Cognitive Impairment Mediates the Association between Dietary Inflammation and Depressive Symptoms in the Elderly
Abstract
1. Introduction
2. Materials and Methods
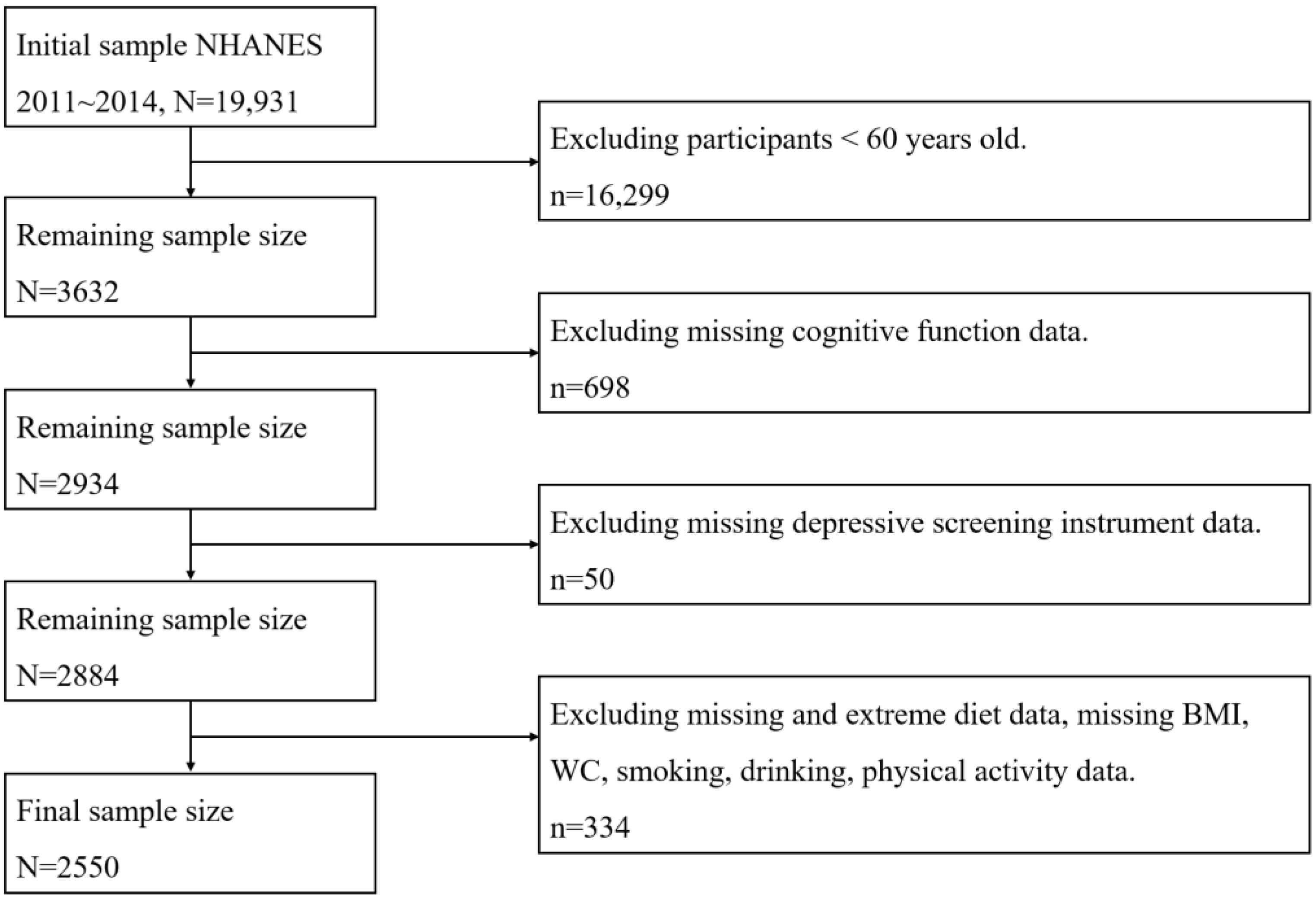
2.1. Sample
2.2. Data Measurement
2.2.1. Outcome Ascertainment
2.2.2. Exposure Measurement
Dietary Inflammatory Index (DII)
Cognitive Function
2.2.3. Covariate Assessment
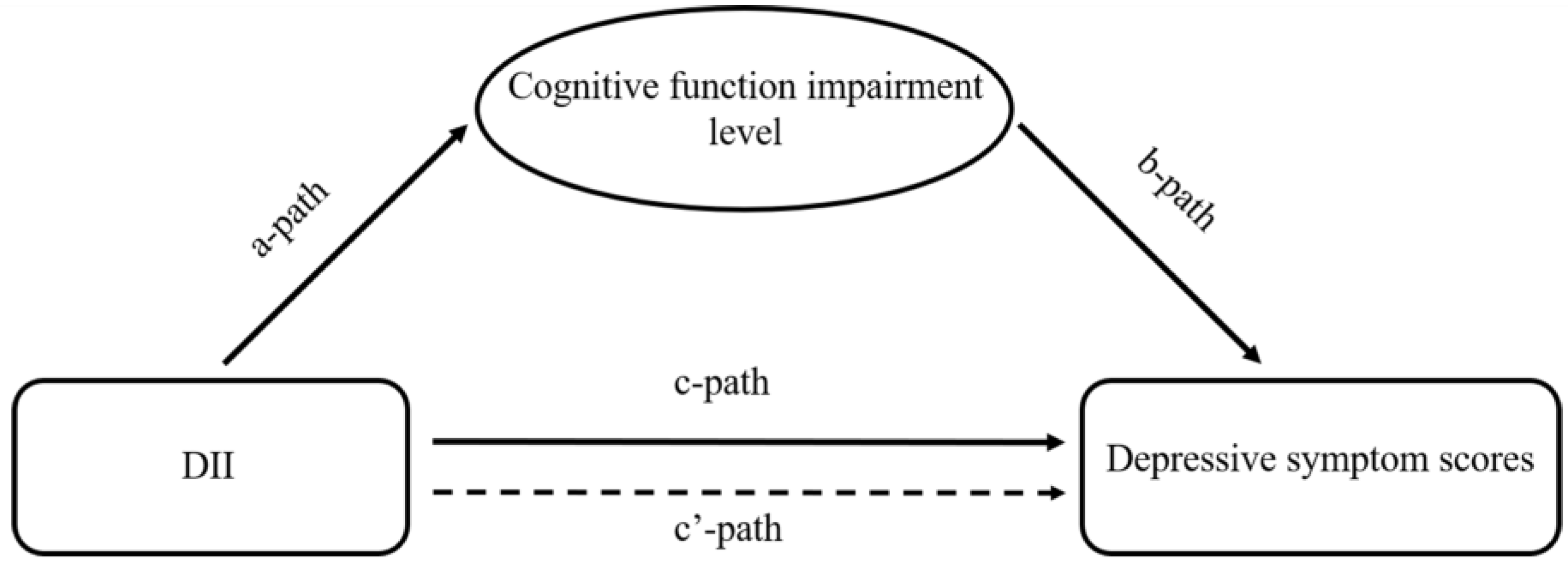
2.3. Statistical Analysis
2.4. Ethics Approval and Consent to Participate
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, R. Editorial: A Systematic Review of Depression. Curr. Neuropharmacol. 2015, 13, 480. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Wang, C.; Sun, M.; Jin, L.; Liu, X. Trends in depression among Adults in the United States, nhanes 2005–2016. J. Affect. Disord. 2020, 263, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Manzouri, L.; Babak, A.; Merasi, M. THE depression status of the elderly and it’s related factors in isfahan in 2007. Iran. J. Ageing 2010, 4. Available online: http://salmandj.uswr.ac.ir/article-1-302-en.html (accessed on 27 November 2022).
