Mid-Arm Muscle Circumference or Body Weight-Standardized Hand Grip Strength in the GLIM Superiorly Predicts Survival in Chinese Colorectal Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Acquisition
2.3. Anthropometric Measurements
2.4. Nutrition Status Assessment
2.5. Selection of Predictors Associated with the OS
2.6. Development and Validation of a Nomogram for Survival Prediction
2.7. Clinical Application
2.8. Statistical Analysis
3. Results
3.1. MAMC or HGS/W Method Has Optimal Performance for the Evaluation of RMM
3.2. Baseline Characteristics
3.3. Kaplan–Meier Analysis
3.4. Predictors Associated with Survival
3.5. GLIM-Diagnosed Malnutrition as an Independent Mortality Risk Factor for Survival
3.6. Nomogram and Its Performance
3.7. Clinical Utilization of the Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| CC | Calf circumference |
| C-index | Concordance index |
| CRC | Colorectal cancer |
| GLIM | Global Leadership Initiative on Malnutrition |
| HGS/W | Hand grip strength |
| HGS/W | Weight-standardized hand grip strength |
| HR | Hazard ratio |
| INSCOC | Investigation on Nutrition Status and its Clinical Outcome of Common Cancers |
| KPS | Karnofsky Performance Score |
| LASSO | Least absolute shrinkage and selection operator |
| MAC | Mid-arm circumference |
| MAMC | Mid-arm muscle circumference |
| 95%CI | 95% confidence intervals |
| NRS 2002 | Nutrition Risk Screening 2002 |
| OS | Overall survival |
| PG-SGA | Patient-Generated Subjective Global Assessment |
| p5 | Fifth percentile |
| p15 | 15th percentile |
| RMM | Reduced muscle mass |
| TSF | Triceps skinfold thickness |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, B.; Luo, C.; Cai, J.; Lu, M.; Zhang, Y.; Chen, H.; Dai, M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021, 522, 255–268. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, T.; Pang, L.; Sharma, S.V.; Li, R.; Nyitray, A.G.; Edwards, B.J. Malnutrition and overall survival in older adults with cancer: A systematic review and meta-analysis. J. Geriatr. Oncol. 2019, 10, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kassa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, C.; Paur, I.; Pedersen, A.; Kværner, A.S.; Ræder, H.; Henriksen, H.B.; Bøhn, S.K.; Wiedswang, G.; Blomhoff, R. Agreement between GLIM and PG-SGA for diagnosis of malnutrition depends on the screening tool used in GLIM. Clin. Nutr. 2022, 41, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ræder, H.; Henriksen, C.; Bøhn, S.K.; Henriksen, H.B.; Kværner, A.S.; Rolid, K.; Paur, I.; Smeland, S.; Blomhoff, R. Agreement between PG-SGA category