Implementation of Nutrigenetics and Nutrigenomics Research and Training Activities for Developing Precision Nutrition Strategies in Malaysia
Abstract
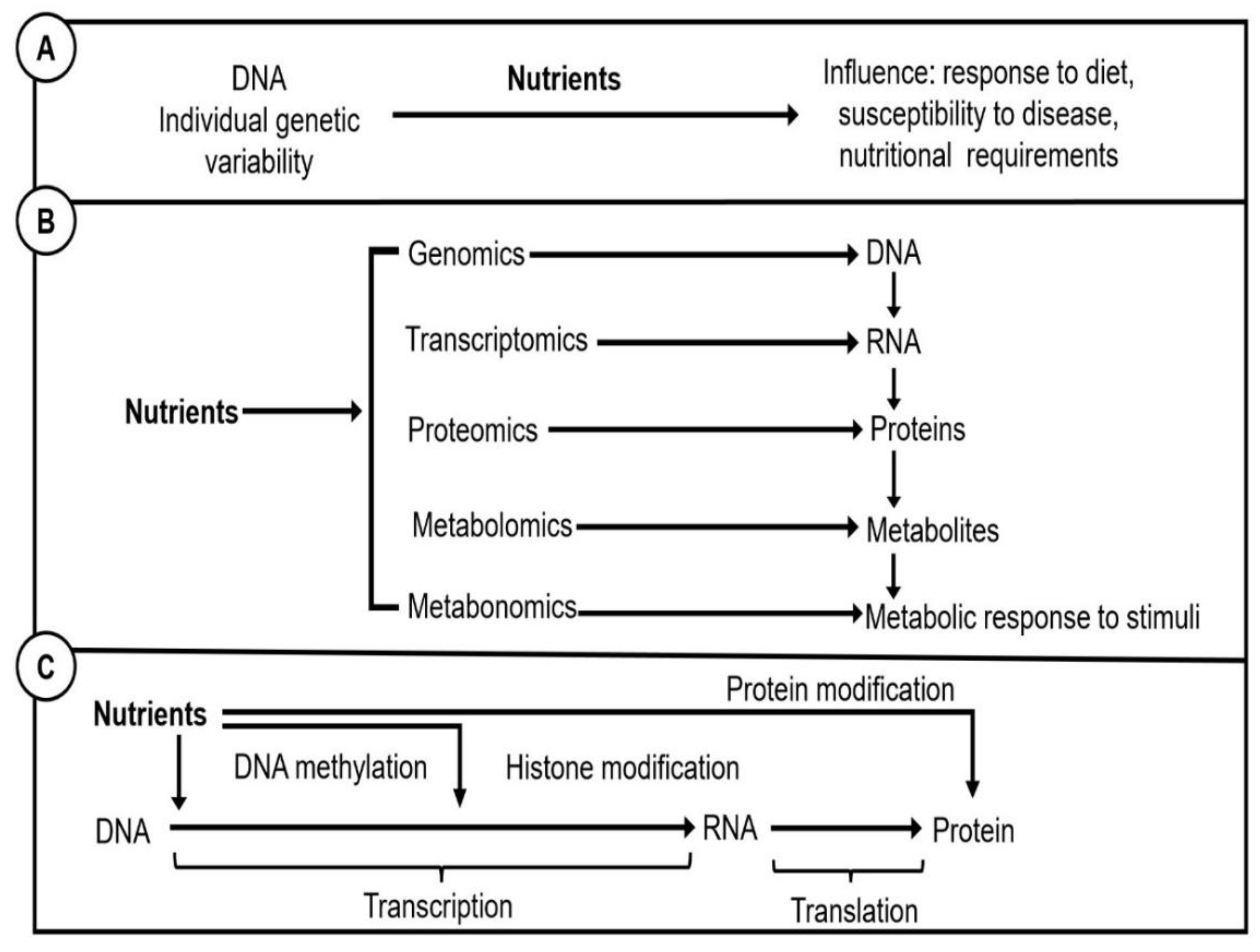
1. Introduction
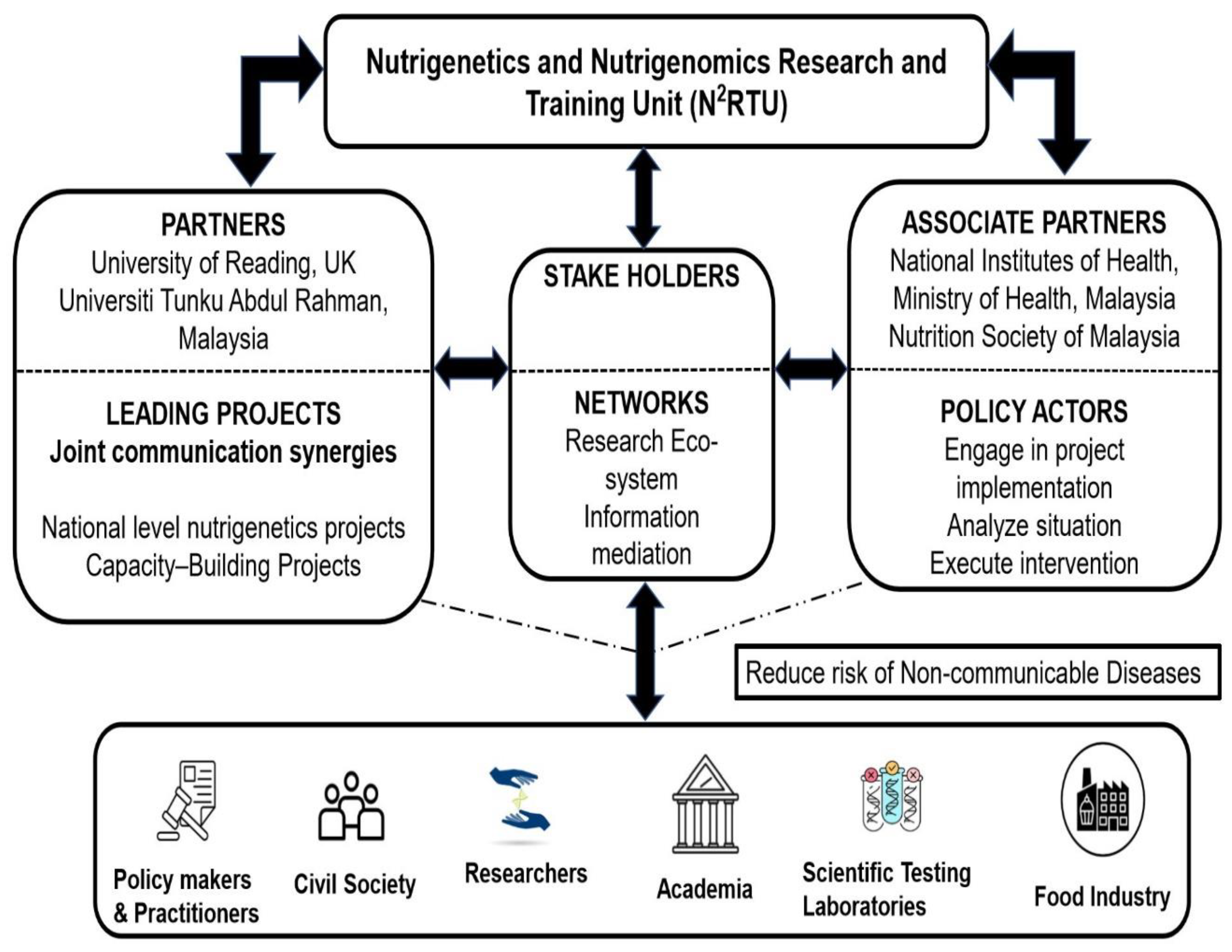
2. N2RTU Framework Implementation Overview
3. Implementing a Nutrigenetics and Nutrigenomics Research Unit
4. Implementing Nutrigenetics and Nutrigenomics Training for Stakeholders for Precision Nutrition in Malaysia
4.1. Academia
4.2. Healthcare Professionals (HCPs)
4.3. Policymakers
4.4. Food Industry
5. Future Implications of Information Systems and N2RTU in Artificial Intelligence
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Nutrition Report. Country Nutrition Profiles, Malaysia: The Burden of Malnutrition at a Glance. Available online: https://globalnutritionreport.org/resources/nutrition-profiles/asia/south-eastern-asia/malaysia/ (accessed on 25 April 2022).
- United Nations Environment Programme: National Plan of Action for Nutrition of Malaysia III (2016–2025). Available online: https://nutrition.moh.gov.my/wpcontent/uploads/2016/12/NPANM_III.pdf (accessed on 26 April 2022).
- Global Health Observatory Data Repository. Children Aged < 5 Years Stunted: Data by Country. Available online: https://apps.who.int/gho/data/view.main.CHILDSTUNTEDv (accessed on 25 April 2022).
- Global Health Observatory Data Repository. Low Birth Weight: Data by Country. Available online: https://apps.who.int/gho/data/view.main.LBWCOUNTRYv (accessed on 25 April 2022).
