Effects of Milk Polar Lipids on DSS-Induced Colitis Severity Are Dependent on Dietary Fat Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Disease Activity Index
2.3. Colon RNA Isolation, cDNA Synthesis, and Real-Time Quantitative Reverse Transcriptase Polymerase Chain Reaction
2.4. Transcriptomics Analysis of Mouse Colon Tissue
2.5. Gut Microbiota Analysis
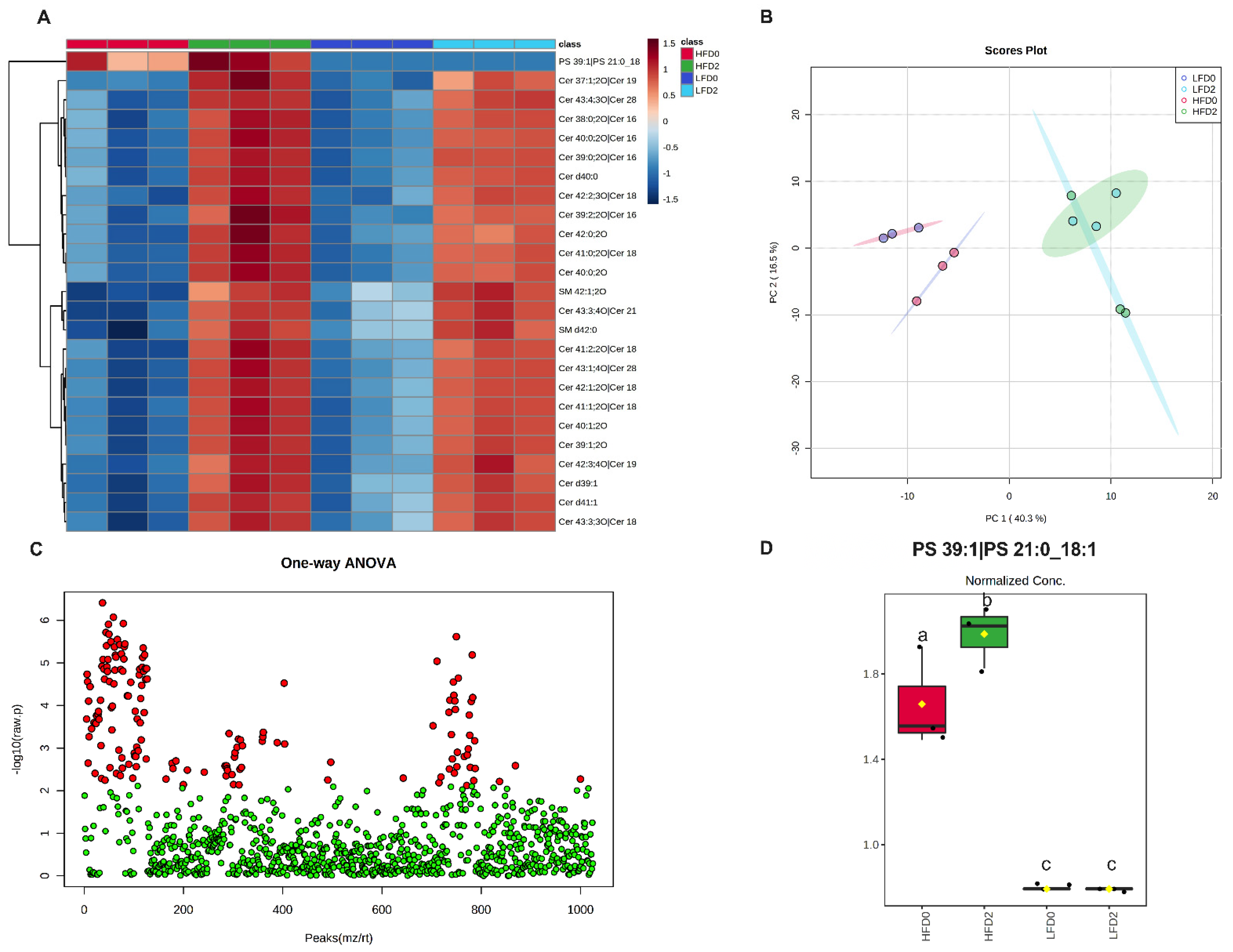
2.6. Fecal Lipidomics Analysis
2.7. Statistical Analysis
3. Results
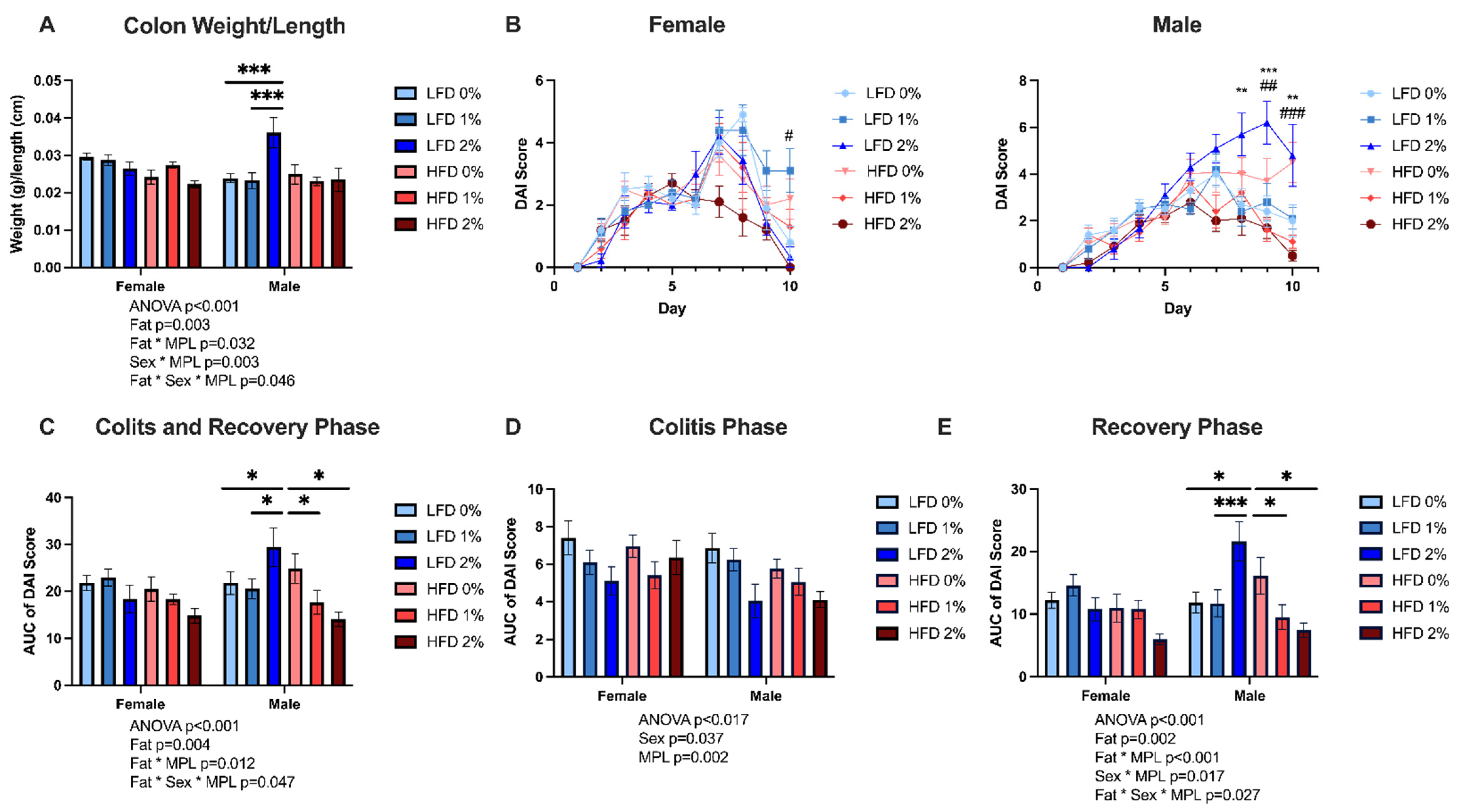
3.1. MPLs Attenuate DSS-Induced Colitis Disease Activity in HFD-Fed Mice, but Exacerbate Disease Activity in LFD-Fed Male Mice
3.2. MPLs in LFD Exacerbate, While MPLs in HFD Attenuate, Colon Inflammation in Male Mice
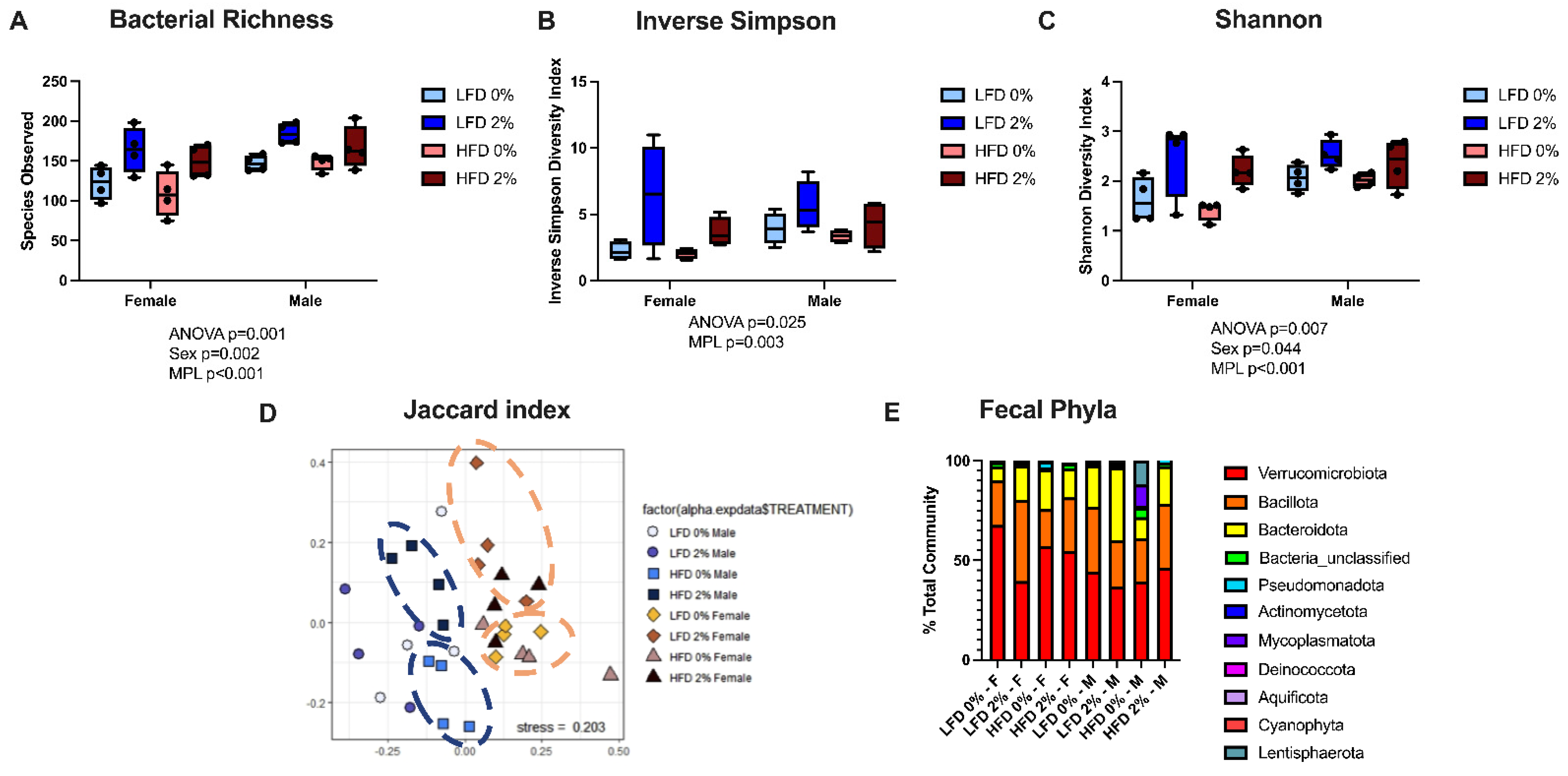
3.3. MPLs and Male Sex Increases Fecal Microbial Diversity but Does Not Alter Microbial Phyla Composition
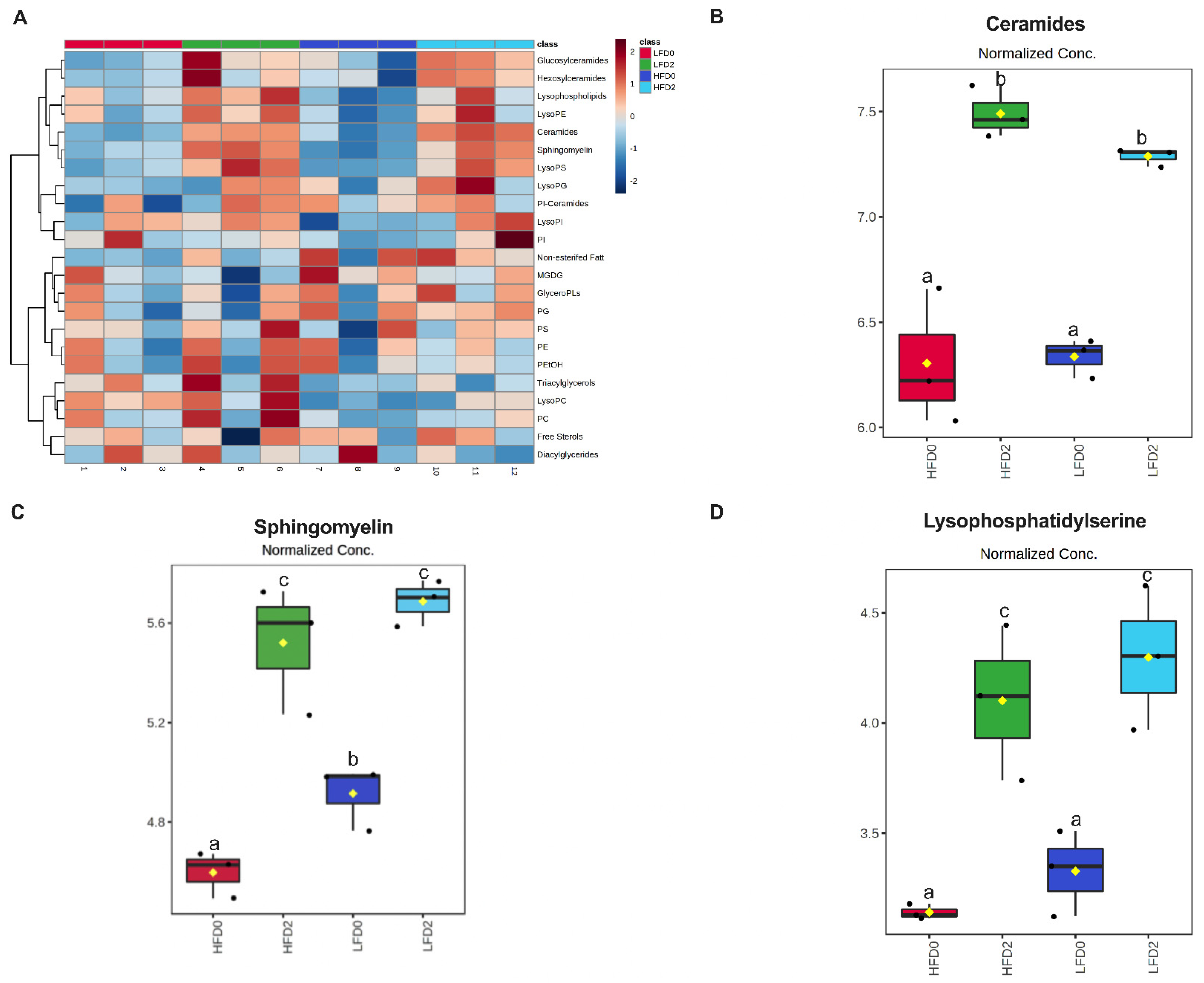
3.4. MPLs Increase Fecal Ceramides and Sphingomyelins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dahlhamer, J.M.; Zammitti, E.P.; Wheaton, A.G.; Croft, J.B. Health-Risk Behaviors and Chronic Conditions Among Adults with Inflammatory Bowel Disease—United States, 2015 and 2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 67, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Giannetti, E.; Martinelli, M.; Sciorio, E.; Staiano, A.; Miele, E. Does cow’s milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis? Acta Paediatr 2013, 102, e273–e278. [Google Scholar] [CrossRef]