and fat-free mass in colorectal cancer patients. Clin. Nutr. ESPEN 2018, 27, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wang, S.; Gao, X.; Gao, T.; Huang, L.; Lian, B.; Wang, X. Poor Pre-operative Nutritional Status Is a Risk Factor of Post-operative Infections in Patients With Gastrointestinal Cancer-A Multicenter Prospective Cohort Study. Front. Nutr. 2022, 9, 850063. [Google Scholar] [CrossRef]
- Almasaudi, A.S.; McSorley, S.T.; Dolan, R.D.; Edwards, C.A.; McMillan, D.C. The relation between Malnutrition Universal Screening Tool (MUST), computed tomography-derived body composition, systemic inflammation, and clinical outcomes in patients undergoing surgery for colorectal cancer. Am. J. Clin. Nutr. 2019, 110, 1327–1334. [Google Scholar] [CrossRef]
- Yamano, T.; Yoshimura, M.; Kobayashi, M.; Beppu, N.; Hamanaka, M.; Babaya, A.; Tsukamoto, K.; Noda, M.; Matsubara, N.; Tomita, N. Malnutrition in rectal cancer patients receiving preoperative chemoradiotherapy is common and associated with treatment tolerability and anastomotic leakage. Int. J. Colorectal Dis. 2016, 31, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Xu, L.B.; Shi, M.M.; Huang, Z.X.; Zhang, W.T.; Zhang, H.H.; Shen, X.; Chen, X.D. Impact of malnutrition diagnosed using Global Leadership Initiative on Malnutrition criteria on clinical outcomes of patients with gastric cancer. JPEN J. Parenter. Enteral. Nutr. 2022, 46, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Z.; Xu, D.; Peng, Y.; Liu, X. The GLIM Criteria Represent a More Appropriate Tool for Nutritional Assessment in Patients With Crohn’s Disease. Front. Nutr. 2022, 9, 826028. [Google Scholar] [CrossRef] [PubMed]
- Okada, G.; Matsumoto, Y.; Habu, D.; Matsuda, Y.; Lee, S.; Osugi, H. Relationship between GLIM criteria and disease-specific symptoms and its impact on 5-year survival of esophageal cancer patients. Clin. Nutr. 2021, 40, 5072–5078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, M.; Zhang, Q.; Zhang, K.P.; Guo, Z.Q.; Xu, H.X.; Yuan, K.T.; Yu, M.; Braga, M.; Cederholm, T.; et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin. Nutr. 2021, 40, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Song, C.; Yin, L.; Wang, C.; Fu, Z.; Guo, Z.; Lin, Y.; Shi, Y.; Hu, W.; Ba, Y.; et al. Extension protocol for the Investigation on Nutrition Status and Clinical Outcome of Patients with Common Cancers in China (INSCOC) study: 2021 update. Precis. Nutr. 2022, 1, e00014. [Google Scholar]
- Maeda, K.; Ishida, Y.; Nonogaki, T.; Mori, N. Reference body mass index values and the prevalence of malnutrition according to the Global Leadership Initiative on Malnutrition criteria. Clin. Nutr. 2020, 39, 180–184. [Google Scholar] [CrossRef]