- Global Health Observatory Data Repository. Prevalence of Anaemia in Pregnant Women: Estimates by Country. Available online: https://apps.who.int/gho/data/view.main.ANAEMIAWOMENPWv (accessed on 25 April 2022).
- Global Health Observatory Data Repository. Anaemia Women of Reproductive Age: Estimates by Country. Available online: https://apps.who.int/gho/data/view.main.ANAEMIAWOMENREPRODUCTIVECOUNTRYv (accessed on 25 April 2022).
- Global Health Observatory Data Repository: Prevalence of Obesity among Adults, BMI ≥ 30: Crude Estimates by Country. Available online: https://apps.who.int/gho/data/view.main.BMI30Cv (accessed on 25 April 2022).
- Ministry of Health Malaysia: National Health and Morbidity Survey (NHMS). 2019. Available online: https://iku.moh.gov.my/nhms-2019 (accessed on 26 April 2022).
- Institute for Health Metrics and Evaluation, Malaysia. Available online: https://www.healthdata.org/malaysia (accessed on 29 April 2022).
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; De Luis, D.A.; Gil, Á. Guide and position of the international society of nutrigenetics/nutrigenomics on personalised nutrition: Part 1-fields of precision nutrition. Lifestyle Genom. 2016, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and diet in the prevention of chronic diseases in future generations. Int. J. Mol. Sci. 2020, 21, 2633. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Ong, T.J. Nutritional genomics. BMJ 2002, 324, 1438–1442. [Google Scholar] [CrossRef]
- Abete, I.; Navas-Carretero, S.; Marti, A.; Martinez, J.A. Nutrigenetics and nutrigenomics of caloric restriction. Prog. Mol. Biol. Transl. Sci. 2012, 108, 323–346. [Google Scholar]
- Hatin, W.I.; Nur-Shafawati, A.R.; Etemad, A.; Jin, W.; Qin, P.; Xu, S.; Jin, L.; Tan, S.-G.; Limprasert, P.; Feisal, M.A. A genome wide pattern of population structure and admixture in peninsular Malaysia Malays. HUGO J. 2014, 8, 1–18. [Google Scholar] [CrossRef]
- Rampal, S.; Mahadeva, S.; Guallar, E.; Bulgiba, A.; Mohamed, R.; Rahmat, R.; Arif, M.T.; Rampal, L. Ethnic differences in the prevalence of metabolic syndrome: Results from a multi-ethnic population-based survey in Malaysia. PLoS ONE 2012, 7, e46365. [Google Scholar] [CrossRef] [PubMed]
- Ihab, A.N.; Rohana, A.; Manan, W.W.; Suriati, W.W.; Zalilah, M.S.; Rusli, A.M. The coexistence of dual form of malnutrition in a sample of rural Malaysia. Int. J. Prev. Med. 2013, 4, 690–699. [Google Scholar]
- Blankenship, J.L.; Rudert, C.; Aguayo, V.M. Triple trouble: Understanding the burden of child undernutrition, micronutrient deficiencies, and overweight in East Asia and the Pacific. Matern. Child Nutr. 2020, 16, e12950. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Selamat, R.; Salleh, R.; Majid, N.L.A.; Zainuddin, A.A.; Bakar, W.A.M.A.; Aris, T. Food insecurity situation in Malaysia: Findings from malaysian adult nutrition survey (MANS) 2014. Malays. J. Public Health Med. 2020, 20, 167–174. [Google Scholar] [CrossRef]
- Ali, A.; Gan, H.J.; Yusof, H.; Kamarudin, K.S.; Zainudin, A. Food classification system based on food processing and its relationship with nutritional status of adults in Terengganu, Malaysia. J. Food Sci. 2019, 4, 539–546. [Google Scholar]
- Khasbullah, N.A.; Ahmad, F.T.; Yusof, H.M. Ultra-processed food consumption in relation to BMI and body fat percentage of adults in Terengganu. Malays. J. Med. Health Sci. 2020, 16, 37–43. [Google Scholar]
- Ali, A.; Wan Syakirah Alia, W.M.S.; Aziz, Y.; Yusof, H. Energy contribution of NOVA food groups and socio-demographic determinants of ultra-processed groups among adults in Terengganu, Malaysia. Food Res. 2019, 3, 640–648. [Google Scholar] [CrossRef]