- Wright, R.; Truelove, S.C. A Controlled Therapeutic Trial of Various Diets in Ulcerative Colitis. Br. Med. J. 1965, 2, 138. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Ament, M.; Artinian, L.; Ridgeway, J.; Shanahan, F. Milk tolerance in adults with ulcerative colitis. Am. J. Gastroenterol. 1994, 89, 872–877. [Google Scholar]
- Lopes, M.B.; Rocha, R.; Lyra, A.C.; Oliveira, V.R.; Coqueiro, F.G.; Almeida, N.S.; Valois, S.S.; Santana, G.O. Restriction of dairy products; a reality in inflammatory bowel disease patients. Nutr. Hosp. 2014, 29, 575–581. [Google Scholar]
- Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients 2020, 12, 1001. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Mazzei, J.C.; Zhou, H.; Brayfield, B.P.; Hontecillas, R.; Bassaganya-Riera, J.; Schmelz, E.M. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: Importance of peroxisome proliferator-activated receptor γ expression. J. Nutr. Biochem. 2011, 22, 1160–1171. [Google Scholar] [CrossRef]
- Millar, C.L.; Jiang, C.; Norris, G.H.; Garcia, C.; Seibel, S.; Anto, L.; Lee, J.-Y.; Blesso, C.N. Cow’s milk polar lipids reduce atherogenic lipoprotein cholesterol, modulate gut microbiota and attenuate atherosclerosis development in LDL-receptor knockout mice fed a Western-type diet. J. Nutr. Biochem. 2020, 79, 108351. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.H.; Porter, C.M.; Jiang, C.; Blesso, C.N. Dietary Milk Sphingomyelin Reduces Systemic Inflammation in Diet-Induced Obese Mice and Inhibits LPS Activity in Macrophages. Beverages 2017, 3, 37. [Google Scholar] [CrossRef]
- Norris, G.H.; Milard, M.; Michalski, M.C.; Blesso, C.N. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J. Nutr. Biochem. 2019, 73, 108224. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef]
