Preparation, Characterization, Wound Healing, and Cytotoxicity Assay of PEGylated Nanophytosomes Loaded with 6-Gingerol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Plant Preparation, Extraction, and Phytochemical Analysis
2.3. HPLC Analysis of the Ginger Crude Extracts
Sample Preparation
2.4. PEGylated Nanophytosome Formation
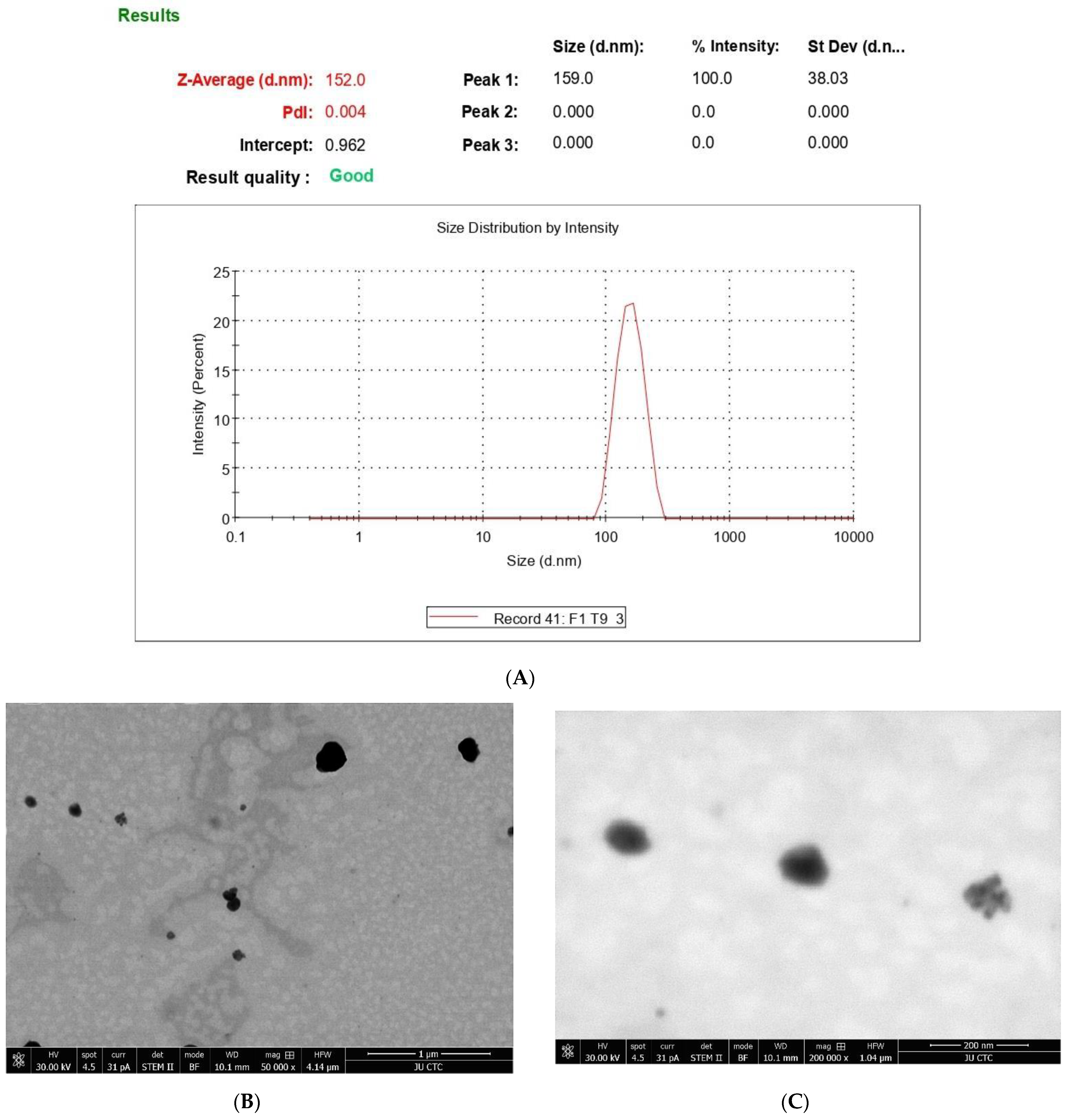
2.5. Characterization of PEGylated Nanophytosomes
2.5.1. Size and Charge
2.5.2. Encapsulation Efficiency (%EE) and Drug Loading (%DL)
2.5.3. Transmission Electron Microscopy (TEM)
2.6. Stability Study (Lyophilization of Loaded PEGylated Nanophytosomes)
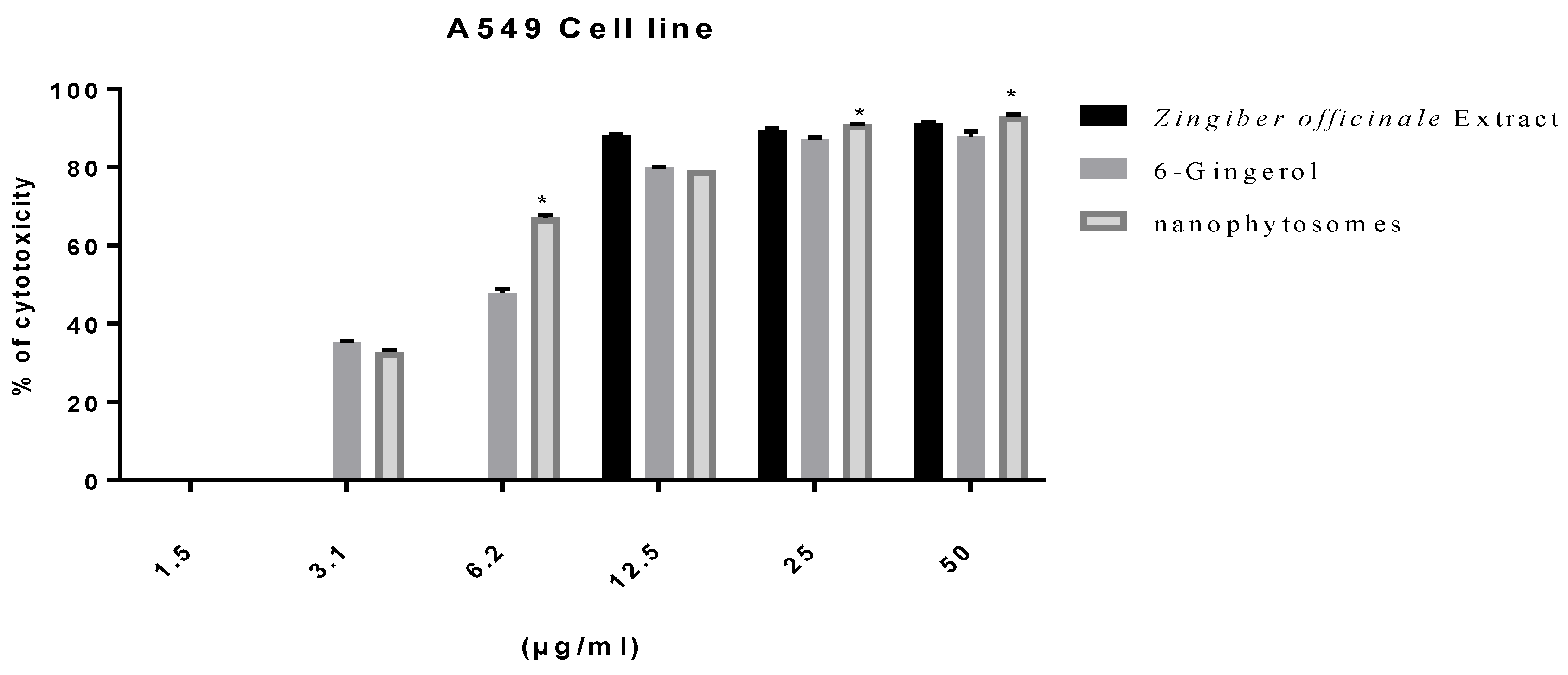
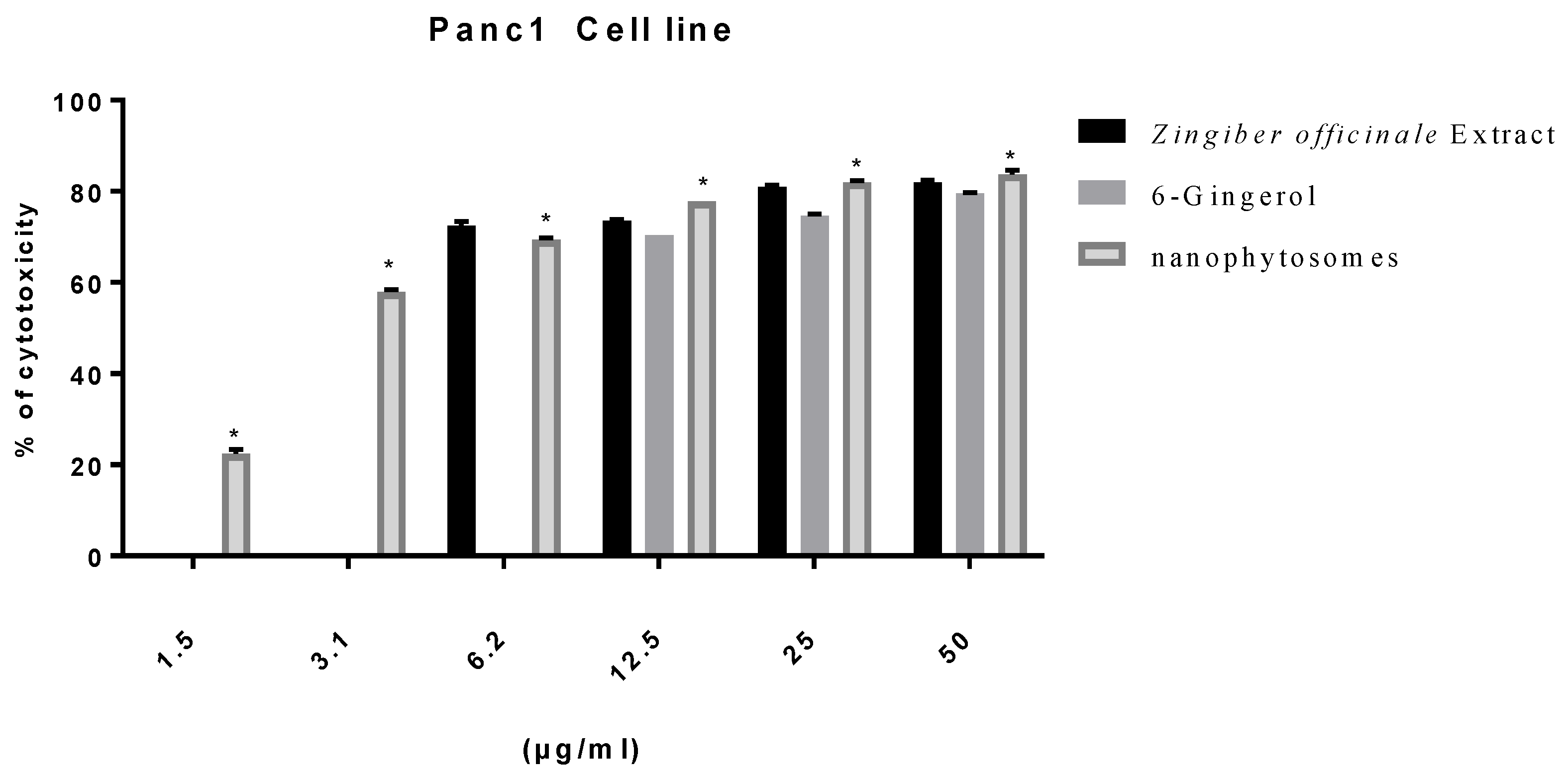
2.7. Viability Assay
2.8. Gene Expression Level Assay
2.9. Ribonucleic Acid (RNA) Extraction and Analysis
2.10. Statistical Analysis
3. Results
3.1. Extraction and Total Phenol, Flavonoid, and Antioxidant Contents of Crude Extract
3.2. Effect of 6-Gingerol on Particle Size, PDI, and Zeta Potential
3.2.1. Stability after Lyophilization
3.2.2. Modulation of Proliferation of Lung and Pancreatic Cancer Cell Lines, as Well as Fibroblasts, by Ginger Extract, 6-Gingerol, and 6-Gingerol Particles Carried by PEGylated Nanophytosomes
3.2.3. Gene Expression of Pro-Inflammatory Cytokines
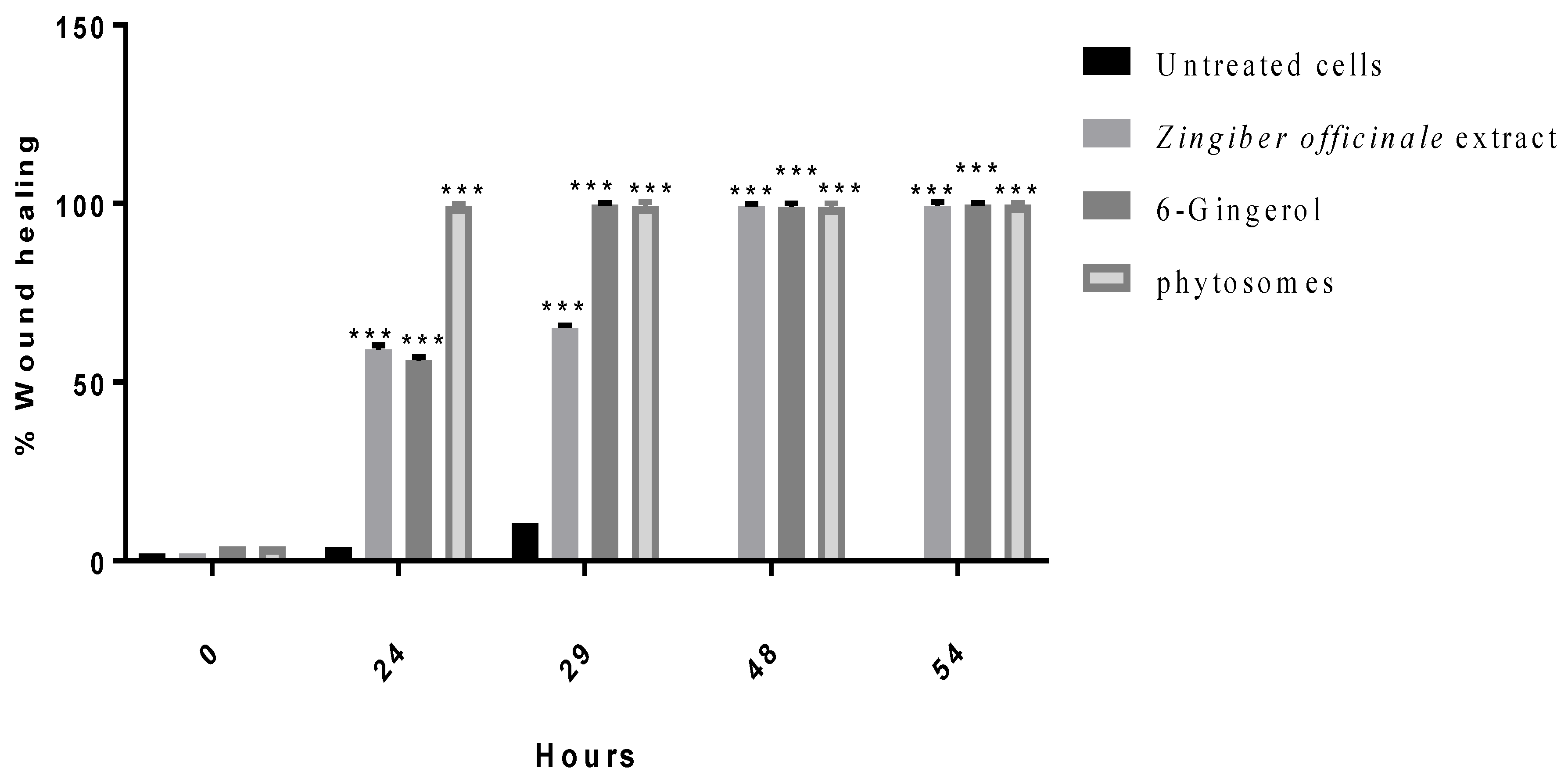
3.2.4. Wound Healing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Lung cancer cells |
| CAP | Capsaicin |
| DDs | Drug delivery systems |
| DLS | Dynamic light scattering |
