Anti-Obesity Effects of Multi-Strain Probiotics in Mice with High-Carbohydrate Diet-Induced Obesity and the Underlying Molecular Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Strains
2.2. Oil Red O Staining
2.3. Experimental Murine Model
2.4. Sample Preparation
2.4.1. RNA Extraction
2.4.2. cDNA Synthesis
2.5. Quantitative Real-Time PCR
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Microbiome Analysis
2.7.1. Microbial DNA Extraction and Library Construction
2.7.2. Sequencing and Processing of 16S rRNA
2.8. Statistical Analysis
3. Results
3.1. MSPs Suppress Lipid Accumulation In Vitro
3.2. MSPs Ameliorate Weight Gain and Modulate Body Fat Composition in HCD-Fed Mice
3.3. MSPs Reduce Visceral Adipocyte Number and Attenuate Adipogenesis-Related and Lipogenesis-Related Gene Expression in HCD-Fed Mice
3.4. MSPs Upregulate the Expression of Lipolysis-Related Genes and Fatty Acid Oxidation-Promoting Factors in HCD-Fed Mice
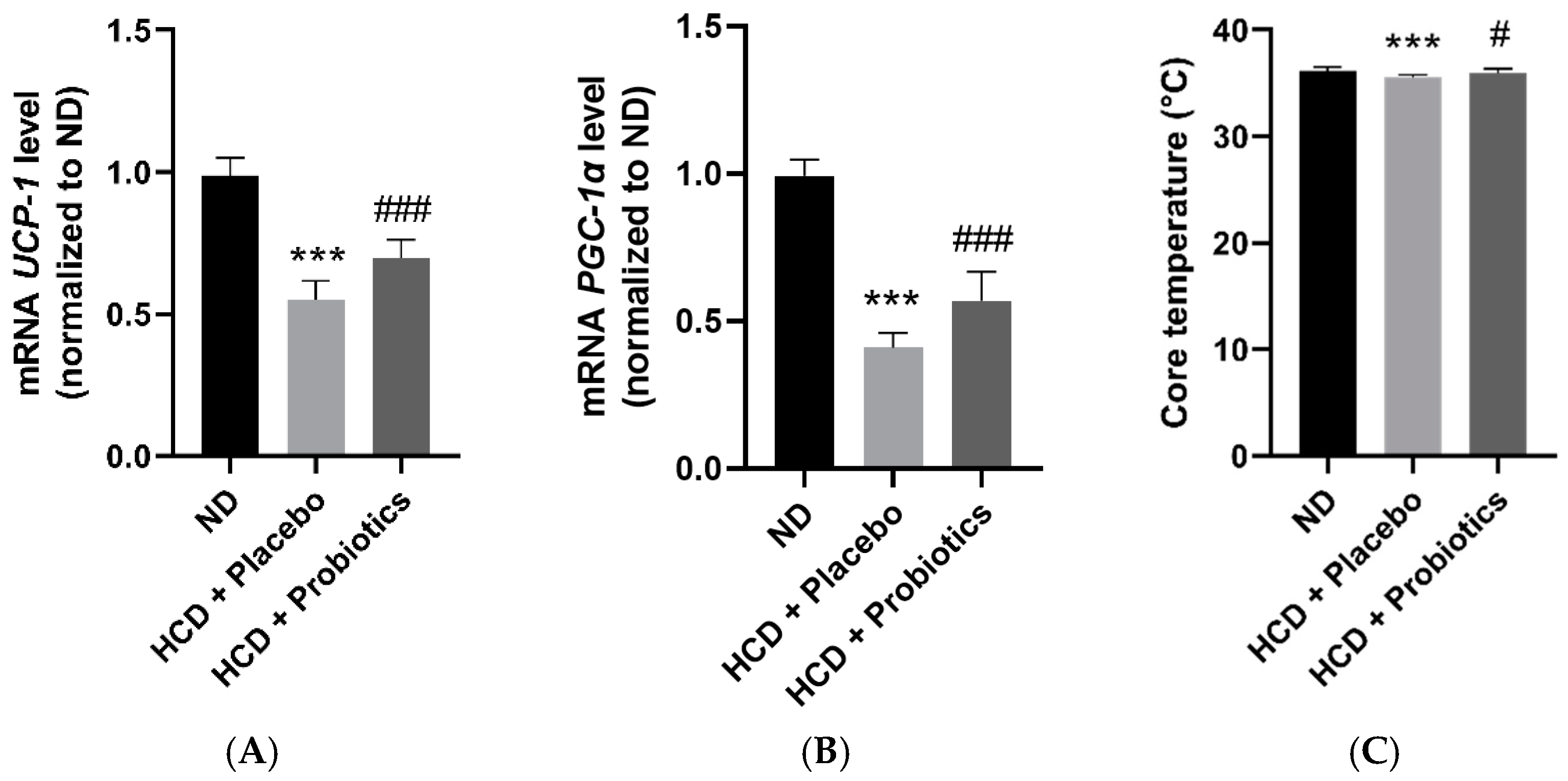
3.5. MSPs Promote the Expression of Thermogenesis-Related Genes in HCD-Fed Mice
3.6. MSPs Regulate the Levels of Obesity-Related Hormones in HCD-Fed Mice
3.7. MSPs Modulate the Intestinal Microbiome in HCD-Fed Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nam, G.E.; Kim, Y.-H.; Han, K.; Jung, J.-H.; Rhee, E.-J.; Lee, S.-S.; Kim, D.J.; Lee, K.-W.; Lee, W.-Y.; on behalf of the Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Obesity Fact Sheet in Korea, 2019: Prevalence of Obesity and Abdominal Obesity from 2009 to 2018 and Social Factors. J. Obes. Metab. Syndr. 2020, 29, 124–132. [Google Scholar] [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berticat, C.; Durand, V.; Raymond, M. Refined Carbohydrate Consumption and Facial Attractiveness. Evol. Psychol. 2020, 18, 1474704920960440. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, B.; O’Keefe, E.L.; O’Keefe, J.H. Death by Carbs: Added Sugars and Refined Carbohydrates Cause Diabetes and Cardiovascular Disease in Asian Indians. Mo. Med. 2016, 113, 395–400. [Google Scholar] [PubMed]
- Ludwig, D.S.; Ebbeling, C.B.; Rimm, E.B. Carbohydrates, Insulin Secretion, and “Precision Nutrition”. Diabetes Care 2022, 45, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Ebbeling, C.B.; Feldman, H.A.; Klein, G.L.; Wong, J.M.W.; Bielak, L.; Steltz, S.K.; Luoto, P.K.; Wolfe, R.R.; Wong, W.W.; Ludwig, D.S. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: Randomized trial. BMJ 2018, 363, k4583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A.; on behalf of the Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Yoo, S.R.; Kim, Y.J.; Park, D.Y.; Jung, U.J.; Jeon, S.M.; Ahn, Y.T.; Huh, C.S.; McGregor, R.; Choi, M.S. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity 2013, 21, 2571–2578. [Google Scholar] [CrossRef]