- Norris, G.H.; Porter, C.M.; Jiang, C.; Millar, C.L.; Blesso, C.N. Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 40, 36–43. [Google Scholar] [CrossRef]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe 2019, 25, 668. [Google Scholar] [CrossRef]
- National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, 3678. [Google Scholar] [CrossRef]
- Millar, C.L.; Anto, L.; Garcia, C.; Kim, M.-B.; Jain, A.; Provatas, A.A.; Clark, R.B.; Lee, J.-Y.; Nichols, F.C.; Blesso, C.N. Gut Microbiome-Derived Glycine Lipids Are Diet-Dependent Modulators of Hepatic Injury and Atherosclerosis. J. Lipid Res. 2022, 63, 100192. [Google Scholar] [CrossRef]
- Millar, C.L.; Norris, G.H.; Vitols, A.; Garcia, C.; Seibel, S.; Anto, L.; Blesso, C.N. Dietary Egg Sphingomyelin Prevents Aortic Root Plaque Accumulation in Apolipoprotein-E Knockout Mice. Nutrients 2019, 11, 1124. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwon, D.; Son, S.W.; Jeong, T.B.; Lee, S.; Kwak, J.H.; Cho, J.-Y.; Hwang, D.Y.; Seo, M.-S.; Kim, K.S.; et al. Inflammatory responses of C57BL/6NKorl mice to dextran sulfate sodium-induced colitis: Comparison between three C57BL/6 N sub-strains. Lab. Anim. Res. 2021, 37, 8. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Fischbeck, A.; Leucht, K.; Frey-Wagner, I.; Bentz, S.; Pesch, T.; Kellermeier, S.; Krebs, M.; Fried, M.; Rogler, G.; Hausmann, M.; et al. Sphingomyelin induces cathepsin D-mediated apoptosis in intestinal epithelial cells and increases inflammation in DSS colitis. Gut 2011, 60, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, G.; Zhu, D.; Zhang, W.; Qi, S.; Xue, X.; Wang, K.; Wu, L. Effects of dietary phosphatidylcholine and sphingomyelin on DSS-induced colitis by regulating metabolism and gut microbiota in mice. J. Nutr. Biochem. 2022, 105, 109004. [Google Scholar] [CrossRef] [PubMed]