| DMSO | Dimethyl sulfoxide |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-NPEG2000 |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| EE | Encapsulation efficiency |
| EGFR | Epidermal growth factor receptor |
| EMEM | Eagle’s minimum essential medium |
| FBS | Fetal bovine serum |
| FDA | Food and Drug Administration |
| HPLC | High-performance liquid chromatography |
| IC50 | Half-maximal inhibitory concentration |
| (COX)-2 | Cyclooxygenase |
| IL-1 | Active human IL-1 beta protein |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 |
| MEM | Minimum essential medium |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide solution |
| NP | Nanoparticle |
| NO | Nitric oxide |
| Panc1 | Pancreatic cancer cells |
| PBS | Phosphate-buffered saline |
| PANC1 | Pancreas cancer |
| PDI | Polydispersity index |
| RPMI | RPMI 1640 growth medium |
| RNA | Ribonucleic acid |
| TEM | Transmission electron microscopy |
| TNBC | Triple-negative breast cancer |
| TNFα | Tumor necrosis factor-alpha |
| Z | Zeta |
References
- Percival, N.J. Classification of Wounds and their Management. Surgery 2002, 20, 114–117. [Google Scholar] [CrossRef]
- Friedstat, J.; Brown, D.A.; Levi, B. Chemical, Electrical, and Radiation Injuries. Clin. Plast. Surg. 2017, 44, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Fentahun, N.; Anteneh, Y.; Menber, Y. Malnutrition in the Outcome of Wound Healing at Public Hospitals in Bahir Dar City, Northwest Ethiopia: A Prospective Cohort Study. J. Nutr. Metab. 2021, 2021, 8824951. [Google Scholar] [CrossRef] [PubMed]