- Pothuraju, R.; Sharma, R.K.; Chagalamarri, J.; Kavadi, P.K.; Jangra, S. Influence of milk fermented with Lactobacillus rhamnosus NCDC 17 alone and in combination with herbal ingredients on diet induced adiposity and related gene expression in C57BL/6J mice. Food Funct. 2015, 6, 3576–3584. [Google Scholar] [CrossRef]
- Ejtahed, H.-S.; Angoorani, P.; Soroush, A.-R.; Atlasi, R.; Hasani-Ranjbar, S.; Mortazavian, A.M.; Larijani, B. Probiotics supplementation for the obesity management; A systematic review of animal studies and clinical trials. J. Funct. Foods 2019, 52, 228–242. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Schütz, F.; Figueiredo-Braga, M.; Barata, P.; Cruz-Martins, N. Obesity and gut microbiome: Review of potential role of probiotics. Porto Biomed. J. 2021, 6, e111. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Nam, M.H.; Lee, H.; Lee, J.S.; Kim, H.; Chung, M.J.; Seo, J.G. Amelioration of obesity-related characteristics by a probiotic formulation in a high-fat diet-induced obese rat model. Eur. J. Nutr. 2018, 57, 2081–2090. [Google Scholar] [CrossRef]
- Lee, E.; Jung, S.-R.; Lee, S.-Y.; Lee, N.-K.; Paik, H.-D.; Lim, S.-I. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolls, B.J. The relationship between dietary energy density and energy intake. Physiol. Behav. 2009, 97, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Yun, S.-I.; Park, M.-H.; Park, J.-H.; Jeong, S.-Y.; Park, H.-O. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Lu, T.Y.; Cheng, M.C.; Lu, H.C.; Wu, M.F.; Hsu, C.L. Deep Sea Water Improves Abnormalities in Lipid Metabolism through Lipolysis and Fatty Acid Oxidation in High-Fat Diet-Induced Obese Rats. Mar. Drugs 2017, 15, 386. [Google Scholar] [CrossRef]
- Pan, R.; Zhu, X.; Maretich, P.; Chen, Y. Combating Obesity With Thermogenic Fat: Current Challenges and Advancements. Front. Endocrinol. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Xie, M.; Cao, L.; Shi, J.; Xu, G.; Hu, C.; Wang, C. Browning of Pig White Preadipocytes by Co-Overexpressing Pig PGC-1α and Mice UCP1. Cell. Physiol. Biochem. 2018, 48, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-S.; Lee, Y.-J.; Kang, H.; Yang, G.; Hong, E.J.; Lim, J.Y.; Oh, S.; Kim, E. