Identifying Predictors for Minimum Dietary Diversity and Minimum Meal Frequency in Children Aged 6–23 Months in Uganda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Indicators of Complementary Feeding
2.3. Descriptive Analysis of Complementary Feeding Indicators
2.4. Multivariable Analysis of the Determinants of Complementary Feeding
2.5. Selection of Predictors
2.6. Control Variables
2.7. Multivariable Model
3. Results
3.1. Sample Characteristics
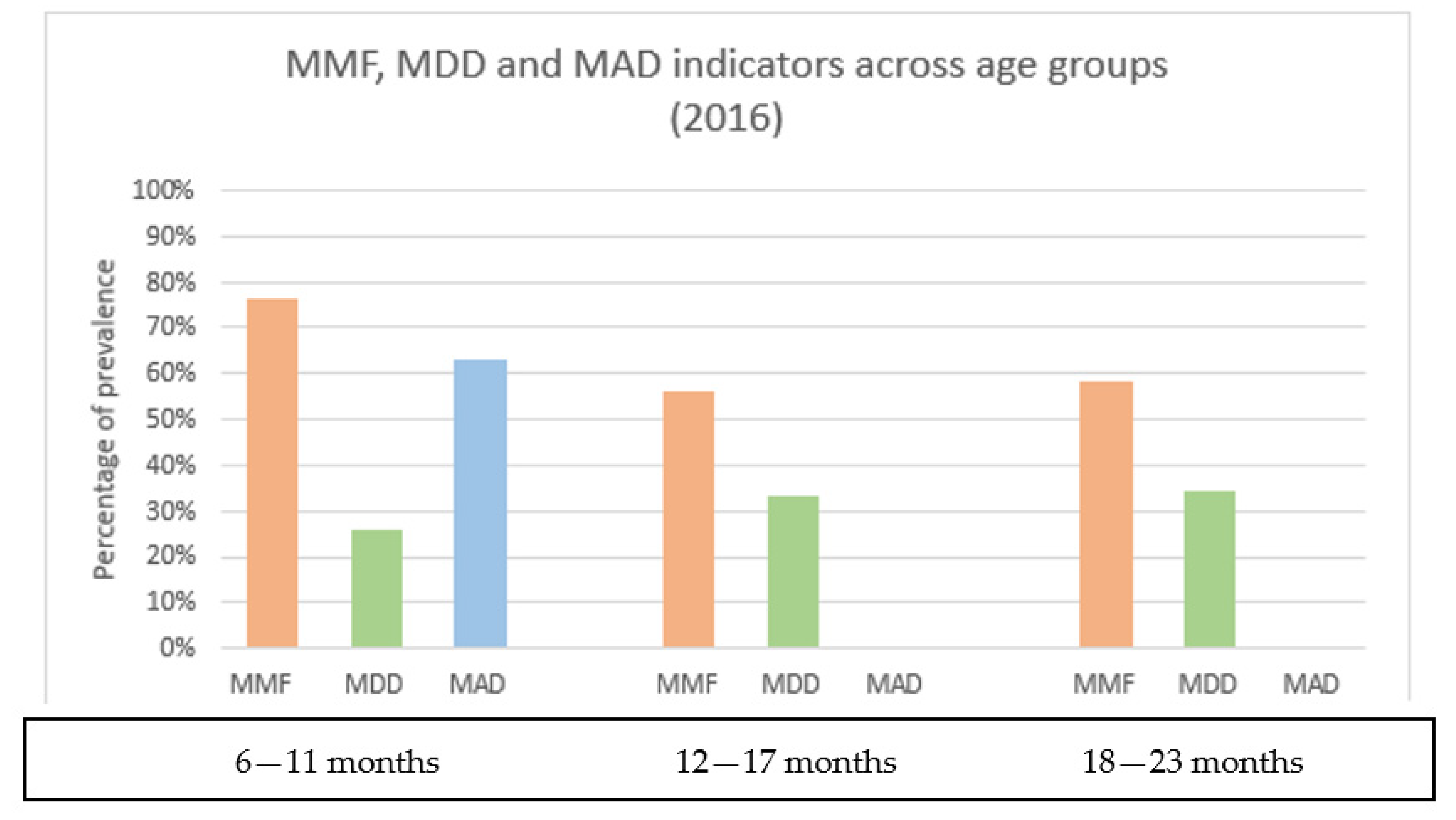
3.2. Description of Complementary Feeding Foods and Distribution of CF Indicators by Child Age Groups and by Region
3.3. Determinants of MMF and MDD
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Work Programme of the United Nations Decade of Action on Nutrition (2016–2025). Available online: https://www.un.org/nutrition/ (accessed on 4 September 2020).