- Herman, T.F.; Bordoni, B. Wound Classification. In StatPearl; StatPearl Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Komosinska-Vassev, K.; Rzepecka-Stojko, A.; Olczyk, P. Bee venom in wound healing. Molecules 2020, 26, 148. [Google Scholar] [CrossRef]
- Baliga, M.S.; Latheef, L.; Haniadka, R.; Fazal, F.; Chacko, J.; Arora, R. Ginger (Zingiber officinale Roscoe) in the Treatment and Prevention of Arthritis. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 529–544. [Google Scholar] [CrossRef]
- Tripathi, S.; Maier, K.G.; Bruch, D.; Kittur, D.S. Effect of 6-Gingerol on Pro-Inflammatory Cytokine Production and Costim-ulatory Molecule Expression in Murine Peritoneal Macrophages. J. Surg. Res. 2007, 138, 209–213. [Google Scholar] [CrossRef]
- Al-Rawaf, H.A.; Gabr, S.A.; Alghadir, A.H. Molecular Changes in Diabetic Wound Healing following Administration of Vitamin D and Ginger Supplements: Biochemical and Molecular Experimental Study. Evid.-Based Complement. Altern. Med. 2019, 2019, 4352470. [Google Scholar] [CrossRef]
- Naderi, N.; Karponis, D.; Mosahebi, A.; Seifalian, A.M. Nanoparticles in wound healing; from hope to promise, from prom-ise to routine. Front. Biosci.-Landmark 2018, 23, 1038–1059. [Google Scholar]
- Abu Hajleh, M.N.; Abu-Huwaij, R.; AL-Samydai, A.; Al-Halaseh, L.K.; Al-Dujaili, E.A. The Revolution of Cosmeceuti-cals Delivery by Using Nanotechnology: A Narrative Review of Advantages and Side Effects. J. Cosmet. Dermatol. 2021, 20, 3818–3828. [Google Scholar] [CrossRef]