- Dumas, J.A. Strategies for Preventing Cognitive Decline in Healthy Older Adults. Can. J. Psychiatry 2017, 62, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Manly, J.J.; Jones, R.N.; Langa, K.M.; Ryan, L.H.; Levine, D.A.; McCammon, R.; Heeringa, S.G.; Weir, D. Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Choi, K.M.; Park, S.; Lee, S.H.; Im, C.H. Selection of the optimal channel configuration for implementing wearable EEG devices for the diagnosis of mild cognitive impairment. Alzheimers Res. Ther. 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, Z.; Reppermund, S.; Brodaty, H.; Crawford, J.D.; Draper, B.; Trollor, J.N.; Sachdev, P.S. Prevalence and characteristics of depression in mild cognitive impairment: The Sydney Memory and Ageing Study. Acta Psychiatr. Scand. 2013, 127, 394–402. [Google Scholar] [CrossRef]
- Ma, L. Depression, Anxiety, and Apathy in Mild Cognitive Impairment: Current Perspectives. Front. Aging Neurosci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Ott, C.V.; Vinberg, M.; Kessing, L.V.; Miskowiak, K.W. The effect of erythropoietin on cognition in affective disorders—Associations with baseline deficits and change in subjective cognitive complaints. Eur. Neuropsychopharmacol. 2016, 26, 1264–1273. [Google Scholar] [CrossRef]
- Chen, G.Q.; Peng, C.L.; Lian, Y.; Wang, B.W.; Chen, P.Y.; Wang, G.P. Association Between Dietary Inflammatory Index and Mental Health: A Systematic Review and Dose-Response Meta-Analysis. Front Nutr. 2021, 8, 662357. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Chen, K.; Jing, Y.; He, J.; Sun, H.; Hu, X. Dietary inflammatory index and depression: A meta-analysis. Public Health Nutr. 2018, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Beckett, C.W.; Niklison-Chirou, M.V. The role of immunomodulators in treatment-resistant depression: Case studies. Cell Death Discov. 2022, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Alderete, T.L. Targeting adipose tissue inflammation to treat the underlying basis of the metabolic complications of obesity. Nestle Nutr. Inst. Workshop. Ser. 2012, 73, 49–60. [Google Scholar] [PubMed]
- De Felice, F.G.; Ferreira, S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar] [CrossRef]
- Soory, M. Nutritional antioxidants and their applications in cardiometabolic diseases. Infect. Disord. Drug Targets 2012, 12, 388–401. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Tan, Q.Q.; Du, X.Y.; Gao, C.L.; Xu, Y. Higher Dietary Inflammatory Index Scores Increase the Risk of Diabetes Mellitus: A Meta-Analysis and Systematic Review. Front Endocrinol. 2021, 12, 693144. [Google Scholar] [CrossRef]
- Gialluisi, A.; Santonastaso, F.; Bonaccio, M.; Bracone, F.; Shivappa, N.; Hebert, J.R.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Circulating Inflammation Markers Partly Explain the Link Between the Dietary Inflammatory Index and Depressive Symptoms. J. Inflamm. Res. 2021, 14, 4955–4968. [Google Scholar] [CrossRef]
- Vicente, B.M.; Lucio, D.S.Q.M.; Maria, D.M.C.; Lima, R.S. The dietary inflammatory index (DII(R)) and its association with cognition, frailty, and risk of disabilities in older adults: A systematic review. Clin. Nutr. ESPEN 2020, 40, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Bureau, U.S.C. Current Population Survey (CPS)—Definitions and Explanations; Bureau, U.S.C.: Bluffton, CA, USA, 2008. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Davis, J.A.; Mohebbi, M.; Collier, F.; Loughman, A.; Staudacher, H.; Shivappa, N.; Hebert, J.R.; Pasco, J.A.; Jacka, F.N. The role of diet quality and dietary patterns in predicting muscle mass and function in men over a 15-year period. Osteoporos. Int. 2021, 32, 2193–2203. [Google Scholar] [CrossRef]