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/173412/nutrients (accessed on 15 September 2022).
- Mortensen, B.K. Anhydrous Milk Fat/Butter Oil and Ghee. Ref. Modul. Food Sci. 2016, 515–521. [Google Scholar] [CrossRef]
- Non, S.K.; Koo, S.I. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J. Nutr. 2004, 134, 2611–2616. [Google Scholar]
- Boland, M.P.; O’Neill, L.A.J. Ceramide activates NFκB by inducing the processing of p105. J. Biol. Chem. 1998, 273, 15494–15500. [Google Scholar] [CrossRef]
- Furuya, H.; Ohkawara, S.; Nagashima, K.; Asanuma, N.; Hino, T. Dietary Sphingomyelin Alleviates Experimental Inflammatory Bowel Disease in Mice. Int. J. Vitam. Nutr. Res. 2008, 78, 41–48. [Google Scholar] [CrossRef]
- Ho, S.; Pothoulakis, C.; Koon, H.W. Antimicrobial Peptides and Colitis. Curr. Pharm. Des. 2013, 19, 40. [Google Scholar]
- Okada, K.; Itoh, H.; Ikemoto, M. Circulating S100A8/A9 is potentially a biomarker that could reflect the severity of experimental colitis in rats. Heliyon 2020, 6, e03470. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Szabo, A.; Hsu, C.L.; A Krier-Burris, R.; A Schroeder, H.; Wang, M.Y.; Carter, R.G.; E Velez, T.; Aguiniga, L.M.; Brown, J.B.; et al. Histamine drives severity of innate inflammation via histamine 4 receptor in murine experimental colitis. Mucosal Immunol. 2018, 11, 861. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Lin, F.; Han, K.; Wang, X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch Biochem. Biophys. 2020, 679, 108187. [Google Scholar] [CrossRef]