- Abdelnabi, H.; Alshaer, W.; Azzam, H.; Alqudah, D.; Al-Samydai, A.; Aburjai, T. Loading of capsaicin-in-cyclodextrin inclu-sion complexes into PEGylated liposomes and the inhibitory effect on IL-8 production by MDA-MB-231 and A549 cancer cell lines. Z. Naturforsch. C J. Biosci. 2021, 76, 503–514. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.S.; Azzam, H.; Aburjai, T. Preparation, Characterization, and Anticancer Effects of Capsaicin-Loaded Nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yang, Q.; Wang, Q.; Sun, C.; Zhu, Y.; Niu, Y.; Yu, J.; Xu, X. Formulation, Characterization, and Pharmacokinetic Studies of 6-Gingerol-Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2018, 19, 3661–3669. [Google Scholar] [CrossRef] [PubMed]
- Pawar, N.; Pai, S.; Nimbalkar, M.; Dixit, G. RP-HPLC analysis of phenolic antioxidant compound 6-gingerol from different ginger cultivars. Food Chem. 2011, 26, 1330–1336. [Google Scholar] [CrossRef]
- Roesken, F.; Uhl, E.; Curri, S.B.; Menger, M.D.; Messmer, K. Acceleration of wound healing by topical drug delivery via lip-osomes. Langenbeck’s Arch. Surg. 2000, 385, 42–49. [Google Scholar] [CrossRef]
- Altan, A.; Yuce, H.B.; Karataş, Ő.; Taşkan, M.M.; Gevrek, F.; Çolak, S.; Akbulut, N. Free and liposome form of gallic acid improves calvarial bone wound healing in Wistar rats. Asian Pac. J. Trop. Biomed. 2020, 10, 156. [Google Scholar] [CrossRef]
- Jangde, R.; Singh, D. Preparation and optimization of quercetin-loaded liposomes for wound healing, using response surface methodology. Artif. Cells Nanomed. Biotechnol. 2014, 44, 635–641. [Google Scholar] [CrossRef]