- Chen, S.P.; Bhattacharya, J.; Pershing, S. Association of Vision Loss With Cognition in Older Adults. JAMA Ophthalmol. 2017, 135, 963–970. [Google Scholar] [CrossRef]
- Chang, H.J.; Lin, K.R.; Lin, M.T.; Chang, J.L. Associations Between Lifestyle Factors and Reduced Kidney Function in US Older Adults: NHANES 1999–2016. Int. J. Public Health. 2021, 66, 1603966. [Google Scholar] [CrossRef]
- World Health Organization. Global Physical Acitvity Questionnaire Analysis Guide; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford: New York, NY, USA, 2013. [Google Scholar]
- Lumley, T. Survey: Analysis of Complex Survey Samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Assmann, K.E.; Andreeva, V.A.; Touvier, M.; Neufcourt, L.; Shivappa, N.; Hebert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Long-term association between the dietary inflammatory index and cognitive functioning: Findings from the SU.VI.MAX study. Eur. J. Nutr. 2017, 56, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Leira, Y.; Viana, J.; Machado, V.; Lyra, P.; Aldrey, J.M.; Pias-Peleteiro, J.M.; Blanco, J.; Sobrino, T.; Mendes, J.J. The Role of Inflammatory Diet and Vitamin D on the Link between Periodontitis and Cognitive Function: A Mediation Analysis in Older Adults. Nutrients 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Frith, E.; Shivappa, N.; Mann, J.R.; Hebert, J.R.; Wirth, M.D.; Loprinzi, P.D. Dietary inflammatory index and memory function: Population-based national sample of elderly Americans. Br. J. Nutr. 2018, 119, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. Mind versus metabolism in the control of food intake and energy balance. Physiol. Behav. 2004, 81, 781–793. [Google Scholar] [CrossRef]
- Higgs, S. Cognitive influences on food intake: The effects of manipulating memory for recent eating. Physiol. Behav. 2008, 94, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef]
- Ismail, Z.; Elbayoumi, H.; Fischer, C.E.; Hogan, D.B.; Millikin, C.P.; Schweizer, T.; Mortby, M.E.; Smith, E.E.; Patten, S.B.; Fiest, K.M. Prevalence of Depression in Patients With Mild Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Psychiat. 2017, 74, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Depression in the elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef]
- Phillips, C.M.; Shivappa, N.; Hebert, J.R.; Perry, I.J. Dietary inflammatory index and mental health: A cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin. Nutr. 2018, 37, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhan, W.; Huang, X.; Zhang, L.; Sun, Y.; Zhang, Z.; Bao, W.; Ma, Y. Investigating Associations Between Depressive Symptoms and Anti-/Pro-Inflammatory Nutrients in an Elderly Population in Northern China: A Bayesian Kernel Machine Regression Approach. J. Inflamm. Res. 2021, 14, 5201–5213. [Google Scholar] [CrossRef]
- Mourao, R.J.; Mansur, G.; Malloy-Diniz, L.F.; Castro, C.E.; Diniz, B.S. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: Systematic review and meta-analysis. Int. J. Geriatr. Psychiatry. 2016, 31, 905–911. [Google Scholar] [CrossRef]