- Ching, Y.K.; Chin, Y.S.; Appukutty, M.; Gan, W.Y.; Ramanchadran, V.; Chan, Y.M. Prevalence of Metabolic Syndrome and Its Associated Factors among Vegetarians in Malaysia. Int. J. Environ. Res. Public Health 2018, 15, 2031. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Precision (personalized) nutrition: Understanding metabolic heterogeneity. Annu. Rev. Food Sci. Technol. 2020, 11, 71–92. [Google Scholar] [CrossRef]
- Looi, L.-M.; Prentice, A.; Griffin, G.; Jebb, S.; Khor, G.L.; Poston, L.; Veerakumarasivam, A.; Wareham, N.; Lee, Y.Y.; Zain, A. Addressing the Global Health Challenge of Obesity in Malaysia Workshop Report; The Academy of Medical Sciences: London, UK, 2018. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S. A nutrigenetics approach to study the impact of genetic and lifestyle factors on cardiometabolic traits in various ethnic groups: Findings from the GeNuIne Collaboration. Proc. Nutr. Soc. 2020, 79, 194–204. [Google Scholar] [CrossRef]
- Keathley, J.; Garneau, V.; Zavala-Mora, D.; Heister, R.R.; Gauthier, E.; Morin-Bernier, J.; Green, R.; Vohl, M.-C. A Systematic Review and Recommendations Around Frameworks for Evaluating Scientific Validity in Nutritional Genomics. Front. Nutr. 2021, 8, 789215. [Google Scholar] [CrossRef]
- Zeisel, S.H. A conceptual framework for studying and investing in precision nutrition. Front. Genet. 2019, 10, 200. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Kim, H.-S.; Prakash, V.; Ramos-Lopez, O.; Zotor, F.; Martinez, J.A. Personalised, population and planetary nutrition for precision health. BMJ Nutr. Prev. Health 2021, 4, 355. [Google Scholar] [CrossRef]
- Horne, J.R.; Nielsen, D.E.; Madill, J.; Robitaille, J.; Vohl, M.-C.; Mutch, D.M. Guiding global best practice in personalized nutrition based on genetics: The development of a nutrigenomics care map. J. Acad. Nutr. Diet. 2022, 122, 259–269. [Google Scholar] [CrossRef]
- Halim-Fikri, H.; Etemad, A.; Latif, A.Z.A.; Merican, A.F.; Baig, A.A.; Annuar, A.A.; Ismail, E.; Salahshourifar, I.; Liza-Sharmini, A.T.; Ramli, M. The first Malay database toward the ethnic-specific target molecular variation. BMC Res. Notes 2015, 8, 176. [Google Scholar] [CrossRef][Green Version]
- Mustapa, M.A.C.; Amin, L.; Frewer, L.J. Predictors of stakeholders’ intention to adopt nutrigenomics. Genes Nutr. 2020, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, H.; Liu, S. Applications of systems science in biomedical research regarding obesity and noncommunicable chronic diseases: Opportunities, promise, and challenges. Adv. Nutr. 2015, 6, 88–95. [Google Scholar] [CrossRef][Green Version]
- Khorraminezhad, L.; Leclercq, M.; Droit, A.; Bilodeau, J.F.; Rudkowska, I. Statistical and Machine-Learning Analyses in Nutritional Genomics Studies. Nutrients 2020, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019, 19, 281. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Sokolovska, N.; Brazeilles, R.; Affeldt, S.; Pelloux, V.; Prifti, E.; Chilloux, J.; Verger, E.O.; Kayser, B.D.; Aron-Wisnewsky, J.; et al. A Data Integration Multi-Omics Approach to Study Calorie Restriction-Induced Changes in Insulin Sensitivity. Front. Physiol. 2018, 9, 1958. [Google Scholar] [CrossRef]
