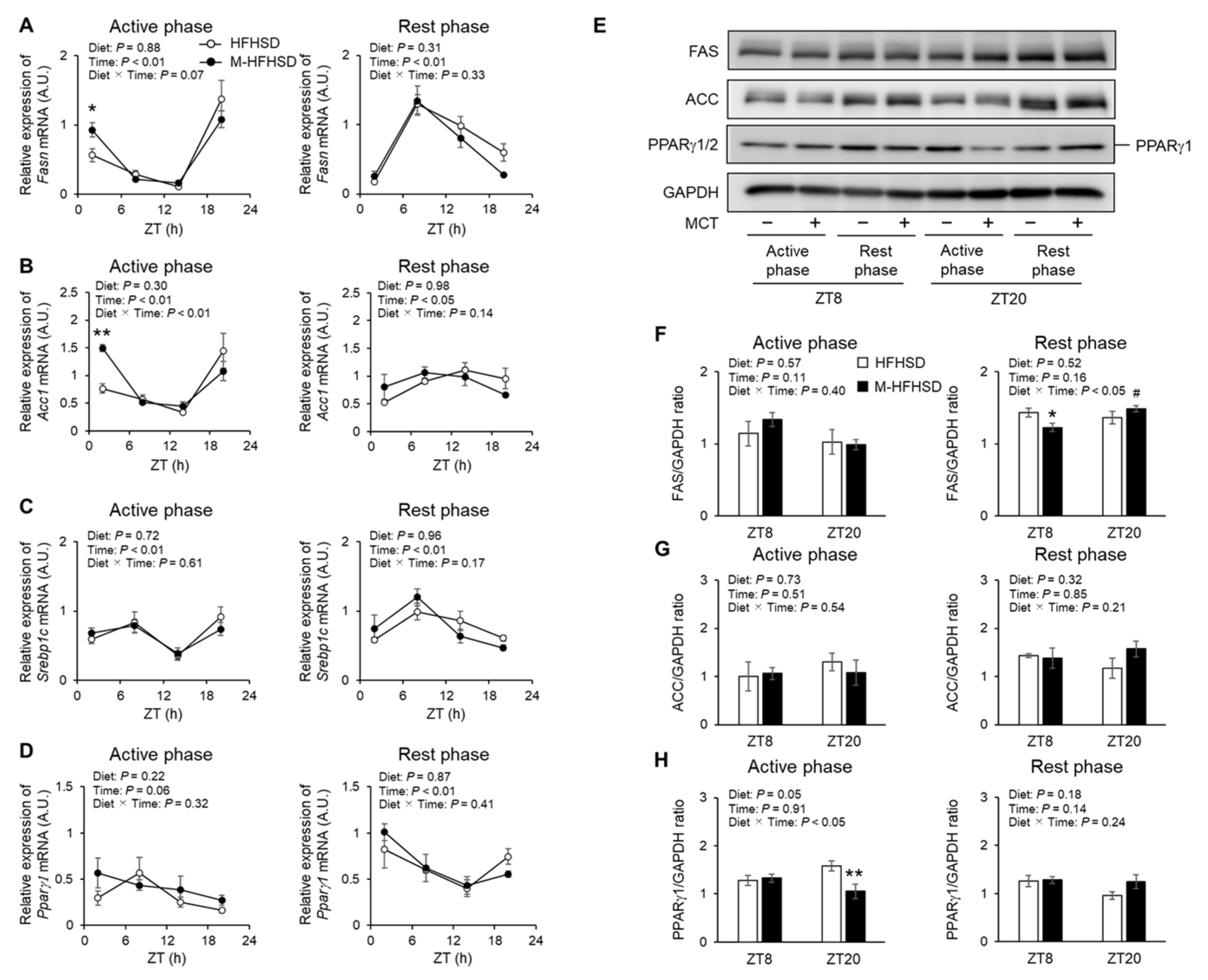
3.5. Expression of Genes and Proteins Associated with Lipid Metabolism in the Liver of Mice Fed with HFHSD or M-HFHSD
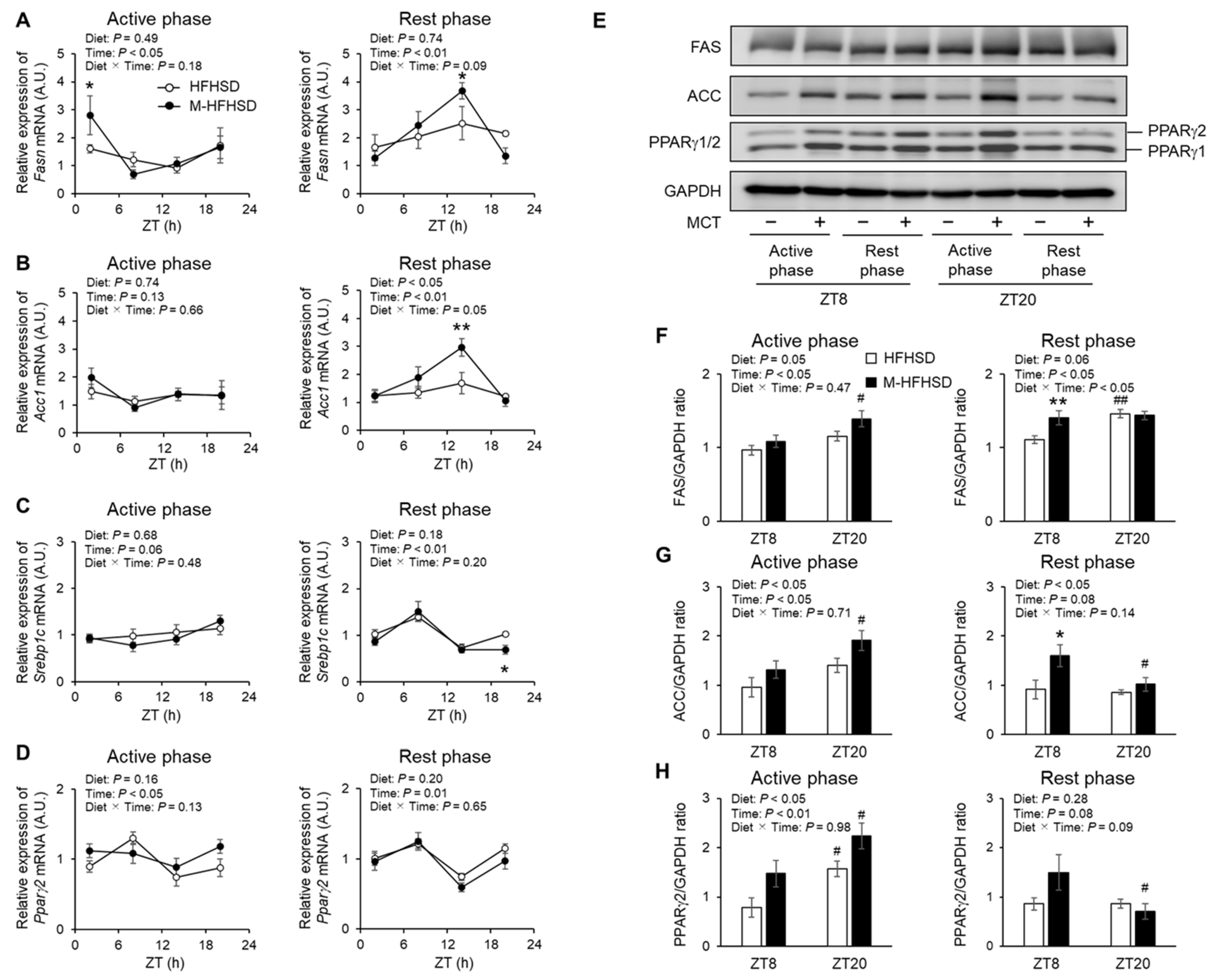
3.6. Expression of Genes and Proteins Associated with Lipid Metabolism in eWAT of Mice Fed with HFHSD or M-HFHSD
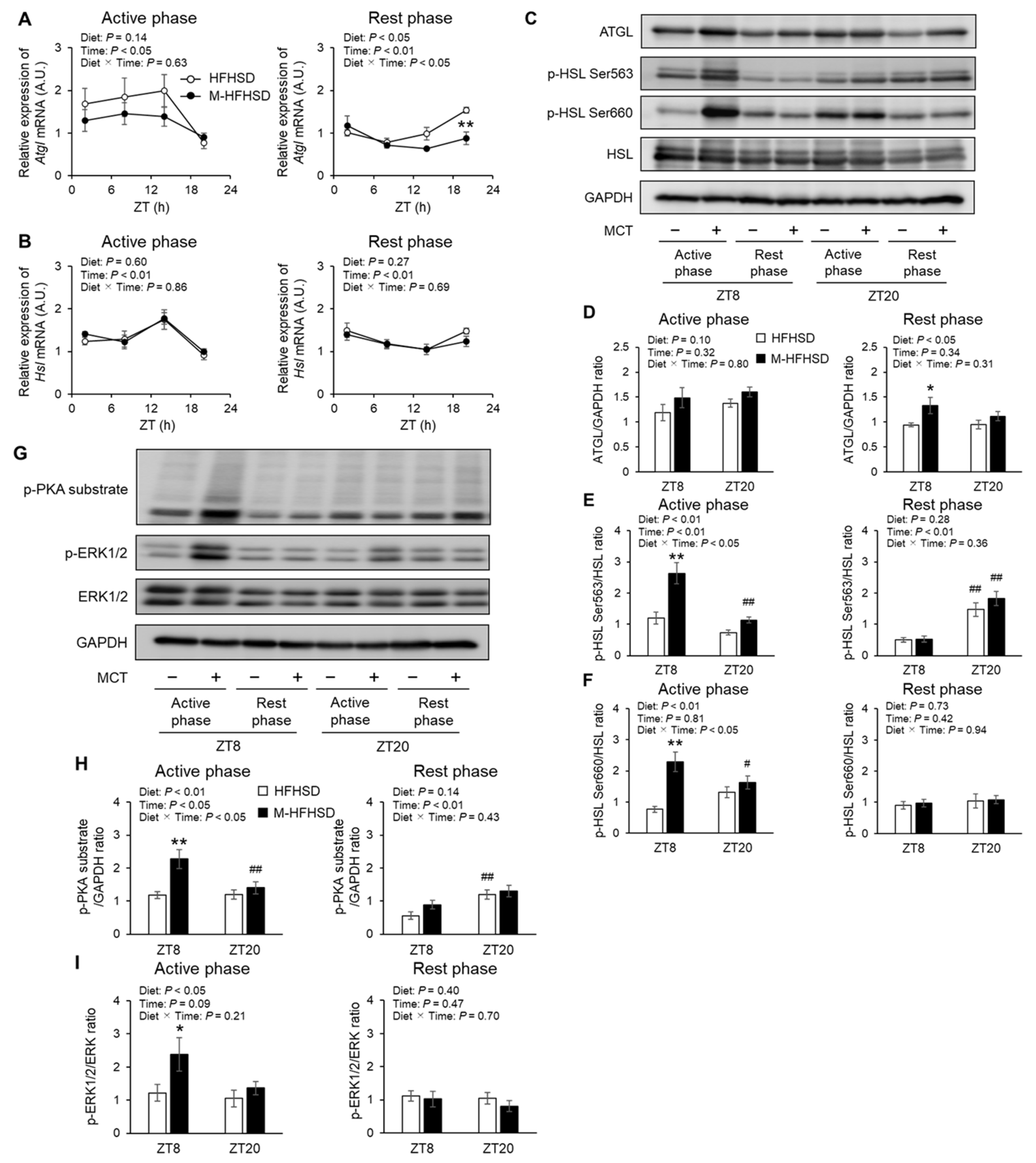
3.7. Expression of Genes and Proteins Associated with Lipolysis in eWAT of Mice Fed with HFHSD or M-HFHSD
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Varady, K.A. Safety of 8-h time restricted feeding in adults with obesity. Appl. Physiol. Nutr. Metab. 2019, 44, 107–109. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Abe, T.; Kazama, R.; Okauchi, H.; Oishi, K. Food deprivation during active phase induces skeletal muscle atrophy via IGF-1 reduction in mice. Arch. Biochem. Biophys. 2019, 677, 108160. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Konishi, T.; Hashimoto, C.; Yamamoto, S.; Takahashi, Y.; Shiina, Y. Dietary fish oil differentially ameliorates high-fructose diet-induced hepatic steatosis and hyperlipidemia in mice depending on time of feeding. J. Nutr. Biochem. 2018, 52, 45–53. [Google Scholar] [CrossRef]
- Konishi, T.; Takahashi, Y.; Shiina, Y.; Oike, H.; Oishi, K. Time-of-day effects of consumption of fish oil-enriched sausages on serum lipid parameters and fatty acid composition in normolipidemic adults: A randomized, double-blind, placebo-controlled, and parallel-group pilot study. Nutrition 2021, 90, 111247. [Google Scholar] [CrossRef]
- Aoyama, S.; Kim, H.K.; Hirooka, R.; Tanaka, M.; Shimoda, T.; Chijiki, H.; Kojima, S.; Sasaki, K.; Takahashi, K.; Makino, S.; et al. Distribution of dietary protein intake in daily meals influences skeletal muscle hypertrophy via the muscle clock. Cell Rep. 2021, 36, 109336. [Google Scholar] [CrossRef]