- Sears, B. Anti-inflammatory Diets. J. Am. Coll. Nutr. 2015, 34 (Suppl. S1), 14–21. [Google Scholar] [CrossRef]
- Shakya, P.R.; Melaku, Y.A.; Shivappa, N.; Hebert, J.R.; Adams, R.J.; Page, A.J.; Gill, T.K. Dietary inflammatory index (DII(R)) and the risk of depression symptoms in adults. Clin. Nutr. 2021, 40, 3631–3642. [Google Scholar] [CrossRef]
| Characteristics | Anti-Inflammatory Diet (N = 1226) | Proinflammatory Diet (N = 1324) | χ2/t | p | |
|---|---|---|---|---|---|
| Age (Mean (SE)) a | 69.15 (0.33) | 68.79 (0.31) | −0.923 | 0.363 | |
| BMI (Mean (SE)) a | 28.56 (0.34) | 29.52 (0.34) | 2.210 | 0.035 | |
| WC (Mean (SE)) a | 101.86 (0.94) | 103.16 (0.66) | 1.237 | 0.226 | |
| Cognitive impairment level (Mean (SE)) a | 0.59 (0.04) | 0.89 (0.05) | 5.009 | <0.001 | |
| CERAD-immediate (Mean (SE)) a | 6.74 (0.09) | 6.45 (0.09) | −3.187 | 0.003 | |
| CERAD-delayed (Mean (SE)) a | 6.49 (0.12) | 6.19 (0.14) | −2.088 | 0.045 | |
| Animal Fluency test (Mean (SE)) a | 19.22 (0.26) | 17.13 (0.24) | −6.596 | <0.001 | |
| DSST (Mean (SE)) a | 55.26 (0.83) | 50.17 (0.79) | −4.861 | <0.001 | |
| Energy intake (Mean (SE)) a | 2239.42 (23.10) | 1552.70 (26.53) | −21.439 | <0.001 | |
| Gender (N (%)) b | 51.078 | <0.001 | |||
| Male | 688 (53.85) | 572 (39.71) | |||
| Female | 538 (46.15) | 752 (60.29) | |||
| Race (N (%)) b | 25.475 | <0.001 | |||
| Mexican American | 117 (3.36) | 112 (4.04) | |||
| Other Hispanic | 116 (3.65) | 150 (4.91) | |||
| Non-Hispanic White | 647 (80.45) | 598 (75.50) | |||
| Non-Hispanic Black | 220 (6.18) | 368 (10.92) | |||
| Other race | 126 (6.36) | 96 (4.63) | |||
| Smoking status (N (%)) b | 34.860 | <0.001 | |||
| Never | 599 (49.86) | 646 (51.98) | |||
| Current Smoker | 109 (6.95) | 209 (12.99) | |||
| Formal Smoker | 518 (43.19) | 469 (35.04) | |||
| Drinking status (N (%)) b | 68.042 | <0.001 | |||
| Never | 150 (8.62) | 225 (17.40) | |||
| Current drinker | 917 (80.08) | 858 (66.05) | |||
| Formal drinker | 159 (11.30) | 241 (16.55) | |||
| Physical activity (N (%)) b | 13.257 | 0.021 | |||
| Inactive | 550 (43.13) | 716 (50.33) | |||
| Active | 676 (56.87) | 608 (49.67) | |||
| Depressive symptoms (N (%)) b | 29.516 | 0.001 | |||
| No | 1138 (95.35) | 1185 (89.74) | |||
| Yes | 88 (4.65) | 139 (10.26) | |||
| Stroke (N (%)) b | 1.128 | 0.388 | |||
| No | 1153 (94.76) | 1239 (93.79) | |||
| Yes | 73 (5.24) | 88 (6.21) | |||
| Arthritis (N (%)) b | 0.370 | 0.772 | |||
| No | 648 (49.13) | 672 (50.34) | |||
| Yes | 578 (50.87) | 652 (49.66) | |||
| Congestive heart failure (N (%)) b | 1.171 | 0.407 | |||
| No | 1162 (94.15) | 1223 (93.10) | |||
| Yes | 64 (5.85) | 101 (6.90) | |||
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Inflammatory diet (reference = Anti-inflammatory) | 2.19 (1.23, 3.89) | 0.009 | 2.82 (0.98, 8.14) | 0.055 | 2.86 (1.01, 8.11) | 0.048 |
| Cognitive impairment level (reference = No) | 1.23 (1.05, 1.46) | 0.013 | 1.40 (1.19, 1.64) | <0.001 | 1.36 (1.16, 1.59) | <0.001 |
| Variables | OR | 95% CI | p | P-Interaction | |
|---|---|---|---|---|---|
| Gender | 0.119 | ||||
| Male (N = 1262) | 1.68 | 1.29–2.18 | <0.001 | ||
| Female (N = 1291) | 1.19 | 0.98–1.44 | 0.076 | ||
| Race | 0.110 | ||||
| Mexican American (N = 229) | 1.12 | 0.84–1.50 | 0.438 | ||