- WHO; UNICEF. Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Rights, U.N.H. Convention on the Rights of the Child. Available online: https://www.ohchr.org/en/professionalinterest/pages/crc.aspx (accessed on 5 February 2022).
- Lartey, A. Maternal and child nutrition in Sub-Saharan Africa: Challenges and interventions. Proc. Nutr. Soc. 2008, 67, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Statistics (UBOS); ICF. Uganda Demographic and Health Survey 2016; UBOS: Kampala, Uganda; ICF: Rockville, MD, USA, 2018.
- UNAP. Uganda Nutrition Action Plan 2018–2025; Ministry of Health, Uganda: Kampala, Uganda, 2018.
- Rakotomanana, H.; Hildebrand, D.; Gates, G.E.; Thomas, D.G.; Fawbush, F.; Stoecker, B.J. Maternal Knowledge, Attitudes, and Practices of Complementary Feeding and Child Undernutrition in the Vakinankaratra Region of Madagascar: A Mixed-Methods Study. Curr. Dev. Nutr. 2020, 4, nzaa162. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Arif, M.; Shah, A.A. Complementary feeding practices and associated factors among children aged 6–23 months in Pakistan. PLoS ONE 2021, 16, e0247602. [Google Scholar] [CrossRef] [PubMed]
- Hector, D.; King, L.; Webb, K.; Heywood, P. Factors affecting breastfeeding practices: Applying a conceptual framework. New South Wales Public Health Bull. 2005, 16, 52–55. [Google Scholar]
- Na, M.; Aguayo, V.M.; Arimond, M.; Mustaphi, P.; Stewart, C.P. Predictors of complementary feeding practices in Afghanistan: Analysis of the 2015 Demographic and Health Survey. Matern. Child Nutr. 2018, 14 (Suppl. S4), e12696. [Google Scholar] [CrossRef]
- Heckert, J.; Olney, D.K.; Ruel, M.T. Is women’s empowerment a pathway to improving child nutrition outcomes in a nutrition-sensitive agriculture program?: Evidence from a randomized controlled trial in Burkina Faso. Soc. Sci. Med. 2019, 233, 93–102. [Google Scholar] [CrossRef]
- Na, M.; Jennings, L.; Talegawkar, S.A.; Ahmed, S. Association between women’s empowerment and infant and child feeding practices in sub-Saharan Africa: An analysis of Demographic and Health Surveys. Public Health Nutr. 2015, 18, 3155–3165. [Google Scholar] [CrossRef] [Green Version]
- Mokori, A.; Schonfeldt, H.; Hendriks, S.L. Child factors associated with complementary feeding practices in Uganda. S. Afr. J. Clin. Nutr. 2017, 30, 7–14. [Google Scholar] [CrossRef]
- Ssemukasa, E.; Kearney, J. Complementary feeding practices in Wakiso district of Uganda. Afr. J. Food Agric. Nutr. Dev. 2014, 14, 9085–9103. [Google Scholar] [CrossRef]
- Mokori, A. Nutritional status, complementary feeding practices and feasible strategies to promote nutrition in returnee children aged 6–23 months in northern Uganda. S. Afr. J. Clin. Nutr. 2012, 25, 173–179. [Google Scholar] [CrossRef]
- Wamani, H.; Åstrøm, A.N.; Peterson, S.; Tylleskär, T.; Tumwine, J.K. Infant and Young Child Feeding in Western Uganda: Knowledge, Practices and Socio-economic Correlates. J. Trop. Pediatr. 2005, 51, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Bagaaya, S.; Wamani, H.; Kajura, R. Complementary Feeding Practices and Associated Factors Among Infants and Young Children 6–23 Months in Fort Portal Kabarole District Uganda (P11-049-19). Curr. Dev. Nutr. 2019, 3, nzz048.P11-049-19. [Google Scholar] [CrossRef] [Green Version]
- Kajjura, R.B.; Veldman, F.J.; Kassier, S.M. Maternal socio-demographic characteristics and associated complementary feeding practices of children aged 6–18 months with moderate acute malnutrition in Arua, Uganda. J. Hum. Nutr. Diet. 2019, 32, 303–310. [Google Scholar] [CrossRef] [PubMed]
- OCHA. Uganda-Subnational Administrative Boundaries. Available online: https://data.humdata.org/dataset/cod-ab-uga? (accessed on 18 April 2022).