- Boulesteix, A.-L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2006, 8, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S. Gene–nutrient interactions on metabolic diseases: Findings from the GeNuIne Collaboration. Nutr. Bull. 2017, 42, 80–86. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Bodhini, D.; Lakshmipriya, N.; Ramya, K.; Anjana, R.M.; Sudha, V.; Lovegrove, J.A.; Kinra, S.; Mohan, V.; Radha, V. Interaction between FTO gene variants and lifestyle factors on metabolic traits in an Asian Indian population. Nutr. Metab. 2016, 13, 39. [Google Scholar] [CrossRef]
- Surendran, S.; Vimaleswaran, K. A nutrigenetic approach to examine the relationship between vitamin B12 status and cardio-metabolic traits in multiple ethnic groups–findings from the GeNuIne Collaboration. Nutr. Bull. 2021, 46, 185–194. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S. GeNuIne (gene-nutrient interactions) Collaboration: Towards implementing multi-ethnic population-based nutrigenetic studies of vitamin B(12) and D deficiencies and metabolic diseases. Proc. Nutr. Soc. 2021, 80, 435–445. [Google Scholar] [CrossRef]
- Alathari, B.E.; Bodhini, D.; Jayashri, R.; Lakshmipriya, N.; Shanthi Rani, C.S.; Sudha, V.; Lovegrove, J.A.; Anjana, R.M.; Mohan, V.; Radha, V.; et al. A Nutrigenetic Approach to Investigate the Relationship between Metabolic Traits and Vitamin D Status in an Asian Indian Population. Nutrients 2020, 12, 1357. [Google Scholar] [CrossRef] [PubMed]
- Ayyappa, K.A.; Shatwan, I.; Bodhini, D.; Bramwell, L.R.; Ramya, K.; Sudha, V.; Anjana, R.M.; Lovegrove, J.A.; Mohan, V.; Radha, V.; et al. High fat diet modifies the association of lipoprotein lipase gene polymorphism with high density lipoprotein cholesterol in an Asian Indian population. Nutr. Metab. 2017, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Bodhini, D.; Gaal, S.; Shatwan, I.; Ramya, K.; Ellahi, B.; Surendran, S.; Sudha, V.; Anjana, M.R.; Mohan, V.; Lovegrove, J.A.; et al. Interaction between TCF7L2 polymorphism and dietary fat intake on high density lipoprotein cholesterol. PLoS ONE 2017, 12, e0188382. [Google Scholar] [CrossRef]
- Shatwan, I.M.; Minihane, A.M.; Williams, C.M.; Lovegrove, J.A.; Jackson, K.G.; Vimaleswaran, K.S. Impact of Lipoprotein Lipase Gene Polymorphism, S447X, on Postprandial Triacylglycerol and Glucose Response to Sequential Meal Ingestion. Int. J. Mol. Sci. 2016, 17, 397. [Google Scholar] [CrossRef]
- Shatwan, I.M.; Weech, M.; Jackson, K.G.; Lovegrove, J.A.; Vimaleswaran, K.S. Apolipoprotein E gene polymorphism modifies fasting total cholesterol concentrations in response to replacement of dietary saturated with monounsaturated fatty acids in adults at moderate cardiovascular disease risk. Lipids Health Dis. 2017, 16, 222. [Google Scholar] [CrossRef]
- Shatwan, I.M.; Winther, K.H.; Ellahi, B.; Elwood, P.; Ben-Shlomo, Y.; Givens, I.; Rayman, M.P.; Lovegrove, J.A.; Vimaleswaran, K.S. Association of apolipoprotein E gene polymorphisms with blood lipids and their interaction with dietary factors. Lipids Health Dis. 2018, 17, 98. [Google Scholar] [CrossRef]
- Surendran, S.; Vimaleswaran, K.S. The influence of one-carbon metabolism gene polymorphisms and gene-environment interactions on homocysteine, Vitamin B12, folate and lipids in a Brazilian adolescent population. J. Diabetol. 2019, 10, 110–122. [Google Scholar]
- Vimaleswaran, K.S.; Minihane, A.M.; Li, Y.; Gill, R.; Lovegrove, J.A.; Williams, C.M.; Jackson, K.G. The APOB insertion/deletion polymorphism (rs17240441) influences postprandial lipaemia in healthy adults. Nutr. Metab. 2015, 12, 7. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Zhou, A.; Cavadino, A.; Hyppönen, E. Evidence for a causal association between milk intake and cardiometabolic disease outcomes using a two-sample Mendelian Randomization analysis in up to 1,904,220 individuals. Int. J. Obes. 2021, 45, 1751–1762. [Google Scholar] [CrossRef]