- Chun, S.-W.; Kim, W.; Choi, K.H. Comparison between grip strength and grip strength divided by body weight in their relationship with metabolic syndrome and quality of life in the elderly. PLoS ONE 2019, 14, e0222040. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Liang, C.H.; He, L.; Tian, J.; Liang, C.S.; Chen, X.; Ma, Z.L.; Liu, Z.Y. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef]
- Mayr, A.; Hofner, B.; Schmid, M. Boosting the discriminatory power of sparse survival models via optimization of the concordance index and stability selection. BMC Bioinform. 2016, 17, 288. [Google Scholar] [CrossRef] [Green Version]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [Green Version]
- Ramspek, C.L.; Jager, K.J.; Dekker, F.W.; Zoccali, C.; van Diepen, M. External validation of prognostic models: What, why, how, when and where? Clin. Kidney J. 2021, 14, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Saville, B.R.; Lewis, R.J. Decision curve analysis. JAMA 2015, 313, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Serón-Arbeloa, C.; Labarta-Monzón, L.; Puzo-Foncillas, J.; Mallor-Bonet, T.; Lafita-López, A.; Bueno-Vidales, N.; Montoro-Huguet, M. Malnutrition Screening and Assessment. Nutrients 2022, 14, 2392. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E. Cancer-associated malnutrition. Eur. J. Clin. Nutr. 2018, 72, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Håkonsen, S.J.; Pedersen, P.U.; Bath-Hextall, F.; Kirkpatrick, P. Diagnostic test accuracy of nutritional tools used to identify undernutrition in patients with colorectal cancer: A systematic review. JBI Database Syst. Rev. Implement Rep. 2015, 13, 141–187. [Google Scholar] [CrossRef]
- Dávalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Duran, X.; Meza-Valderrama, D.; Rodríguez, D.A.; Muñoz, E.; Tejero-Sánchez, M.; Muns, M.D.; Guillén-Solà, A.; et al. Malnutrition According to GLIM Criteria Is Associated with Mortality and Hospitalizations in Rehabilitation Patients with Stable Chronic Obstructive Pulmonary Disease. Nutrients 2021, 13, 369. [Google Scholar] [CrossRef]
- Theilla, M.; Rattanachaiwong, S.; Kagan, I.; Rigler, M.; Bendavid, I.; Singer, P. Validation of GLIM malnutrition criteria for diagnosis of malnutrition in ICU patients: An observational study. Clin. Nutr. 2021, 40, 3578–3584. [Google Scholar] [CrossRef]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Bàràny, P.; Heimbürger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef] [Green Version]