- Nagao, T.; Hase, T.; Tokimitsu, I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 2007, 15, 1473–1483. [Google Scholar] [CrossRef]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef]
- Takahashi, M.; Ozaki, M.; Tsubosaka, M.; Kim, H.K.; Sasaki, H.; Matsui, Y.; Hibi, M.; Osaki, N.; Miyashita, M.; Shibata, S. Effects of Timing of Acute and Consecutive Catechin Ingestion on Postprandial Glucose Metabolism in Mice and Humans. Nutrients 2020, 12, 565. [Google Scholar] [CrossRef] [Green Version]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef]
- Turner, N.; Hariharan, K.; TidAng, J.; Frangioudakis, G.; Beale, S.M.; Wright, L.E.; Zeng, X.Y.; Leslie, S.J.; Li, J.Y.; Kraegen, E.W.; et al. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: Potent tissue-specific effects of medium-chain fatty acids. Diabetes 2009, 58, 2547–2554. [Google Scholar] [CrossRef] [Green Version]
- Matualatupauw, J.C.; Bohl, M.; Gregersen, S.; Hermansen, K.; Afman, L.A. Dietary medium-chain saturated fatty acids induce gene expression of energy metabolism-related pathways in adipose tissue of abdominally obese subjects. Int. J. Obes. 2017, 41, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, C.; Zhang, Y.; Xu, Q.; Yu, X.; Zhang, X.; Wang, J.; Zhang, R.; Gong, X.; Guo, C. Triglyceride with medium-chain fatty acids increases the activity and expression of hormone-sensitive lipase in white adipose tissue of C57BL/6J mice. Biosci. Biotechnol. Biochem. 2011, 75, 1939–1944. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Zhang, Y.; Xu, Q.; Yu, X.M.; Zhang, X.S.; Wang, J.; Xue, C.; Yang, X.Y.; Zhang, R.X.; Xue, C.Y. Increased norepinephrine by medium-chain triglyceride attributable to lipolysis in white and brown adipose tissue of C57BL/6J mice. Biosci. Biotechnol. Biochem. 