- Geoportal, I. Lake Victoria Basin. Available online: http://geoportal.icpac.net/layers/geonode%3Alv_basin (accessed on 18 April 2022).
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Part 2: Measurement; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- WHO; USAID; AED; UNICEF; IFPRI; UCDAVIS. Indicators for Assessing Infant and Young Child Feeding Practices. Part 1 Definitions; USAID: Washington, DC, USA, 2007.
- Stewart, C.P.; Iannotti, L.; Dewey, K.G.; Michaelsen, K.F.; Onyango, A.W. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child Nutr. 2013, 9 (Suppl. S2), 27–45. [Google Scholar] [CrossRef]
- Jennings, L.; Na, M.; Cherewick, M.; Hindin, M.; Mullany, B.; Ahmed, S. Women’s empowerment and male involvement in antenatal care: Analyses of Demographic and Health Surveys (DHS) in selected African countries. BMC Pregnancy Childbirth 2014, 14, 297. [Google Scholar] [CrossRef] [Green Version]
- Rutsein, S.O.; Kiersten, J. The DHS Wealth Index. DHS Comparative Reports No.6; ORC Macro: Calverton, MD, USA, 2004. [Google Scholar]
- Krebs, N.F.; Hambidge, K.M. Complementary feeding: Clinically relevant factors affecting timing and composition. Am. J. Clin. Nutr. 2007, 85, 639s–645s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arimond, M.; Daelmans, B.; Dewey, K. Indicators for feeding practices in children. Lancet 2008, 371, 541–542. [Google Scholar] [CrossRef]
- Caetano, M.C.; Ortiz, T.T.; Silva, S.G.; Souza, F.I.; Sarni, R.O. Complementary feeding: Inappropriate practices in infants. J. Pediatr. (Rio J.) 2010, 86, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, G.; Berrang-Ford, L.; Twesigomwe, S.; Kakwangire, P.; Galazoula, M.; Zavaleta-Cortijo, C.; Patterson, K.; Namanya, D.B.; Lwasa, S.; Nowembabazi, E.; et al. Socio-economic and environmental factors affecting breastfeeding and complementary feeding practices among Batwa and Bakiga communities in south-western Uganda. PLoS Glob. Public Health 2022, 2, e0000144. [Google Scholar] [CrossRef]
- Brown, K.H. Diarrhea and malnutrition. J. Nutr. 2003, 133, 328s–332s. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Marquardt, M.; Bwibo, N. The impact of morbidity on food intake in rural Kenyan children. South Afr. J. Clin. Nutr. 2012, 25, 142–148. [Google Scholar] [CrossRef]
- Alderman, H.; Haddad, L.; Headey, D.D.; Smith, L. Association between economic growth and early childhood nutrition. Lancet Glob. Health 2014, 2, e500. [Google Scholar] [CrossRef] [Green Version]
- Alderman, H.; Headey, D. The timing of growth faltering has important implications for observational analyses of the underlying determinants of nutrition outcomes. PLoS ONE 2018, 13, e0195904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, A.; Oh, J.; Lee, J.-K.; Lee, H.-Y.; Perkins, J.M.; Heo, J.; Ro, Y.S.; Subramanian, S.V. Short-term and long-term associations between household wealth and physical growth: A cross-comparative analysis of children from four low- and middle-income countries. Glob. Health Action 2015, 8, 26523. [Google Scholar] [CrossRef] [PubMed]
- Zere, E.; McIntyre, D. Inequities in under-five child malnutrition in South Africa. Int. J. Equity Health 2003, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Thang, N.M.; Popkin, B.M. In an era of economic growth, is inequity holding back reductions in child malnutrition in Vietnam? Asia Pac. J. Clin. Nutr. 2003, 12, 405–410. [Google Scholar]
- Jones, R.; Haardörfer, R.; Ramakrishnan, U.; Yount, K.M.; Miedema, S.; Girard, A.W. Women’s empowerment and child nutrition: The role of intrinsic agency. SSM-Popul. Health 2019, 9, 100475. [Google Scholar] [CrossRef]
- Hodgson, D.L. Pastoralism, patriarchy and history: Changing gender relations among Maasai in Tanganyika, 1890–1940. J. Afr. Hist. 1999, 40, 41–65. [Google Scholar] [CrossRef]
- Holtzman, J. Politics and gastropolitics: Gender and the power of food in two African pastoralist societies. J. R. Anthropol. Inst. 2002, 8, 259–278. [Google Scholar] [CrossRef]