- Gort-van Dijk, D.; Weerink, L.B.; Milovanovic, M.; Haveman, J.W.; Hemmer, P.H.; Dijkstra, G.; Lindeboom, R.; Campmans-Kuijpers, M.J.E. Bioelectrical Impedance Analysis and Mid-Upper Arm Muscle Circumference Can Be Used to Detect Low Muscle Mass in Clinical Practice. Nutrients 2021, 13, 2350. [Google Scholar] [CrossRef]
- He, L.; Yang, N.; Wang, J.; Huang, J.; Li, W.; Xu, L.; Ping, F.; Li, Y.; Zhang, H. Mid-Arm Muscle and Subcutaneous Fat Associated with All-Cause Mortality Independent of BMI: A Prospective Cohort Study. Obesity 2021, 29, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Kaegi-Braun, N.; Tribolet, P.; Baumgartner, A.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Hoess, C.; et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: Secondary analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, Q.; Tang, M.; Zhang, X.; Ruan, G.; Zhang, X.; Shi, H. Associations of low hand grip strength with 1 year mortality of cancer cachexia: A multicentre observational study. J. Cachexia Sarcopenia Muscle 2021, 12, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, A.; Deftereos, I.; Carter, V.M.; Morris, S.; Shannon, R.; Kiss, N.; Yeung, J.M. Ability of malnutrition screening and assessment tools to identify computed tomography defined low muscle mass in colorectal cancer surgery. Nutr. Clin. Pract. 2022, 37, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, Y.; Qin, X.; Lv, Z.; Wang, H.; Wu, D.; Yuan, Z.; Wang, H. Development and Validation of a Prognostic Nomogram for Colorectal Cancer Patients With Synchronous Peritoneal Metastasis. Front. Oncol. 2021, 11, 615321. [Google Scholar] [CrossRef]
- Zhang, N.; Ning, F.; Guo, R.; Pei, J.; Qiao, Y.; Fan, J.; Jiang, B.; Liu, Y.; Chi, Z.; Mei, Z.; et al. Prognostic Values of Preoperative Inflammatory and Nutritional Markers for Colorectal Cancer. Front. Oncol. 2020, 10, 585083. [Google Scholar] [CrossRef]
- Teka, M.A.; Yesuf, A.; Hussien, F.M.; Hassen, H.Y. Histological characteristics, survival pattern and prognostic determinants among colorectal cancer patients in Ethiopia: A retrospective cohort study. Heliyon 2021, 7, e06366. [Google Scholar] [CrossRef]
- Wu, G.; Liu, J.; Liu, H.; Jin, L.; Huang, X.; Mo, X.; Zhong, H.; Li, Y.; Zhang, Y.; Tang, W. An Applicable Inflammation-Joined and Nutrition-Related Prognostic Indicator in Patients With Colorectal Cancer. Front. Oncol. 2021, 11, 644670. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, W.; Hou, H.; Ning, S.; Li, J.; Huang, B.; Zhang, L. Prognostic Value of C-Reactive Protein, Glasgow Prognostic Score, and C-Reactive Protein-to-Albumin Ratio in Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 637650. [Google Scholar] [CrossRef]