- Kapoor, B.; Singh, S.; Gulati, M.; Gupta, R.; Vaidya, Y. Application of Liposomes in Treatment of Rheumatoid Arthritis: Quo Vadis. Sci. World J. 2014, 2014, 978351. [Google Scholar] [CrossRef]
- Cafino, E.; Lirazan, M.; Marfori, E. A simple HPLC method for the analysis of [6]-gingerol produced by multiple shoot culture of ginger (Zingiber officinale). Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 38–42. [Google Scholar]
- Ghanbarzadeh, S.; Valizadeh, H.; Zakerimilani, P. The Effects of Lyophilization on the Physico-Chemical Stability of Sirolimus Liposomes. Adv. Pharm. Bull. 2013, 3, 25–29. [Google Scholar] [CrossRef]
- Yousef, I.; Oran, S.; Alqaraleh, M.; Bustanji, M. Evaluation of cytotoxic, antioxidant and antibacterial activities of Origanum dayi, Salvia palaestina and Bongardia chrysogonum plants growing wild in Jordan. Trop. J. Nat. Prod. Res. 2021, 5, 66–70. [Google Scholar]
- Al-Tawarah, N.M.; Qaralleh, H.; Khlaifat, A.M.; Nofal, M.N.; Khleifat, K.M.; Al-Limoun, M.O.; Alqaraleh, M.; Al Shhab, M.A. Anticancer and Antibacterial Properties of Verthemia Iphionides Essential Oil/Silver Nanoparticles. Biomed. Pharmacol. J. 2020, 13, 1175–1184. [Google Scholar] [CrossRef]
- Alqaraleh, M.; Kasabri, V.; AL-Majali, I.; Aljaafreh, A.; AL-Othman, N.; Khleifat, K.; AL-Tawarah, N.; Qaralleh, H.; Khwaldeh, A.; Alalawi, S. Branched chain amino Acids as in vitro and in vivo Anti-Oxidation Compounds. Res. J. Pharm. Technol. 2021, 14, 3899–3904. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Furlan, V.; Bren, U. Protective Effects of [6]-Gingerol against Chemical Carcinogens: Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 695. [Google Scholar] [CrossRef]