- Bose, S. The Effect of Women’s Status and Community on the Gender Differential in Children’s Nutrition in India. J. Biosoc. Sci. 2011, 43, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Ickes, S.B.; Hurst, T.E.; Flax, V.L. Maternal Literacy, Facility Birth, and Education Are Positively Associated with Better Infant and Young Child Feeding Practices and Nutritional Status among Ugandan Children. J. Nutr. 2015, 145, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Shroff, M.R.; Griffiths, P.L.; Suchindran, C.; Nagalla, B.; Vazir, S.; Bentley, M.E. Does maternal autonomy influence feeding practices and infant growth in rural India? Soc. Sci. Med. 2011, 73, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Semahegn, A.; Tesfaye, G.; Bogale, A. Complementary feeding practice of mothers and associated factors in Hiwot Fana Specialized Hospital, Eastern Ethiopia. Pan Afr. Med J. 2014, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.A.; Phalke, D.B. Beliefs and practices regarding diet in common childhood illnesses among rural caregivers. J. Med. Nutr. Nutraceuticals 2014, 3, 99. [Google Scholar] [CrossRef]
- Kanjilal, B.; Mazumdar, P.G.; Mukherjee, M.; Rahman, M.H. Nutritional status of children in India: Household socio-economic condition as the contextual determinant. Int. J. Equity Health 2010, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Malapit, H.J.L.; Kadiyala, S.; Quisumbing, A.R.; Cunningham, K.; Tyagi, P. Women’s empowerment mitigates the negative effects of low production diversity on maternal and child nutrition in Nepal. J. Dev. Stud. 2015, 51, 1097–1123. [Google Scholar] [CrossRef] [Green Version]
- Das, J.; Das, S.K.; Hasan, T.; Ahmed, S.; Ferdous, F.; Begum, R.; Chisti, M.J.; Malek, M.A.; Mamun, A.A.; Faruque, A.S.G. Childhood malnutrition in households with contemporary siblings: A scenario from urban Bangladesh. Eur. J. Clin. Nutr. 2015, 69, 1178–1179. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, V.M. Complementary feeding practices for infants and young children in South Asia. A review of evidence for action post-2015. Matern. Child Nutr. 2017, 13, e12439. [Google Scholar] [CrossRef] [Green Version]
- Schönfeldt, H.C.; Gibson Hall, N. Dietary protein quality and malnutrition in Africa. Br. J. Nutr. 2012, 108, S69–S76. [Google Scholar] [CrossRef] [Green Version]
- Lowder, S.K.; Carisma, B.; Skoet, J. Who invests in agriculture and how much? An empirical review of the relative size of various investments in agriculture in low- and middle-income countries. Eur. J. Dev. Res. 2012, 27, 371–390. [Google Scholar] [CrossRef]
- Gallegos-Riofrío, C.A.; Waters, W.F.; Salvador, J.M.; Carrasco, A.M.; Lutter, C.K.; Stewart, C.P.; Iannotti, L.L. The Lulun Project’s social marketing strategy in a trial to introduce eggs during complementary feeding in Ecuador. Matern. Child Nutr. 2018, 14, e12700. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Chapnick, M.; Nicholas, J.; Gallegos-Riofrio, C.A.; Moreno, P.; Douglas, K.; Habif, D.; Cui, Y.; Stewart, C.; Lutter, C.K.; et al. Egg intervention effect on linear growth no longer present after two years. Matern. Child Nutr. 2020, 16, e12925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, S.E.; Lewis, D.; Travis, A.J. Small-scale egg production centres increase children’s egg consumption in rural Zambia. Matern. Child Nutr. 2018, 14, e12662. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Banta, J.E.; Betancourt, J.A. Relationship between household wealth inequality and chronic childhood under-nutrition in Bangladesh. Int. J. Equity Health 2006, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Dhami, M.V.; Ogbo, F.A.; Osuagwu, U.L.; Agho, K.E. Prevalence and factors associated with complementary feeding practices among children aged 6–23 months in India: A regional analysis. BMC Public Health 2019, 19, 1034. [Google Scholar] [CrossRef] [Green Version]
- Gatica-Domínguez, G.; Neves, P.A.R.; Barros, A.J.D.; Victora, C.G. Complementary Feeding Practices in 80 Low- and Middle-Income Countries: Prevalence of and Socioeconomic Inequalities in Dietary Diversity, Meal Frequency, and Dietary Adequacy. J. Nutr. 2021, 151, 1956–1964. [Google Scholar] [CrossRef]
- Nwosu, C.O.; Ataguba, J.E.-O. Explaining changes in wealth inequalities in child health: The case of stunting and wasting in Nigeria. PLoS ONE 2020, 15, e0238191. [Google Scholar] [CrossRef]