| Other Hispanic (N = 266) | 1.66 | 1.20–2.30 | 0.003 | ||
| Non-Hispanic White (N = 1246) | 1.25 | 0.94–1.67 | 0.117 | ||
| Non-Hispanic Black (N = 590) | 1.88 | 1.30–2.72 | 0.001 | ||
| Other race (N = 222) | 2.56 | 1.51–4.35 | 0.001 | ||
| Physical exercise | 0.744 | ||||
| Inactive (N = 1269) | 1.45 | 1.21–1.73 | <0.001 | ||
| Active (N = 1284) | 1.26 | 0.87–1.81 | 0.219 | ||
| Smoking status | 0.169 | ||||
| Non-smoker (N = 1247) | 1.25 | 0.97–1.60 | 0.078 | ||
| Current smoker (N = 318) | 0.92 | 0.64–1.33 | 0.656 | ||
| Former smoker (N = 988) | 1.75 | 1.38–2.21 | <0.001 | ||
| Drinking status | 0.113 | ||||
| Non-drinker (N = 375) | 1.49 | 1.04–2.13 | 0.030 | ||
| Current drinker (N = 1775) | 1.39 | 1.20–1.62 | <0.001 | ||
| Former drinker (N = 400) | 1.75 | 1.10–2.79 | 0.019 | ||
| Inflammatory Diet | 0.060 | ||||
| Proinflammation (N = 1327) | 1.32 | 1.04–1.67 | 0.023 | ||
| Anti-inflammation (N = 1226) | 1.50 | 1.21–1.85 | <0.001 | ||
| Stroke | 0.479 | ||||
| Yes (N = 161) | 1.59 | 1.02–2.46 | 0.039 | ||
| No (N = 2392) | 1.36 | 1.12–1.65 | 0.003 | ||
| Arthritis | 0.639 | ||||
| Yes (N = 1230) | 1.29 | 1.05–1.58 | 0.016 | ||
| No (N = 1323) | 1.38 | 1.08–1.75 | 0.010 | ||
| Congestive heart failure | 0.116 | ||||
| Yes (N = 165) | 0.69 | 0.37–1.29 | 0.235 | ||
| No (N = 2388) | 1.45 | 1.23–1.71 | <0.001 | ||
| Mediator | Exposure to Mediator | Mediator to Outcome | Direct Effect | Mediated (Indirect Effect) | Total Effect (Exposure to Outcome) | Proportion Mediated (%) |
|---|---|---|---|---|---|---|
| CERAD-immediate | −0.079 (0.019) p < 0.001 | −0.329 (0.059) p < 0.001 | 0.233 (0.057) p < 0.001 | 0.026 (0.008) 95% CI (0.011, 0.044) p = 0.002 | 0.259 (0.057) p < 0.001 | 10.0 |
| CERAD-delayed | −0.158 (0.029) p < 0.001 | −0.170 (0.040) p < 0.001 | 0.232 (0.058) p < 0.001 | 0.027 (0.009) 95% CI (0.012, 0.046) p = 0.002 | 0.259 (0.057) p < 0.001 | 10.4 |
| Animal fluency test | −0.490 (0.070) p < 0.001 | −0.089 (0.013) p < 0.001 | 0.215 (0.058) p < 0.001 | 0.044 (0.010) 95% CI (0.026, 0.066) p < 0.001 | 0.259 (0.057) p < 0.001 | 17.0 |
| DSST | −2.014 (0.205) p < 0.001 | −0.047 (0.006) p < 0.001 | 0.163 (0.058) p = 0.005 | 0.095 (0.015) 95% CI (0.068, 0.126) p < 0.001 | 0.259 (0.057) p < 0.001 | 36.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Wang, L.; Hu, Y.; Wang, X.; Yan, S.; Guo, Y.; Li, J.; Xie, Z.; Li, B. Cognitive Impairment Mediates the Association between Dietary Inflammation and Depressive Symptoms in the Elderly. Nutrients 2022, 14, 5118. https://doi.org/10.3390/nu14235118
Sun M, Wang L, Hu Y, Wang X, Yan S, Guo Y, Li J, Xie Z, Li B. Cognitive Impairment Mediates the Association between Dietary Inflammation and Depressive Symptoms in the Elderly. Nutrients. 2022; 14(23):5118. https://doi.org/10.3390/nu14235118
Chicago/Turabian StyleSun, Mengzi, Ling Wang, Yueyang Hu, Xuhan Wang, Shoumeng Yan, Yinpei Guo, Jing Li, Zechun Xie, and Bo Li. 2022. "Cognitive Impairment Mediates the Association between Dietary Inflammation and Depressive Symptoms in the Elderly" Nutrients 14, no. 23: 5118. https://doi.org/10.3390/nu14235118
APA StyleSun, M., Wang, L., Hu, Y., Wang, X., Yan, S., Guo, Y., Li, J., Xie, Z., & Li, B. (2022). Cognitive Impairment Mediates the Association between Dietary Inflammation and Depressive Symptoms in the Elderly. Nutrients, 14(23), 5118. https://doi.org/10.3390/nu14235118