- Vasarri, M.; Degl’innocenti, D. Antioxidant and Anti-Inflammatory Agents from the Sea: A Molecular Treasure for New Po-tential Drugs. Mar. Drugs 2022, 20, 132. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, P. Inflammation: Biochemistry, cellular targets, anti-inflammatory agents and challenges with special empha-sis on cyclooxygenase 2. Bioorg. Chem. 2022, 121, 105663. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Nascimento, S.; Carvalho DE Queiroz, J.; Fernandes De Medeiros, A.; De França Nunes, A.; Piuvezam, G.; Lima Maciel, B.; Souza Passos, T.; Morais, A. Anti-inflammatory agents as modulators of the inflammation in adipose tissue: A systematic review. PLoS ONE 2022, 17, e0273942. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Fazelian, S.; Agah, S.; Khazdouz, M.; Rahimlou, M.; Agh, F.; Potter, E.; Heshmati, S.; Heshmati, J. Effect of ginger (Zingiber officinale) on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cytokine 2020, 135, 155224. [Google Scholar] [CrossRef]
- Al-Radadi, N.S.; Abdullah; Faisal, S.; Alotaibi, A.; Ullah, R.; Hussain, T.; Rizwan, M.; Saira; Zaman, N.; Iqbal, M.; et al. Zingiber officinale driven bioproduction of ZnO nanoparticles and their anti-inflammatory, anti-diabetic, anti-Alzheimer, anti-oxidant, and anti-microbial applications. Inorg. Chem. Commun. 2022, 140, 109274. [Google Scholar] [CrossRef]
- Daniels, C.; Isaacs, Z.; Finelli, R.; Leisegang, K. The Efficacy of Zingiber officinale on Dyslipidaemia, Blood Pressure, and In-flammation as Cardiovascular Risk Factors: A Systematic Review. Clin. Nutr. ESPEN 2022, 51, 72–82. [Google Scholar] [CrossRef]