- Kobayashi, H.; Uchimura, K.; Ishii, T.; Takahashi, K.; Mori, K.; Tsuchiya, K.; Furuya, F. Intelectin1 ameliorates macrophage activation via inhibiting the nuclear factor kappa B pathway. Endocr. J. 2022, 69, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Shea-Donohue, T.; Notari, L.; Sun, R.; Zhao, A. Mechansims of Smooth Muscle Repsonses to Inflammation. Neurogastroenterol. Motil. 2012, 24, 802. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Vrees, M.D.; Potenti, F.M.; Harnett, K.M.; Fiocchi, C.; Pricolo, V.E. Interleukin 1beta-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J. Pharmacol. Exp. Ther. 2004, 311, 60–70. [Google Scholar] [CrossRef]
- Grewal, S.S.; Fass, D.M.; Yao, H.; Ellig, C.L.; Goodman, R.H.; Stork, P.J.S. Calcium and cAMP Signals Differentially Regulate cAMP-responsive Element-binding Protein Function via a Rap1-Extracellular Signal-regulated Kinase Pathway. J. Biol. Chem. 2000, 275, 34433–34441. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Wang, H.; Cao, M.; Li, M.; Zhao, J.; Hu, Y.; Wang, Y.; Li, S.; Xie, Y.; et al. Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function. Cell Prolif. 2019, 52, e12673. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yuan, Q.; Gong, H.; Du, M.; Mao, X. Gut microbiota mediates the alleviative effect of polar lipids-enriched milk fat globule membrane on obesity-induced glucose metabolism disorders in peripheral tissues in rat dams. Int. J. Obes. 2021, 46, 793–801. [Google Scholar] [CrossRef]
- Miguel Aguilera, J.; Miguel Aguilera, J. The food matrix: Implications in processing, nutrition and health The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2018, 59, 3612–3629. [Google Scholar] [CrossRef]