- Darteh, E.K.M.; Acquah, E.; Kumi-Kyereme, A. Correlates of stunting among children in Ghana. BMC Public Health 2014, 14, 504. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, P.; Dongre, A.; Garg, B. Childhood morbidity, household practices and health care seeking for sick children in a tribal district of Maharashtra, India. Indian J. Med. Sci. 2010, 64, 7–16. [Google Scholar] [CrossRef]
- Degefa, N.; Tadesse, H.; Aga, F.; Yeheyis, T. Sick Child Feeding Practice and Associated Factors among Mothers of Children Less Than 24 Months Old, in Burayu Town, Ethiopia. Int. J. Pediatr. 2019, 2019, 3293516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.H.; Peerson, J.M.; Rivera, J.; Allen, L.H. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2002, 75, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.S.; Ryman, T.K.; Dietz, V. Experiences Integrating Delivery of Maternal and Child Health Services with Childhood Immunization Programs: Systematic Review Update. J. Infect. Dis. 2012, 205, S6–S19. [Google Scholar] [CrossRef] [PubMed]
- Kishor, S.; Subaiya, L. Understanding Women’s Empowerment: A Comparative Analysis of Demographic and Health Surveys (DHS) Data; Macro International: Calverton, MD, USA, 2008. [Google Scholar]
- Pratley, P. Associations between quantitative measures of women’s empowerment and access to care and health status for mothers and their children: A systematic review of evidence from the developing world. Soc. Sci. Med. 2016, 169, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Yount, K.M.; DiGirolamo, A.M.; Ramakrishnan, U. Impacts of domestic violence on child growth and nutrition: A conceptual review of the pathways of influence. Soc. Sci. Med. 2011, 72, 1534–1554. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.J.; Kordas, K.; Murray-Kolb, L.E. Associations between women’s autonomy and child nutritional status: A review of the literature. Matern. Child Nutr. 2015, 11, 452–482. [Google Scholar] [CrossRef]
- Scarpa, G.; Berrang-Ford, L.; Bawajeeh, A.O.; Twesigomwe, S.; Kakwangire, P.; Peters, R.; Beer, S.; Williams, G.; Zavaleta-Cortijo, C.; Namanya, D.B.; et al. Developing an online food composition database for an Indigenous population in south-western Uganda. Public Health Nutr. 2021, 24, 2455–2464. [Google Scholar] [CrossRef]
- Scarpa, G.; Berrang-Ford, L.; Twesigomwe, S.; Kakwangire, P.; Peters, R.; Zavaleta-Cortijo, C.; Patterson, K.; Namanya, D.B.; Lwasa, S.; Nowembabazi, E.; et al. A Community-Based Approach to Integrating Socio, Cultural and Environmental Contexts in the Development of a Food Database for Indigenous and Rural Populations: The Case of the Batwa and Bakiga in South-Western Uganda. Nutrients 2021, 13, 3503. [Google Scholar] [CrossRef]


| Indicators | Indicator Definition | 6–8 Months | 9–23 Months |
|---|---|---|---|
| Introduction of solid, semi-solid or soft food 6–8 months | Percentage of infants 6–8 months of age who consumed solid, semi-solid or soft foods during the previous day. | Same for all age groups | Same for all age groups |
| Minimum dietary diversity (MDD) | Percentage of children 6–23 months of age who consumed foods and beverages from at least four out of seven defined food groups during the previous day | Same for all age groups | Same for all age groups |
| Minimum meal frequency (MMF) | Percentage of children 6–23 months of age who consumed solid, semi-solid or soft foods (also including milk feeds for non-breastfed children) at least the minimum number of times during the previous day. | Breastfed children: Number of solid, semi-solid, or soft foods ≥2 Non-breastfed children: Total of solid, semi-solid, or soft foods AND milk feeds ≥4 | Number of solid, semi-solid, or soft foods ≥3 |
| Minimum acceptable diet (MAD) | Percentage of children 6–23 months of age who consumed a minimum acceptable diet (see columns to right for definitions according to age group and breastfeeding status) during the previous day. | Breastfed children: Number of food categories ≥4 AND Number of solid, semi-solid, or soft foods ≥2 Non-breastfed children: Number of food categories ≥4, AND Number of milk feeds ≥2, AND Total number of solid, semi-solid, or soft foods AND milk feeds ≥4 | Number of food categories ≥4 AND Number of solid, semi-solid, or soft foods ≥3 |