- Yeshi, K.; Turpin, G.; Jamtsho, T.; Wangchuk, P. Indigenous Uses, Phytochemical Analysis, and Anti-Inflammatory Properties of Australian Tropical Medicinal Plants. Molecules 2022, 27, 3849. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Basnet, P.; Hussain, H.; Tho, I.; Skalko-Basnet, N. Liposomal delivery system enhances anti-inflammatory properties of cur-cumin. J. Pharm. Sci. 2012, 101, 598–609. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Jamaluddin, A.; Lestari, A. Effectiveness of Ginger Ointment (Zingiber officinale roscoe) on Incision Wound Healing in White Rats (Rattus norvegicus). J. Indones. Vet. Res. 2021, 5, 20–26. [Google Scholar]
- Rahayu, K.I.N.; Suharto, I.P.S.; Etika, A.N.; Nurseskasatmata, S.E. The Effect of Ginger Extract (Zingiber officinale roscoe) on the Number of Neutrophil Cells, Fibroblast and Epithelialization on Incision Wound. J. Phys. Conf. Ser. 2020, 1569, 032063. [Google Scholar] [CrossRef]

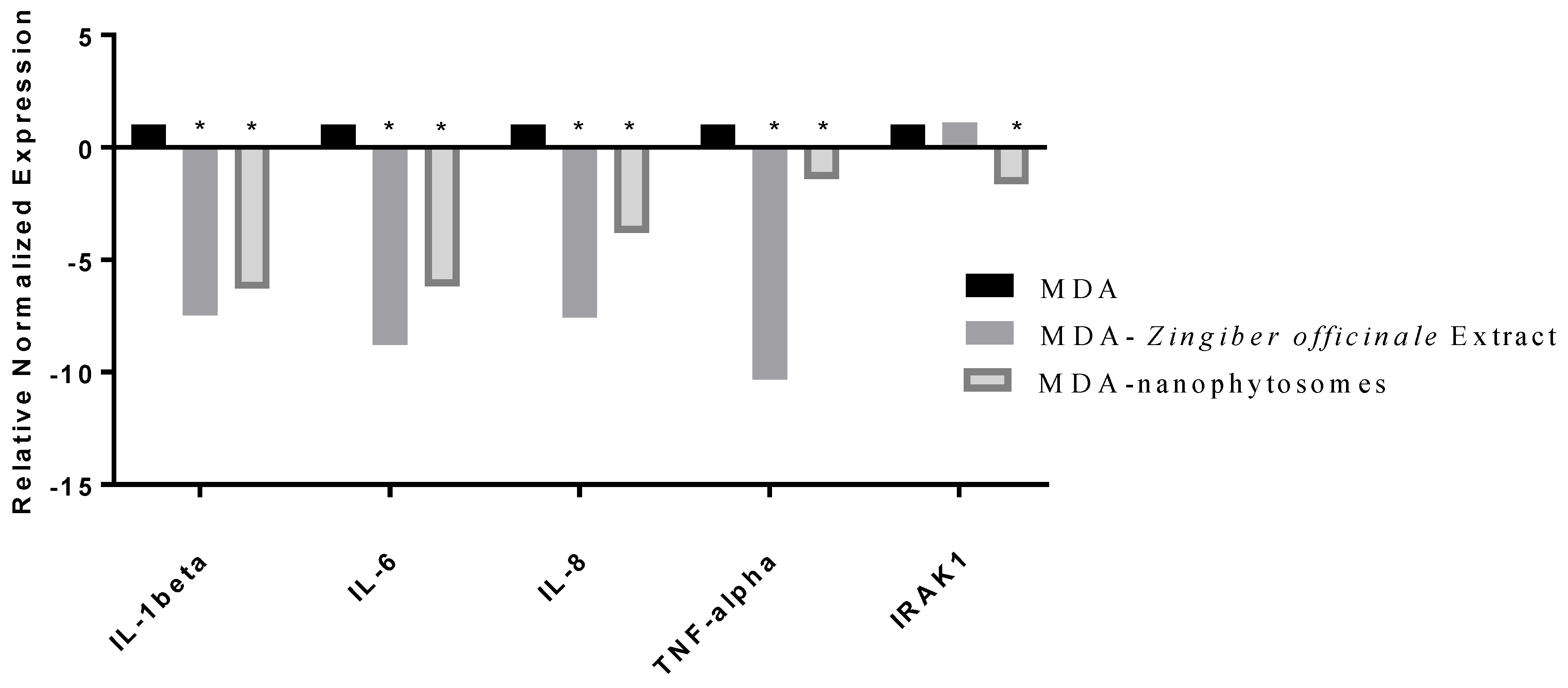
| Description | Two-Sample t-Test | |||||
|---|---|---|---|---|---|---|
| Test | Group | Mean | Std. Deviation | Minimum | Maximum | Sig. |
| Size | 1 | 126.01 | 0.435 | 125.70 | 126.50 | |
| 2 | 150.16 | 1.650 | 148.80 | 152.00 | 0.96 ns | |
| Charge | 1 | −11.13 | 0.152 | −11.00 | −11.30 | |
| 2 | −13.36 | 1.266 | −12.40 | −14.80 | 0.04 * | |
| PDI | 1 | 0.127 | 0.017 | 0.11 | 0.14 | |
| 2 | 0.060 | 0.050 | 0.004 | 0.09 | 0.71 ns | |
| Description | Two-Sample t-Test | |||