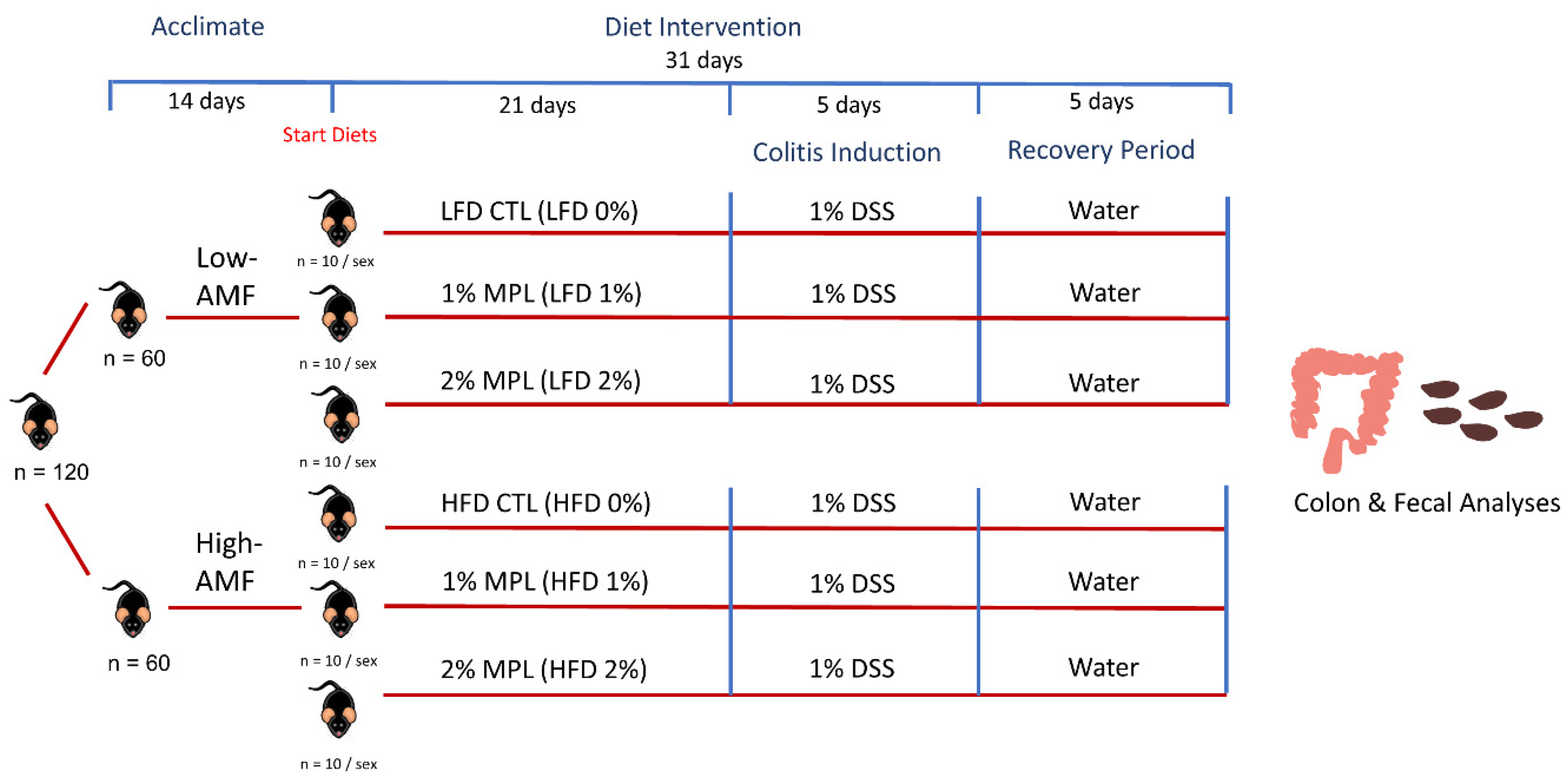


| Diet Component (g per 1 kg) | LFD 0% | LFD 1% | LFD 2% | HFD 0% | HFD 1% | HFD 2% |
|---|---|---|---|---|---|---|
| Casein | 80 | 80 | 80 | 80 | 80 | 80 |
| L-Cystine | 3 | 3 | 3 | 3 | 3 | 3 |
| Sucrose | 200 | 200 | 200 | 200 | 200 | 200 |
| Corn Starch | 175 | 172.5 | 170 | 20 | 17.5 | 15 |
| Lactose | 21.35 | 10.68 | 0 | 21.35 | 10.68 | 0 |
| Anhydrous Milkfat | 52 | 26 | 0 | 207 | 181 | 155 |
| Soybean Oil | 20 | 20 | 20 | 20 | 20 | 20 |
| Cellulose | 50 | 50 | 50 | 50 | 50 | 50 |
| Mineral Mix, AIN-93G-MX (94046) | 43 | 43 | 43 | 43 | 43 | 43 |
| Vitamin Mix, AIN-93-VX (94047) | 19 | 19 | 19 | 19 | 19 | 19 |
| Choline Bitartrate | 3 | 3 | 3 | 3 | 3 | 3 |
| TBHQ, antioxidant | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Cholesterol | 1.5 | 1.3 | 1.12 | 1.5 | 1.3 | 1.12 |
| Skim milk powder—Dyets 403150 | 332 | 166 | 0 | 332 | 166 | 0 |
| Beta serum powder—Tatua BSP2 | 0 | 205.5 | 411 | 0 | 205.5 | 411 |
| Calcium Carbonate (40% calcium by wt) | 0 | 0.68 | 1.36 | 0 | 0.68 | 1.36 |
| Potassium Chloride (53% potassium by wt) | 0 | 0.19 | 0.38 | 0 | 0.19 | 0.38 |
| Sodium Chloride (40% sodium by wt) | 0 | 0.25 | 0.51 | 0 | 0.25 | 0.51 |
| Diet Component | LFD 0% | LFD 1% | LFD 2% | HFD 0% | HFD 1% | HFD 2% |
|---|---|---|---|---|---|---|
| Total Protein (g/kg) | 200.2 | 200.3 | 200.4 | 200.2 | 200.3 | 200.4 |
| Total Carbohydrate (g/kg) | 568.7 | 566.5 | 564.0 | 414.0 | 411.5 | 409.0 |
| Total Fat (g/kg) | 74.7 | 76.3 | 78.0 | 229.7 | 231.3 | 233.0 |
| Total Lactose (g/kg) | 194.0 | 194.0 | 194.0 | 194.0 | 194.0 | 194.0 |
| Total Cholesterol (g/kg) | 1.6 | 1.68 | 1.68 | 2.06 | 2.06 | 2.06 |