- Isgin-Atici, K.; Alathari, B.E.; Turan-Demirci, B.; Sendur, S.N.; Lay, I.; Ellahi, B.; Alikasifoglu, M.; Erbas, T.; Buyuktuncer, Z.; Vimaleswaran, K.S. Interaction between Dietary Fat Intake and Metabolic Genetic Risk Score on 25-Hydroxyvitamin D Concentrations in a Turkish Adult Population. Nutrients 2022, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Cavadino, A.; Verweij, N.; Nolte, I.M.; Leach, I.M.; Auvinen, J.; Veijola, J.; Elliott, P.; Penninx, B.W.; Snieder, H. Interactions between uncoupling protein 2 gene polymorphisms, obesity and alcohol intake on liver function: A large meta-analysed population-based study. Eur. J. Endocrinol. 2015, 173, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.; Aji, A.; Ariyasra, U.; Sari, S.; Malik, S.; Tasrif, N.; Yani, F.; Lovegrove, J.A.; Sudji, I.; Lipoeto, N. A nutrigenetic approach for investigating the relationship between vitamin B12 status and metabolic traits in Indonesian women. J. Diabetes Metab. Disord. 2019, 18, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Cruvinel, N.T.; da Silva, N.R.; Chandrabose, M.; Lovegrove, J.A.; Horst, M.A.; Vimaleswaran, K.S. Impact of Genetic Risk Score and Dietary Protein Intake on Vitamin D Status in Young Adults from Brazil. Nutrients 2022, 14, 1015. [Google Scholar] [CrossRef]
- Wuni, R.; Adela Nathania, E.; Ayyappa, A.K.; Lakshmipriya, N.; Ramya, K.; Gayathri, R.; Geetha, G.; Anjana, R.M.; Kuhnle, G.G.C.; Radha, V.; et al. Impact of Lipid Genetic Risk Score and Saturated Fatty Acid Intake on Central Obesity in an Asian Indian Population. Nutrients 2022, 14, 2713. [Google Scholar] [CrossRef]
- de Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef]
- Alsulami, S.; Nyakotey, D.A.; Dudek, K.; Bawah, A.M.; Lovegrove, J.A.; Annan, R.A.; Ellahi, B.; Vimaleswaran, K.S. Interaction between Metabolic Genetic Risk Score and Dietary Fatty Acid Intake on Central Obesity in a Ghanaian Population. Nutrients 2020, 12, 1906. [Google Scholar] [CrossRef]
- Alathari, B.E.; Aji, A.S.; Ariyasra, U.; Sari, S.R.; Tasrif, N.; Yani, F.F.; Sudji, I.R.; Lovegrove, J.A.; Lipoeto, N.I.; Vimaleswaran, K.S. Interaction between Vitamin D-Related Genetic Risk Score and Carbohydrate Intake on Body Fat Composition: A Study in Southeast Asian Minangkabau Women. Nutrients 2021, 13, 326. [Google Scholar] [CrossRef]
- Connaugton, R.M.; McMorrow, A.M.; Healy, M.L.; McGillicuddy, F.C.; Lithander, F.E.; Roche, H.M. An anti-inflammatory nutritional intervention selectively improves insulin sensitivity in overweight and obese adolescents wherein baseline metabotype predicts response. Proc. Nutr. Soc. 2014, 73, E84. [Google Scholar] [CrossRef]
- Riedl, A.; Gieger, C.; Hauner, H.; Daniel, H.; Linseisen, J. Metabotyping and its application in targeted nutrition: An overview. Br. J. Nutr. 2017, 117, 1631–1644. [Google Scholar] [CrossRef]
- Muda, W.M.W.; Sundaram, J.K.; Gen, T.Z. Addressing Malnutrition in Malaysia; Khazanah Research Institute: Kuala Lumpur, Malaysia, 2019. [Google Scholar]
- Zayts, O.; Sarangi, S.; Thong, M.K.; Chung, B.H.-Y.; Lo, I.F.-M.; Kan, A.S.-Y.; Lee, J.M.H.; Padilla, C.D.; Cutiongco-de la Paz, E.M.; Faradz, S.M. Genetic counseling/consultation in South-East Asia: A report from the workshop at the 10th Asia Pacific conference on human genetics. J. Genet. Couns. 2013, 22, 917–924. [Google Scholar] [CrossRef] [PubMed]
- MyHVP. The Malaysian Node of The Human Variome Project. Available online: http://hvpmalaysia.kk.usm.my/about.php (accessed on 17 May 2022).