|---|---|---|---|---|
| Test | Group | Mean | Std. Deviation | Sig. |
| Size | 1 | 150.1667 | 1.65025 | 0.102 ns |
| 2 | 160.0667 | 9.31254 | ||
| PDI | 1 | 0.0623 | 0.05054 | 0.628 ns |
| 2 | 0.2400 | 0.06207 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Samydai, A.; Qaraleh, M.A.; Alshaer, W.; Al-Halaseh, L.K.; Issa, R.; Alshaikh, F.; Abu-Rumman, A.; Al-Ali, H.; Al-Dujaili, E.A.S. Preparation, Characterization, Wound Healing, and Cytotoxicity Assay of PEGylated Nanophytosomes Loaded with 6-Gingerol. Nutrients 2022, 14, 5170. https://doi.org/10.3390/nu14235170
Al-Samydai A, Qaraleh MA, Alshaer W, Al-Halaseh LK, Issa R, Alshaikh F, Abu-Rumman A, Al-Ali H, Al-Dujaili EAS. Preparation, Characterization, Wound Healing, and Cytotoxicity Assay of PEGylated Nanophytosomes Loaded with 6-Gingerol. Nutrients. 2022; 14(23):5170. https://doi.org/10.3390/nu14235170
Chicago/Turabian StyleAl-Samydai, Ali, Moath Al Qaraleh, Walhan Alshaer, Lidia K. Al-Halaseh, Reem Issa, Fatima Alshaikh, Aseel Abu-Rumman, Hayat Al-Ali, and Emad A. S. Al-Dujaili. 2022. "Preparation, Characterization, Wound Healing, and Cytotoxicity Assay of PEGylated Nanophytosomes Loaded with 6-Gingerol" Nutrients 14, no. 23: 5170. https://doi.org/10.3390/nu14235170
APA StyleAl-Samydai, A., Qaraleh, M. A., Alshaer, W., Al-Halaseh, L. K., Issa, R., Alshaikh, F., Abu-Rumman, A., Al-Ali, H., & Al-Dujaili, E. A. S. (2022). Preparation, Characterization, Wound Healing, and Cytotoxicity Assay of PEGylated Nanophytosomes Loaded with 6-Gingerol. Nutrients, 14(23), 5170. https://doi.org/10.3390/nu14235170