2012, 76, 1213–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Ezaki, O.; Suzuki, M. Effects of Timing of Medium-Chain Triglycerides (8:0 and 10:0) Supplementation during the Day on Muscle Mass, Function and Cognition in Frail Elderly Adults. J. Frailty Aging 2022, 11, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Anthonsen, M.W.; Rönnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.J.; Patel, S.; Natu, V.; Kraemer, F.B. Mutational analysis of structural features of rat hormone-sensitive lipase. Biochemistry 1998, 37, 8973–8979. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Shen, W.J.; Muliro, K.; Patel, S.; Souza, S.C.; Roth, R.A.; Kraemer, F.B. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J. Biol. Chem. 2001, 276, 45456–45461. [Google Scholar] [CrossRef] [Green Version]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Malapaka, R.R.; Khoo, S.; Zhang, J.; Choi, J.H.; Zhou, X.E.; Xu, Y.; Gong, Y.; Li, J.; Yong, E.L.; Chalmers, M.J.; et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J. Biol. Chem. 2012, 287, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.; Saidemberg, D.; Deng, T.; et al. Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) γ activators and pan-PPAR partial agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef] [Green Version]
- Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Hasty, A.H.; Osuga, J.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 1999, 274, 35832–35839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schadinger, S.E.; Bucher, N.L.; Schreiber, B.M.; Farmer, S.R. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E1195–E1205. [Google Scholar] [CrossRef]