| Total Phospholipid (g/kg) | 0.5 | 10.3 | 20.1 | 0.5 | 10.3 | 20.1 |
| % kcal from Protein | 21.4 | 21.3 | 21.3 | 17.7 | 17.7 | 17.7 |
| % kcal from Carbohydrate | 60.7 | 60.4 | 60.0 | 36.6 | 36.3 | 36.1 |
| % kcal from Fat | 17.9 | 18.3 | 18.7 | 45.7 | 46 | 46.2 |
| Calorie Density (kcal/g) | 3.75 | 3.75 | 3.75 | 4.52 | 4.52 | 4.52 |
| p-Value (Three-Way ANOVA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LFD 0% | LFD 1% | LFD 2% | HFD 0% | HFD 1% | HFD 2% | MPL | Fat | Sex | Interaction | |
| End Body Weight (g) | ||||||||||
| Female | 21.52 ± 1.30 | 21.02 ± 1.46 | 21.27 ± 1.11 | 21.24 ± 1.49 | 22.87 ± 2.60 | 21.48 ± 1.56 | n.s. | <0.001 | <0.001 | MPL*Fat (0.018) MPL*Sex (0.009) Fat*Sex*MPL (0.036) |
| Male | 24.48 ± 1.60 | 25.48 ± 1.29 | 24.69 ± 1.88 | 26.65 ± 1.89 | 27.53 ± 1.29 | 27.87 ± 1.72 | ||||
| Total Food Intake (g) | ||||||||||
| Female | 300.4 ± 24.7 | 331.6 ± 41.2 | 304.5 ± 59.2 | 295.9 ± 37.0 | 387.3 ± 53.6 | 362.0 ± 98.2 | n.s. | n.s. | n.s. | n.s. |
| Male | 307.9 ± 7.3 | 325.4 ± 55.3 | 292.7 ± 10.6 | 354.5 ± 149.8 | 322.0 ± 25.9 | 346.8 ± 108.3 | ||||
| Spleen (g) | ||||||||||
| Female | 0.11 ± 0.02 | 0.13 ± 0.04 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.09 ± 0.01 | n.s. | n.s. | n.s. | n.s. |
| Male | 0.09 ± 0.03 | 0.08 ± 0.01 | 0.09 ± 0.04 | 0.10 ± 0.04 | 0.17 ± 0.25 | 0.09 ± 0.02 | ||||
| Liver (g) | ||||||||||
| Female | 0.97 ± 0.11 | 0.87 ± 0.12 | 0.89 ± 0.12 | 0.88 ± 0.10 | 0.85 ± 0.12 | 0.82 ± 0.07 | 0.046 | n.s. | <0.001 | n.s. |
| Male | 1.06 ± 0.19 | 0.92± 0.09 | 0.98 ± 0.14 | 0.97 ± 0.17 | 0.99 ± 0.11 | 0.97 ± 0.12 | ||||
| Adipose (g) | ||||||||||