- Cao, Y.; Deng, S.; Yan, L.; Gu, J.; Yang, J.; Yang, M.; Liu, L.; Cai, K. A nomogram based on pretreatment levels of serum bilirubin and total bile acid levels predicts survival in colorectal cancer patients. BMC Cancer 2021, 21, 85. [Google Scholar] [CrossRef]
- Zhu, B.; Luo, J.; Jiang, Y.; Yu, L.; Liu, M.; Fu, J. Prognostic significance of nomograms integrating IL-37 expression, neutrophil level, and MMR status in patients with colorectal cancer. Cancer Med. 2018, 7, 3682–3694. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Lin, X.; Li, N.; Zhang, M.; He, X.; Liu, J.; Kang, J.; Chen, X.; Wang, C.; Wang, X.; et al. Evaluation of the Global Leadership Initiative on Malnutrition Criteria Using Different Muscle Mass Indices for Diagnosing Malnutrition and Predicting Survival in Lung Cancer Patients. JPEN J. Parenter. Enteral. Nutr. 2021, 45, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Tang, M.; Song, M.; Zhang, Q.; Zhang, K.; Ruan, G.; Zhang, X.; Ge, Y.; Yang, M.; et al. Different muscle mass indices of the Global Leadership Initiative on Malnutrition in diagnosing malnutrition and predicting survival of patients with gastric cancer. Nutrition 2021, 89, 111286. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y.; Miyazaki, K.; Yoshikawa, M.; Yamada, S.; Saito, Y.; Ikemoto, T.; Imura, S.; Morine, Y.; Shimada, M. Value of the CRP-albumin ratio in patients with resectable pancreatic cancer. J. Med. Invest. 2021, 68, 244–255. [Google Scholar] [CrossRef]
- Ayrancı, M.K.; Küçükceran, K.; Dundar, Z.D. NLR and CRP to albumin ratio as a predictor of in-hospital mortality in the geriatric ED patients. Am. J. Emerg. Med. 2021, 44, 50–55. [Google Scholar] [CrossRef]
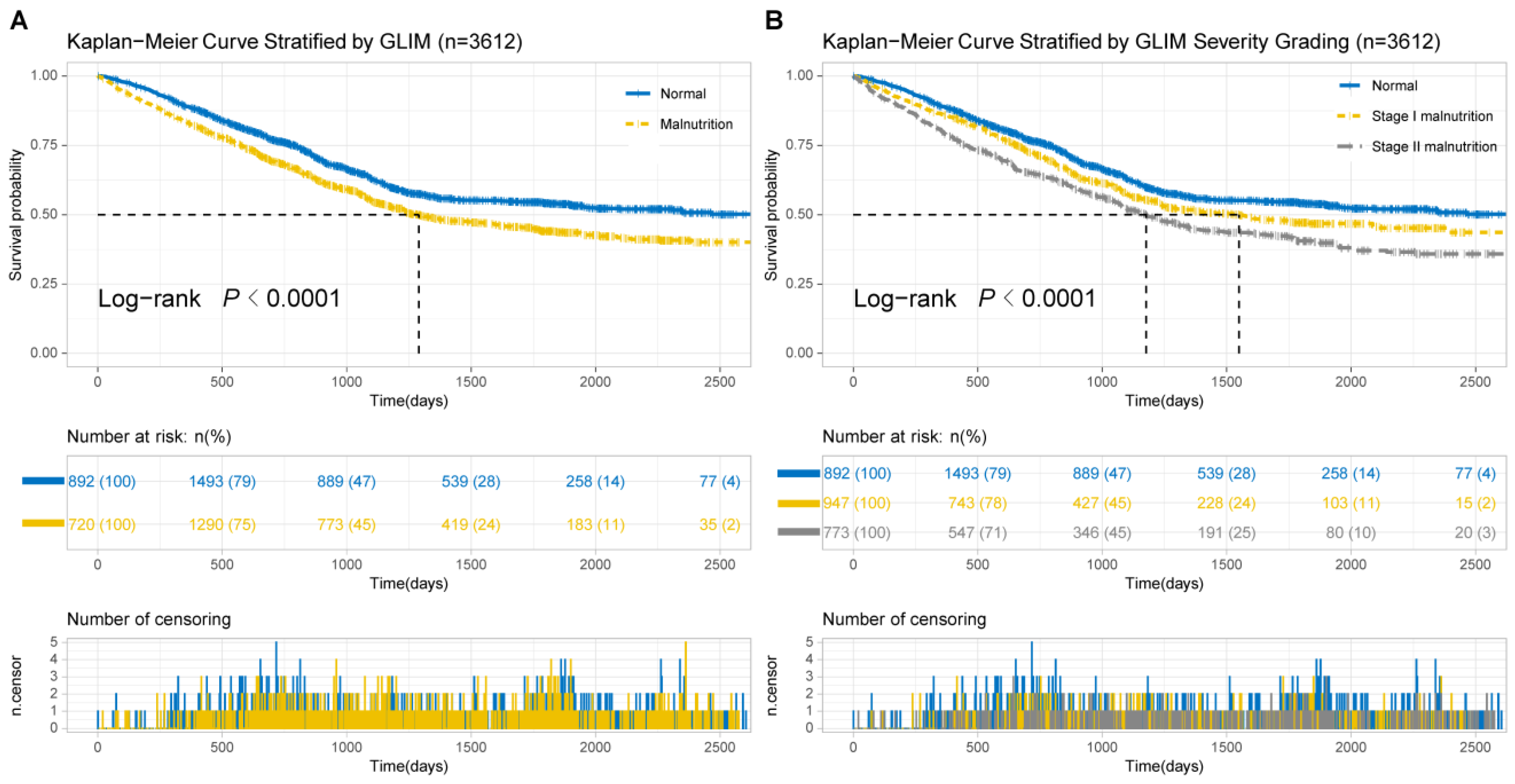
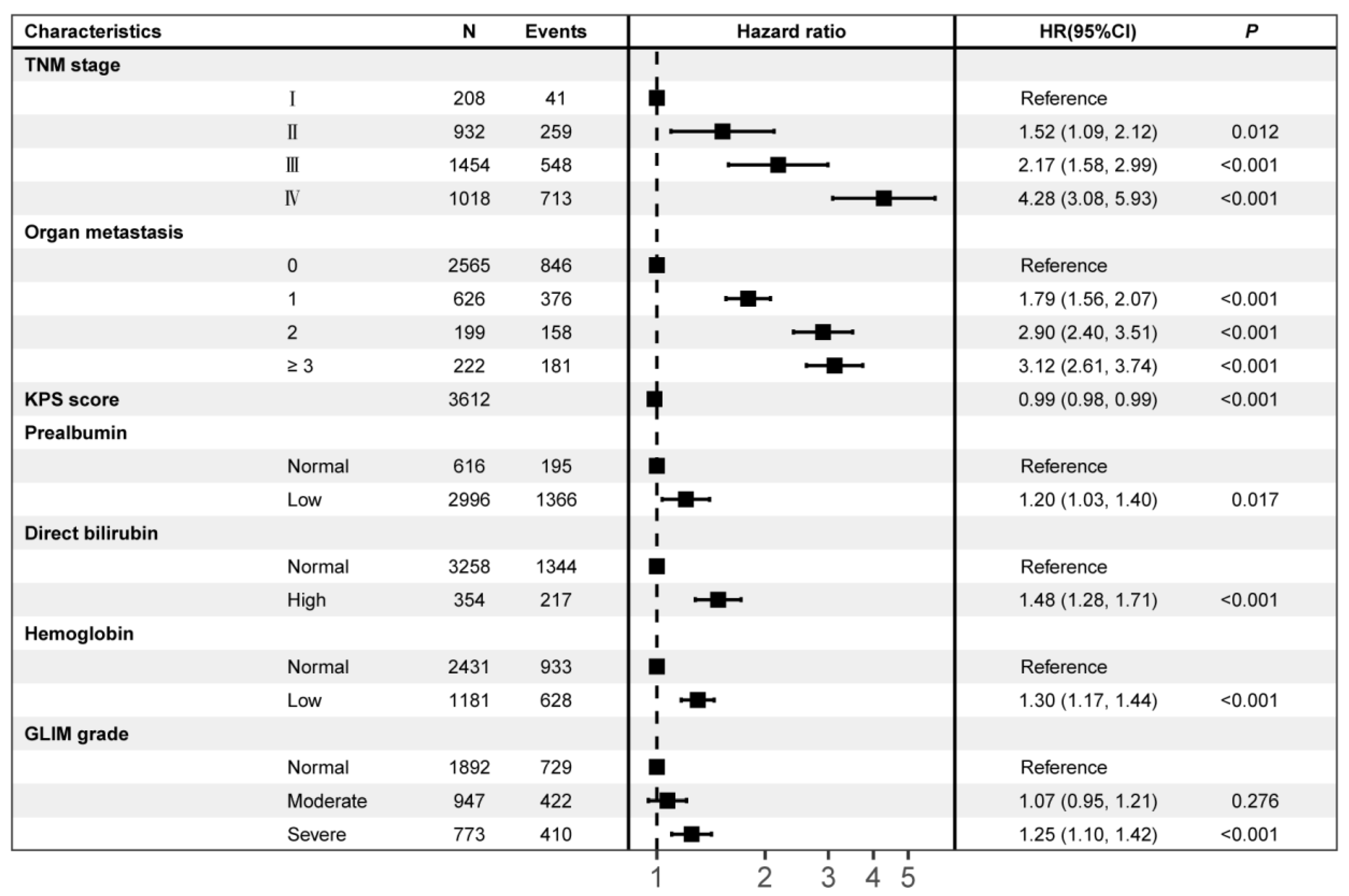
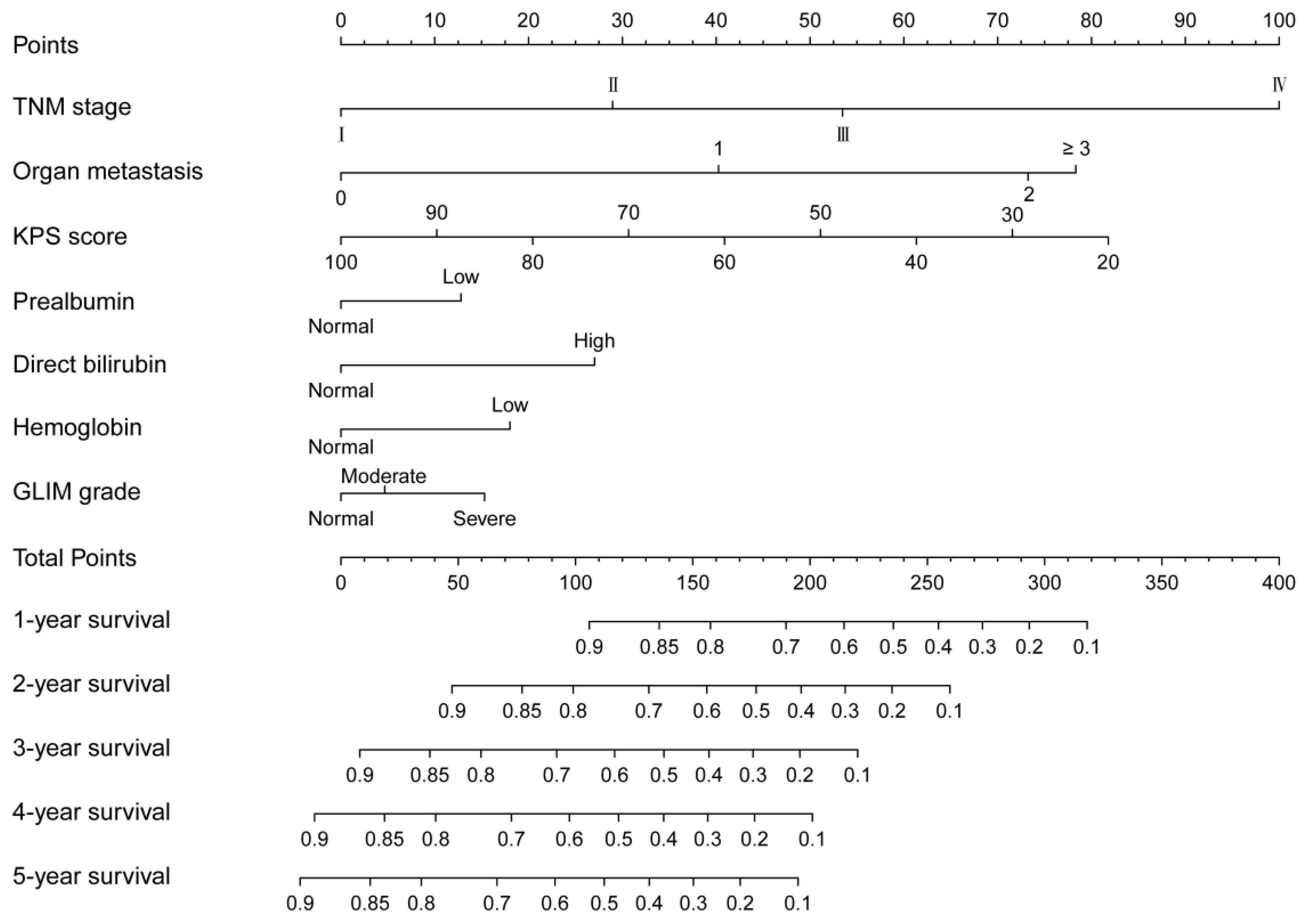

| Parameters to Assess RMM | Normal | Stage I (Moderate Malnutrition) | Stage II (Severe Malnutrition) | p | |||
|---|---|---|---|---|---|---|---|
| n (%) | Median (95% CI) | n (%) | Median (95% CI) | n (%) | Median (95% CI) | ||
| No RMM assessment * | 2140 (59.3) | 2352 (1893, NA) | 876 (24.3) | 1455 (1230, 1770) | 596 (16.5) | 1176 (1054, 1412) | <0.001 |
| MAMC | 2027 (56.1) | 2464 (1949, NA) | 908 (25.1) | 1373 (1224, 1754) | 677 (18.7) | 1180 (1076, 1353) | <0.001 |
| CC | 2051 (56.8) | 2464 (1908, NA) | 747 (20.7) | 1589 (1255, 2118) | 814 (22.5) | 1180 (1074, 1350) | <0.001 |
| HGS/W | 1988 (55.0) | 2464 (1948, NA) | 930 (25.8) | 1549 (1248, 2118) | 694 (19.2) | 1160 (1056, 1346) | <0.001 |