- Kroker, A.J.; Bruning, J.B. Review of the Structural and Dynamic Mechanisms of PPARγ Partial Agonism. PPAR Res. 2015, 2015, 816856. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Hirasaka, K.; Kohno, S.; Tomida, C.; Haruna, M.; Uchida, T.; Ohno, A.; Oarada, M.; Teshima-Kondo, S.; Okumura, Y.; et al. Capric Acid Up-Regulates UCP3 Expression without PDK4 Induction in Mouse C2C12 Myotubes. J. Nutr. Sci. Vitaminol. 2016, 62, 32–39. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Sjøberg, K.A.; Kleinert, M.; Richter, E.A.; Kiens, B. Small Amounts of Dietary Medium-Chain Fatty Acids Protect Against Insulin Resistance During Caloric Excess in Humans. Diabetes 2021, 70, 91–98. [Google Scholar] [CrossRef]
- Kreymann, B.; Williams, G.; Ghatei, M.A.; Bloom, S.R. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet 1987, 2, 1300–1304. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Harada, N.; Yamane, S.; Iwasaki, K.; Ikeguchi, E.; Kanemaru, Y.; Harada, T.; Sankoda, A.; Shimazu-Kuwahara, S.; Joo, E.; et al. Medium-chain triglyceride diet stimulates less GIP secretion and suppresses body weight and fat mass gain compared with long-chain triglyceride diet. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E53–E64. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Harada, N.; Kishino, S.; Iwasaki, K.; Ikeguchi-Ogura, E.; Yamane, S.; Kato, T.; Kanemaru, Y.; Sankoda, A.; Hatoko, T.; et al. Medium-chain triglycerides inhibit long-chain triglyceride-induced GIP secretion through GPR120-dependent inhibition of CCK. iScience 2021, 24, 102963. [Google Scholar] [CrossRef]