| Female | 0.37 ± 0.09 | 0.22 ± 0.13 | 0.22 ± 0.11 | 0.33 ± 0.11 | 0.41 ± 0.24 | 0.30 ± 0.17 | n.s. | <0.001 | <0.001 | Fat*Sex (0.008) |
| Male | 0.48 ± 0.15 | 0.60 ± 0.11 | 0.37 ± 0.19 | 0.65 ± 0.35 | 0.83 ± 0.42 | 0.87 ± 0.36 | ||||
| Colon Length (cm) | ||||||||||
| Female | 6.06 ± 0.60 | 6.14 ± 0.62 | 6.60 ± 0.84 | 6.67 ± 0.69 | 6.43 ± 0.64 | 6.59 ± 0.87 | n.s. | n.s. | n.s. | n.s. |
| Male | 6.52 ± 0.93 | 6.60 ± 1.10 | 6.15 ± 1.05 | 6.95 ± 0.69 | 6.73 ± 0.90 | 7.18 ± 0.84 | ||||
| Colon Weight (g) | ||||||||||
| Female | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.18± 0.02 | 0.16 ± 0.03 | 0.18 ± 0.02 | 0.15 ± 0.02 | 0.001 | 0.021 | n.s. | MPL*Fat (0.031) MPL*Sex (0.002) |
| Male | 0.16 ± 0.02 | 0.15 ± 0.02 | 0.21 ± 0.04 | 0.17 ± 0.04 | 0.16 ± 0.02 | 0.16 ± 0.04 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, C.; Anto, L.; Blesso, C.N. Effects of Milk Polar Lipids on DSS-Induced Colitis Severity Are Dependent on Dietary Fat Content. Nutrients 2022, 14, 5145. https://doi.org/10.3390/nu14235145
Garcia C, Anto L, Blesso CN. Effects of Milk Polar Lipids on DSS-Induced Colitis Severity Are Dependent on Dietary Fat Content. Nutrients. 2022; 14(23):5145. https://doi.org/10.3390/nu14235145
Chicago/Turabian StyleGarcia, Chelsea, Liya Anto, and Christopher N. Blesso. 2022. "Effects of Milk Polar Lipids on DSS-Induced Colitis Severity Are Dependent on Dietary Fat Content" Nutrients 14, no. 23: 5145. https://doi.org/10.3390/nu14235145
APA StyleGarcia, C., Anto, L., & Blesso, C. N. (2022). Effects of Milk Polar Lipids on DSS-Induced Colitis Severity Are Dependent on Dietary Fat Content. Nutrients, 14(23), 5145. https://doi.org/10.3390/nu14235145