| MAMC or HGS/W | 1892 (52.4) | NA (1971, NA) | 947 (26.2) | 1549 (1241, 2086) | 773 (21.4) | 1176 (1074, 1328) | <0.001 |
| MAMC and HGS/W | 2123 (58.8) | 2464 (1904, NA) | 891 (24.7) | 1373 (1224, 1754) | 598 (16.6) | 1176 (1056, 1373) | <0.001 |
| CC or HGS/W | 1912 (52.9) | NA (1949, NA) | 807 (22.3) | 1615 (1300, NA) | 893 (24.7) | 1180 (1076, 1328) | <0.001 |
| CC and HGS/W | 2127 (58.9) | 2464 (1904, NA) | 870 (24.1) | 1455 (1231, 1833) | 615 (17.0) | 1160 (1047, 1350) | <0.001 |
| MAMC or CC or HGS/W | 1839 (50.9) | NA (1971, NA) | 831 (23.0) | 1615 (1296, NA) | 942 (26.1) | 1183 (1077, 1310) | <0.001 |
| MAMC and CC and HGS/W | 2136 (59.1) | 2352 (1895, NA) | 878 (24.3) | 1408 (1206, 1766) | 598 (16.6) | 1176 (1056, 1373) | <0.001 |
| Parameters to Assess RMM | Normal | Stage I (Moderate Malnutrition) | p | Stage II (Severe Malnutrition) | p | |||
|---|---|---|---|---|---|---|---|---|
| n (%) | Reference | n (%) | HR (95% CI) | n (%) | HR (95% CI) | |||
| No RMM assessment * | 2140 (59.3) | 1 | 876 (24.3) | 1.21 (1.07, 1.36) | 0.002 | 596 (16.5) | 1.44 (1.26, 1.64) | <0.001 |
| MAMC | 2027 (56.1) | 1 | 908 (25.1) | 1.24 (1.10, 1.40) | <0.001 | 677 (18.7) | 1.45 (1.28, 1.65) | <0.001 |
| CC | 2051 (56.8) | 1 | 747 (20.7) | 1.18 (1.03, 1.34) | 0.014 | 814 (22.5) | 1.45 (1.29, 1.63) | <0.001 |
| HGS/W | 1988 (55.0) | 1 | 930 (25.8) | 1.19 (1.06, 1.34) | 0.004 | 694 (19.2) | 1.50 (1.32, 1.70) | <0.001 |
| MAMC or HGS/W | 1892 (52.4) | 1 | 947 (26.2) | 1.21 (1.07, 1.36) | 0.002 | 773 (21.4) | 1.51 (1.34, 1.70) | <0.001 |
| MAMC and HGS/W | 2123 (58.8) | 1 | 891 (24.7) | 1.23 (1.09, 1.38) | 0.001 | 598 (16.6) | 1.44 (1.27, 1.64) | <0.001 |
| CC or HGS/W | 1912 (52.9) | 1 | 807 (22.3) | 1.16 (1.02, 1.32) | 0.026 | 893 (24.7) | 1.48 (1.32, 1.67) | <0.001 |
| CC and HGS/W | 2127 (58.9) | 1 | 870 (24.1) | 1.21 (1.07, 1.36) | 0.002 | 615 (17.0) | 1.47 (1.29, 1.67) | <0.001 |
| MAMC or CC or HGS/W | 1839 (50.9) | 1 | 831 (23.0) | 1.18 (1.04, 1.34) | 0.011 | 942 (26.1) | 1.51 (1.35, 1.69) | <0.001 |
| MAMC and CC and HGS/W | 2136 (59.1) | 1 | 878 (24.3) | 1.22 (1.08, 1.37) | 0.001 | 598 (16.6) | 1.44 (1.26, 1.64) | <0.001 |
| Grade | Phenotypic Criteria | ||
|---|---|---|---|
| Weight Loss (%) | Low BMI (kg/m2) | Reduced Muscle Mass a,b,c | |
| Moderate malnutrition | 5–10% within the past 6 months, or 10–20% beyond 6 months | <18.5 if <70 years, or <20 if ≥70 years | Mid-arm muscle circumference < p15, weight-standardized hand grip strength < p15 |
| Severe malnutrition | >10% within the past 6 months, or >20% beyond 6 months | <17.0 if <70 years, or <17.8 if ≥70 years | Mid-arm muscle circumference < p5, weight-standardized hand grip strength < p5 |