- Williams, R.; Periasamy, M. Genetic and environmental factors contributing to visceral adiposity in Asian populations. Endocrinol. Metab. 2020, 35, 681. [Google Scholar] [CrossRef]
- Lim, S.Y.; Zalilah, M.S.; Chin, Y.S.; Ramachandran, V.; Chan, Y.M. Dietary Acid Load, IGF-1 Single Nucleotide Polymorphism and Bone Resorption among Postmenopausal Chinese Women. Nutrients 2018, 10, 915. [Google Scholar] [CrossRef]
- Say, Y.H.; Sio, Y.Y.; Heng, A.H.S.; Ng, Y.T.; Matta, S.A.; Pang, S.L.; Teh, K.F.; Wong, Y.R.; Rawanan Shah, S.M.; Reginald, K. Golgin A7 family member B (GOLGA7B) is a plausible novel gene associating high glycaemic index diet with acne vulgaris. Exp. Dermatol. 2022, 31, 1208–1219. [Google Scholar] [CrossRef]
- Lee, S.S.; Ling, K.H.; Tusimin, M.; Subramaniam, R.; Rahim, K.F.; Loh, S.P. Interplay between Maternal and Neonatal Vitamin D Deficiency and Vitamin-D-Related Gene Polymorphism with Neonatal Birth Anthropometry. Nutrients 2022, 14, 564. [Google Scholar] [CrossRef]
- Mitra, S.R.; Tan, P.Y.; Amini, F. Association of ADRB2 rs1042713 with obesity and obesity-related phenotypes and its interaction with dietary fat in modulating glycaemic indices in Malaysian adults. J. Nutr. Metab. 2019, 2019, 8718795. [Google Scholar] [CrossRef]
- Abdullah, N.; Murad, N.A.; Haniff, E.M.; Syafruddin, S.E.; Attia, J.; Oldmeadow, C.; Kamaruddin, M.; Abd Jalal, N.; Ismail, N.; Ishak, M. Predicting type 2 diabetes using genetic and environmental risk factors in a multi-ethnic Malaysian cohort. Public Health 2017, 149, 31–38. [Google Scholar] [CrossRef]
- Too, C.L.; Yahya, A.; Murad, S.; Dhaliwal, J.S.; Larsson, P.T.; Muhamad, N.A.; Abdullah, N.A.; Mustafa, A.N.; Klareskog, L.; Alfredsson, L. Smoking interacts with HLA-DRB1 shared epitope in the development of anti-citrullinated protein antibody-positive rheumatoid arthritis: Results from the Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA). Arthritis Res. Ther. 2012, 14, R89. [Google Scholar] [CrossRef] [PubMed]
- NRP Technical Working Group on Nutrition Research; Ministry of Health Malaysia. Nutrition Research Priorities in Malaysia for 12th Malaysia Plan (2021–2025). Available online: https://nutrition.moh.gov.my/wp-content/uploads/2021/07/Nutrition-Research-Priorities-in-Msia-for-12th-MP-2021-2025.pdf (accessed on 25 September 2022).
- Nor Asiah, M.; Fatin Norhasny, L.; Nor Soleha, M.; Chun Lai, T.; Mohamad Zabri, J.; Mohammed Faizal, B.; Jaya, K.P.K. Health Research Priorities for 12th Malaysia Plan (12MP-HRP) 2021–2025; National Institutes of Health, Ministry of Health Malaysia: Shah Alam, Malaysia, 2021.
- Balasopoulou, A.; Mooy, F.-M.; Baker, D.J.; Mitropoulou, C.; Skoufas, E.; Bulgiba, A.; Katsila, T.; Patrinos, G.P. Advancing global precision medicine: An overview of genomic testing and counseling services in Malaysia. OMICS J. Integr. Biol. 2017, 21, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.R.; Gilliland, J.A.; O’Connor, C.P.; Seabrook, J.A.; Madill, J. Change in Weight, BMI, and Body Composition in a Population-Based Intervention Versus Genetic-Based Intervention: The NOW Trial. Obesity 2020, 28, 1419–1427. [Google Scholar] [CrossRef]
- Joffe, Y.; Herholdt, H. What will it take to build an expert group of nutrigenomic practitioners? Lifestyle Genom. 2020, 13, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Malaysia. National Nutrition Policy Malaysia 2.0. Available online: https://jeffreysachs.center/sites/default/files/content/210817%20Puan%20Zalma%20-%20Webinar%20SDG2%20-%20National%20Nutrition%20Policy%20of%20Malaysia.pdf (accessed on 2 August 2022).
- World Health Organisation. Global Database on the Implementation of Nutrition Action (GINA). Available online: https://extranet.who.int/nutrition/gina/en/node/59283 (accessed on 2 August 2022).
- Gibney, M.J.; Gibney, E.R. Diet, genes and disease: Implications for nutrition policy. Proc. Nutr. Soc 2004, 63, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Castle, D.; Ries, N.M. Ethical, legal and social issues in nutrigenomics: The challenges of regulating service delivery and building health professional capacity. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2007, 622, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Healthier Choice Logo. Available online: https://myhcl.moh.gov.my/index.php/site/home (accessed on 28 April 2022).
- Organisation for Economic Co-Operation and Development (OECD). The Heavy Burden of Obesity: The Economics of Prevention. Available online: https://www.oecd.org/health/the-heavy-burden-of-obesity-67450d67-en.htm (accessed on 2 August 2022).