- Nonaka, H.; Ohue-Kitano, R.; Masujima, Y.; Igarashi, M.; Kimura, I. Dietary Medium-Chain Triglyceride Decanoate Affects Glucose Homeostasis Through GPR84-Mediated GLP-1 Secretion in Mice. Front. Nutr. 2022, 9, 848450. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Morgan, L.M.; Tredger, J.A.; Deacon, S.; Wright, J.; Marks, V. Glucagon-like peptide-1 (7-36) amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: Acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993, 138, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Yasumoto, Y.; Higo-Yamamoto, S.; Yamamoto, S.; Ohkura, N. Feeding cycle-dependent circulating insulin fluctuation is not a dominant Zeitgeber for mouse peripheral clocks except in the liver: Differences between endogenous and exogenous insulin effects. Biochem. Biophys. Res. Commun. 2017, 483, 165–170. [Google Scholar] [CrossRef]
| Ingredients (g/100 g) | HFHSD | M-HFHSD |
|---|---|---|
| Casein | 25 | 25 |
| Cystine | 0.375 | 0.375 |
| Cornstarch | 14.869 | 14.869 |
| Sucrose | 20 | 20 |
| Beef tallow | 14 | 7 |
| Lard | 14 | 8 |
| MCT oil | 0 | 13 |
| Soybean oil | 2 | 2 |
| Cellulose | 5 | 5 |
| Mineral mixture, AIN-93 | 3.5 | 3.5 |
| Vitamin mixture, AIN-93 | 1 | 1 |
| Choline bitartrate | 0.25 | 0.25 |
| Tert-butyl hydroquinone | 0.006 | 0.006 |
| Fat (% of energy) | 53.9 | 53.9 |
| Total energy (MJ/100 g diet) | 1.99 | 1.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, T. Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet. Nutrients 2022, 14, 5096. https://doi.org/10.3390/nu14235096
Abe T. Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet. Nutrients. 2022; 14(23):5096. https://doi.org/10.3390/nu14235096
Chicago/Turabian StyleAbe, Tomoki. 2022. "Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet" Nutrients 14, no. 23: 5096. https://doi.org/10.3390/nu14235096
APA StyleAbe, T. (2022). Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet. Nutrients, 14(23), 5096. https://doi.org/10.3390/nu14235096