| Characteristics | Overall | GLIM Diagnosis | p | ||
|---|---|---|---|---|---|
| Normal | Moderate Malnutrition | Severe Malnutrition | |||
| (n = 3612) | (n = 1892) | (n = 947) | (n = 773) | ||
| General information | |||||
| Age, years, mean ± SD | 64.09 ± 12.45 | 63.12 ± 11.95 | 64.80 ± 12.63 | 65.58 ± 13.20 | <0.001 |
| Sex, male, n (%) | 2173 (60.2) | 1125 (59.5) | 564 (59.6) | 484 (62.6) | 0.291 |
| Smoking, yes, n (%) | 1361 (37.7) | 684 (36.2) | 364 (38.4) | 313 (40.5) | 0.095 |
| Alcohol drinker, yes, n (%) | 680 (18.8) | 348 (18.4) | 181 (19.1) | 151 (19.5) | 0.765 |
| Family cancer history, yes, n (%) | 561 (15.5) | 297 (15.7) | 145 (15.3) | 119 (15.4) | 0.958 |
| TNM Stage, n (%) | 0.937 | ||||
| Ⅰ | 212 (5.9) | 113 (6.0) | 58 (6.1) | 41 (5.3) | |
| Ⅱ | 942 (26.1) | 502 (26.5) | 236 (24.9) | 204 (26.4) | |
| Ⅲ | 1482 (41.0) | 764 (40.4) | 400 (42.2) | 318 (41.1) | |
| Ⅳ | 976 (27.0) | 513 (27.1) | 253 (26.7) | 210 (27.2) | |
| Organ metastasis, n (%) | 0.371 | ||||
| 0 | 2565 (71.0) | 1343 (71.0) | 673 (71.1) | 549 (71.0) | |
| 1 | 626 (17.3) | 347 (18.3) | 154 (16.3) | 125 (16.2) | |
| 2 | 199 (5.5) | 97 (5.1) | 59 (6.2) | 43 (5.6) | |
| ≥3 | 222 (6.1) | 105 (5.5) | 61 (6.4) | 56 (7.2) | |
| Differentiation grade, n (%) | 0.017 | ||||
| Well | 141 (3.9) | 81 (4.3) | 33 (3.5) | 27 (3.5) | |
| Moderate | 2791 (77.3) | 1494 (79.0) | 712 (75.2) | 585 (75.7) | |
| Poor | 680 (18.8) | 317 (16.8) | 202 (21.3) | 161 (20.8) | |
| Radical resection, yes, n (%) | 2331 (64.5) | 1211 (64.0) | 615 (64.9) | 505 (65.3) | 0.774 |
| Adjuvant chemotherapy, yes, n (%) | 1304 (36.1) | 725 (38.3) | 322 (34.0) | 257 (33.2) | 0.014 |
| KPS score, mean ± SD | 85.78 ± 13.79 | 88.39 ± 10.97 | 84.37 ± 14.69 | 81.11 ± 17.00 | <0.001 |
| Nutrition-related information | |||||
| BMI, kg/m2, mean ± SD | 22.43 ± 3.32 | 23.48 ± 2.83 | 21.97 ± 3.26 | 20.45 ± 3.45 | <0.001 |
| Mid-arm muscle circumference, cm, mean ± SD | 21.12 ± 3.48 | 21.82 ± 3.33 | 20.91 ± 3.03 | 19.69 ± 3.85 | <0.001 |
| Hand grip strength/weight ratio, mean ± SD | 0.42 ± 0.15 | 0.43 ± 0.14 | 0.41 ± 0.14 | 0.40 ± 0.18 | <0.001 |
| Calf circumference, cm, mean ± SD | 32.82 ± 4.20 | 33.83 ± 4.14 | 32.32 ± 3.68 | 30.96 ± 4.21 | <0.001 |
| PGSGA score, ≥4, n (%) | 2274 (63.0) | 638 (33.7) | 896 (94.6) | 740 (95.7) | <0.001 |
| NRS2002 score, ≥3, n (%) | 1204 (33.3) | 169 (8.9) | 539 (56.9) | 496 (64.2) | <0.001 |
| Parenteral nutritional support, yes, n (%) | 1061 (29.4) | 474 (25.1) | 291 (30.7) | 296 (38.3) | <0.001 |