- Khor, G.L. Food availability and the rising obesity prevalence in Malaysia. Int. e-J. Sci. Med. Educ. 2012, 6, S61–S68. [Google Scholar] [CrossRef]
- Kwon, D.Y. Personalized diet oriented by artificial intelligence and ethnic foods. J. Ethn. Foods 2020, 7, 10. [Google Scholar] [CrossRef]
- Uthpala, T.; Fernando, H.; Thibbotuwawa, A.; Jayasinghe, M. Importance of nutrigenomics and nutrigenetics in food Science. MOJ Food Process. Technol. 2020, 8, 114–119. [Google Scholar]
- Romero-Cortes, T.; López-Pérez, P.; Toledo, A.; Pérez-España, V.; Aparicio-Burgos, J.; Cuervo-Parra, J. Nutrigenomics and Nutrigenetics in Functional Foods. Int. J. Bio-Resour. Stress Manag. 2018, 9, 661–672. [Google Scholar] [CrossRef]
- Doherty, A.; Wall, A.; Khaldi, N.; Kussmann, M. Artificial intelligence in functional food ingredient discovery and characterisation: A focus on bioactive plant and food peptides. Front. Genet. 2021, 12, 768979. [Google Scholar] [CrossRef]
- Nutrition Department, Ministry of Health Malaysia. Nutrition Supervision Program. Available online: https://nutrition.moh.gov.my/en/program-pengawasan-pemakanan-2/ (accessed on 2 August 2022).
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Morgenstern, J.D.; Rosella, L.C.; Costa, A.P.; de Souza, R.J.; Anderson, L.N. Perspective: Big data and machine learning could help advance nutritional epidemiology. Adv. Nutr. 2021, 12, 621–631. [Google Scholar] [CrossRef]
- Srinivasan, B.; Lee, S.; Erickson, D.; Mehta, S. Precision nutrition—Review of methods for point-of-care assessment of nutritional status. Curr. Opin. Biotechnol. 2017, 44, 103–108. [Google Scholar] [CrossRef]
- Lange, K.W.; Hauser, J.; Lange, K.M.; Makulska-Gertruda, E.; Nakamura, Y.; Reissmann, A. Big data approaches to nutrition and health. CICSJ Bull. 2016, 34, 43. [Google Scholar]
- Kirk, D.; Catal, C.; Tekinerdogan, B. Precision nutrition: A systematic literature review. Comput. Biol. Med. 2021, 133, 104365. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanapal, A.C.T.A.; Wuni, R.; Ventura, E.F.; Chiet, T.K.; Cheah, E.S.G.; Loganathan, A.; Quen, P.L.; Appukutty, M.; Noh, M.F.M.; Givens, I.; et al. Implementation of Nutrigenetics and Nutrigenomics Research and Training Activities for Developing Precision Nutrition Strategies in Malaysia. Nutrients 2022, 14, 5108. https://doi.org/10.3390/nu14235108
Dhanapal ACTA, Wuni R, Ventura EF, Chiet TK, Cheah ESG, Loganathan A, Quen PL, Appukutty M, Noh MFM, Givens I, et al. Implementation of Nutrigenetics and Nutrigenomics Research and Training Activities for Developing Precision Nutrition Strategies in Malaysia. Nutrients. 2022; 14(23):5108. https://doi.org/10.3390/nu14235108
Chicago/Turabian StyleDhanapal, Anto Cordelia T. A., Ramatu Wuni, Eduard F. Ventura, Teh Kuan Chiet, Eddy S. G. Cheah, Annaletchumy Loganathan, Phoon Lee Quen, Mahenderan Appukutty, Mohd F. M. Noh, Ian Givens, and et al. 2022. "Implementation of Nutrigenetics and Nutrigenomics Research and Training Activities for Developing Precision Nutrition Strategies in Malaysia" Nutrients 14, no. 23: 5108. https://doi.org/10.3390/nu14235108
APA StyleDhanapal, A. C. T. A., Wuni, R., Ventura, E. F., Chiet, T. K., Cheah, E. S. G., Loganathan, A., Quen, P. L., Appukutty, M., Noh, M. F. M., Givens, I., & Vimaleswaran, K. S. (2022). Implementation of Nutrigenetics and Nutrigenomics Research and Training Activities for Developing Precision Nutrition Strategies in Malaysia. Nutrients, 14(23), 5108. https://doi.org/10.3390/nu14235108