| Variable | Definition | Description |
|---|---|---|
| Outcome variables | ||
| Minimum dietary diversity | Did the child consume food and beverages from at least four out of seven defined food groups during the previous day? Binary variable: Yes/No | This variable was also stratified by age groups (6–11 months, 12–17 months, 18–23 months) based on international indicators. |
| Minimum meal frequency | Did the child eat the minimum number of times which is appropriate for his/her age during the previous day (2, 3 or 4 times depending on breastfeeding status)? Binary variable: Yes/No | This variable was also stratified by age groups (6–11 months, 12–17 months, 18–23 months) based on international indicators. |
| Predictor variables | ||
| Female empowerment | What is the female empowerment score for the mother? Categorical variable: Very low, low, medium, high | This score (0–13 points) was created and previously used as a discrete variable by Jennings et al. [25]; however for this study we grouped the score in 4 categories (very low if the score was lower than 4, low if the score was equal to 5–6, medium if the score was equal to 7–9, high if the score was higher than 10). |
| Family wealth | What is the wealth percentile of the child’s family? Categorical variable: lowest, second, middle, fourth, highest | The family wealth is a composite variable found in the UDHS 2016, which is calculated based on: house’s ownership; materials used to build the house; typology of sanitation facilities and water access. It was generated using principal components analysis. The variable divides household wealth into 5 wealth quintiles. |
| Child health—vaccination status | Has the child completed vaccinations for his/her age? Categorical variable: fully vaccinated, partially vaccinated, not vaccinated | Fully vaccinated children included those who received all vaccinations according to their age group (6–11 months, 12–17 months & 18–23 months). Partially vaccinated children included those who received at least 1 vaccination, but not all vaccinations according to their age group. Not vaccinated children included those who did not receive any vaccinations. |
| Child health—sick in the past 2 weeks | Was the child sick with fever, cough or diarrhoea in the past 2 weeks? Binary variable: Yes/No | This variable is composite, and derives from 3 different variables available in the UDHS 2016: Did the child have fever in the past 2 weeks? Did the child have a cough in the past 2 weeks? Did the child have diarrhoea in the past 2 weeks? A child was considered sick when one or more of these three variables was positive. |
| Control variables | ||
| Sex of child | Which is the sex of the child? Binary variable: Female/Male | Female or male child |
| Current breastfeeding status | Is the child currently breastfeeding? Binary variable: Yes/No | This variable investigates if the child is still being breastfed and not only if he/she was breastfed. |
| N. Antenatal visits | How many antenatal visits did the mother attend during pregnancy? Categorical variable: No visits, 1–3 visits, >4 visits | Number of antenatal visits attended during pregnancy by the mother. |
| Maternal education | What is the mother’s education level? Categorical variable: No education, primary education, secondary education or higher | Highest level of education that the mother acquired in her life, divided into 3 categories: no education, primary education and secondary education/higher. |
| Geographic location | Do the family live in a city or rural area? Binary variable: urban/rural location | Place of residence: urban or rural. The answer is not formulated by the respondent, but it is defined based on the place where the cluster or sample is based. |
| Hypothesis Number | Hypothesis for the MDD Indicator | Hypothesis for the MMF Indicator | Evidence Justifying the Hypothesis |
|---|---|---|---|