| Enteral nutritional support, yes, n (%) | 1217 (33.7) | 558 (29.5) | 336 (35.5) | 323 (41.8) | <0.001 |
| Laboratory findings | |||||
| Total protein, g/L, mean ± SD | 67.75 ± 8.08 | 68.65 ± 7.60 | 67.18 ± 8.38 | 66.23 ± 8.54 | <0.001 |
| Albumin, g/L, mean ± SD | 39.20 ± 10.67 | 40.40 ± 13.59 | 38.42 ± 5.28 | 37.20 ± 6.16 | <0.001 |
| Prealbumin, mg/L, mean ± SD | 211.46 ± 81.56 | 224.36 ± 75.98 | 205.04 ± 87.04 | 187.76 ± 81.67 | <0.001 |
| Direct bilirubin, μmol/L, mean ± SD | 4.55 ± 9.89 | 4.01 ± 7.58 | 5.10 ± 12.91 | 5.21 ± 10.52 | 0.003 |
| C-reactive protein, mg/L, mean ± SD | 19.17 ± 35.64 | 15.16 ± 29.14 | 19.96 ± 35.11 | 28.00 ± 47.25 | <0.001 |
| Hemoglobin, g/L, mean ± SD | 121.25 ± 23.66 | 125.39 ± 21.94 | 118.72 ± 25.54 | 114.23 ± 23.26 | <0.001 |
| White blood cells, 109/L, mean ± SD | 6.40 ± 3.34 | 6.20 ± 3.30 | 6.40 ± 3.03 | 6.90 ± 3.71 | <0.001 |
| Neutrophils, 109/L, mean ± SD | 5.63 ± 9.85 | 5.32 ± 9.52 | 5.56 ± 9.84 | 6.49 ± 10.60 | 0.021 |
| Red blood cells, 1012/L, mean ± SD | 4.29 ± 2.72 | 4.43 ± 3.57 | 4.16 ± 0.63 | 4.12 ± 1.64 | 0.006 |
| Platelets, 109/L, mean ± SD | 224.06 ± 92.95 | 214.87 ± 84.34 | 232.58 ± 99.35 | 236.10 ± 102.23 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Xu, H.; Zou, Y.; Cui, J.; Xu, K.; Zhou, M.; Guo, P.; Cheng, H.; Shi, H.; Song, C.; et al. Mid-Arm Muscle Circumference or Body Weight-Standardized Hand Grip Strength in the GLIM Superiorly Predicts Survival in Chinese Colorectal Cancer Patients. Nutrients 2022, 14, 5166. https://doi.org/10.3390/nu14235166
Wu T, Xu H, Zou Y, Cui J, Xu K, Zhou M, Guo P, Cheng H, Shi H, Song C, et al. Mid-Arm Muscle Circumference or Body Weight-Standardized Hand Grip Strength in the GLIM Superiorly Predicts Survival in Chinese Colorectal Cancer Patients. Nutrients. 2022; 14(23):5166. https://doi.org/10.3390/nu14235166
Chicago/Turabian StyleWu, Tiantian, Hongxia Xu, Yuanlin Zou, Jiuwei Cui, Kedi Xu, Mingming Zhou, Pengxia Guo, Haoqing Cheng, Hanping Shi, Chunhua Song, and et al. 2022. "Mid-Arm Muscle Circumference or Body Weight-Standardized Hand Grip Strength in the GLIM Superiorly Predicts Survival in Chinese Colorectal Cancer Patients" Nutrients 14, no. 23: 5166. https://doi.org/10.3390/nu14235166
APA StyleWu, T., Xu, H., Zou, Y., Cui, J., Xu, K., Zhou, M., Guo, P., Cheng, H., Shi, H., Song, C., & The Investigation on Nutrition Status and its Clinical Outcome of Common Cancers (INSCOC) Group. (2022). Mid-Arm Muscle Circumference or Body Weight-Standardized Hand Grip Strength in the GLIM Superiorly Predicts Survival in Chinese Colorectal Cancer Patients. Nutrients, 14(23), 5166. https://doi.org/10.3390/nu14235166