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARγ signaling. Sci. Rep. 2019, 9, 20152. [Google Scholar] [CrossRef] [Green Version]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [Green Version]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Hall, K.D. Carbohydrates, insulin, and obesity. Science 2021, 372, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Kuppusamy, P.; Srisesharam, S.; Lee, J.C.; Sivanesan, R.; Kim, D.; Choi, K.C. Positive metabolic effects of selected probiotic bacteria on diet-induced obesity in mice are associated with improvement of dysbiotic gut microbiota. FASEB J. 2020, 34, 12289–12307. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Piza, A.; Lee, S.-J. Effects of dietary fibers and prebiotics in adiposity regulation via modulation of gut microbiota. Appl. Biol. Chem. 2020, 63, 2. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Possemiers, S.; Druart, C.; Van de Wiele, T.; De Backer, F.; Cani, P.D.; Larondelle, Y.; Delzenne, N.M. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 2011, 6, e20944. [Google Scholar] [CrossRef] [Green Version]
- Ordiz, M.I.; May, T.D.; Mihindukulasuriya, K.; Martin, J.; Crowley, J.; Tarr, P.I.; Ryan, K.; Mortimer, E.; Gopalsamy, G.; Maleta, K.; et al. The effect of dietary resistant starch type 2 on the microbiota and markers of gut inflammation in rural Malawi children. Microbiome 2015, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.R.; Seo, E.; Oh, S.; Seo, M.; Byun, K.; Kim, B.-Y. Anti-Obesity Effects of Multi-Strain Probiotics in Mice with High-Carbohydrate Diet-Induced Obesity and the Underlying Molecular Mechanisms. Nutrients 2022, 14, 5173. https://doi.org/10.3390/nu14235173
Kim HR, Seo E, Oh S, Seo M, Byun K, Kim B-Y. Anti-Obesity Effects of Multi-Strain Probiotics in Mice with High-Carbohydrate Diet-Induced Obesity and the Underlying Molecular Mechanisms. Nutrients. 2022; 14(23):5173. https://doi.org/10.3390/nu14235173
Chicago/Turabian StyleKim, Hye Rim, Eunsol Seo, Seyeon Oh, MinYeong Seo, Kyunghee Byun, and Byung-Yong Kim. 2022. "Anti-Obesity Effects of Multi-Strain Probiotics in Mice with High-Carbohydrate Diet-Induced Obesity and the Underlying Molecular Mechanisms" Nutrients 14, no. 23: 5173. https://doi.org/10.3390/nu14235173
APA StyleKim, H. R., Seo, E., Oh, S., Seo, M., Byun, K., & Kim, B.-Y. (2022). Anti-Obesity Effects of Multi-Strain Probiotics in Mice with High-Carbohydrate Diet-Induced Obesity and the Underlying Molecular Mechanisms. Nutrients, 14(23), 5173. https://doi.org/10.3390/nu14235173