| H1 | Children who have reported as being sick in the past 2 weeks are more likely to have met the standard for minimum dietary diversity. | Children who have reported as being sick in the past 2 weeks are more likely to have met the standard for minimum meal frequency. | Children, when sick, are more likely to be fed with more and more nutritious food by their mothers [44,45] |
| H2 | Children who have received complete age-appropriate vaccinations are more likely to have met the standard for minimum dietary diversity. | Children from who have received complete age-appropriate vaccinations are more likely to have met the standard for minimum meal frequency. | Mothers attending vaccination clinics get more information on child feeding [11] |
| H3 | Children are more likely to have met the standard for minimum dietary diversity at increasing levels of household wealth. | Children are more likely to have met the standard for minimum meal frequency diversity at increasing levels of household wealth. | Children living in wealthier households are more likely to eat a diverse and balanced diet [46] |
| H4 | Children are more likely to have met the standard for minimum dietary diversity with increasing levels of female empowerment. | Children are more likely to have met the standard for minimum meal frequency with increasing levels of female empowerment. | Mothers with higher level of female empowerment are more likely to have children with better nutritional outcomes [43,47] |
| Sample N | Count (Percentage) | |
|---|---|---|
| Child characteristics | ||
| Child sex | 5485 | |
| Female | 2711(49%) | |
| Male | 2774 (51%) | |
| Breastfeeding status | 5485 | |
| Still breastfed | 4077 (74%) | |
| Not breastfed | 1408 (26%) | |
| Age (in months) | 5485 | |
| 6–11 | 1989 (36%) | |
| 12–17 | 1820 (33%) | |
| 18–23 | 1676 (31%) | |
| Completed age-appropriate vaccination | 5485 | 2196 (40%) |
| Child reported as sick in the past 2 weeks | 5148 | 3112 (61%) |
| Child health: had the following symptom in the past 2 weeks | 5148 | |
| Diarrhoea | 1766 (34%) | |
| Fever | 2118 (41%) | |
| Cough | 2442 (47%) | |
| Maternal characteristics | ||
| Antenatal clinic visits | 4800 | |
| None | 87 (2%) | |
| 1–3 | 1699 (35%) | |
| >=4 | 3014 (63%) | |
| Highest educational level | 5485 | |
| No education | 690 (13%) | |
| Primary | 3332 (61%) | |
| Secondary or higher | 1463 (27%) | |
| Female empowerment—woman involved in decision making on: | 5485 | |
| How woman’s income is used | 2404 (44%) | |
| How man’s income is used | 2313 (42%) | |
| Large household purchases | 2896 (53%) | |
| Visiting family and friends | 3229 (59%) | |
| Regarding own health care | 3334 (61%) | |
| Female empowerment—other | ||
| Woman salary similar/higher than man salary | 562 (10%) | |
| Woman owns a land | 2062 (38%) | |
| Woman owns a house | 2619 (48%) | |
| Attitude towards domestic violence—Beating justified if a woman does the following: | 5485 | |
| Goes out without telling him [male/husband] | 2151 (40%) | |
| Neglects the children | 2247 (61%) | |
| Argues with him [male/husband] | 1650 (30%) | |
| Refuses to have sex with him [male/husband] | 1092 (20%) | |
| Burns the food | 827 (15%) | |
| Women’s empowerment score | 5485 | |
| Very low (score 1–3) | 1374 (29%) | |
| Low (score 4–6) | 2000 (43%) | |
| Medium (score 7–9) | 1115 (24%) | |
| High (score 10–13) | 218 (5%) | |
| Household characteristics | ||
| Household wealth | 5485 | |
| Poorest | 1536 (28%) | |
| Poorer | 1102 (20%) | |
| Middle | 1027 (19%) | |
| Richer | 908 (17%) | |
| Richest | 912 (17%) | |
| Geographical region | 5485 | |
| Capital | 257 (5%) | |
| Central Uganda | 942 (17%) | |
| West Uganda | 1379 (25%) | |
| East Uganda | 1558 (28%) | |
| North Uganda | 1349 (25%) | |
| Type of residence | 4707 | |
| Rural | 3809 (81%) | |
| Urban | 898 (19%) |
| (a) MMF | ||||||||||||||||
| All Ages | 6–11 Months | 12–17 Months | 18–23 Months | |||||||||||||
| MINIMUM MEAL FREQUENCY | UNADJUSTED | ADJUSTED | UNADJUSTED | ADJUSTED | UNADJUSTED | ADJUSTED | UNADJUSTED | ADJUSTED | ||||||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Vaccination status | ||||||||||||||||
| Not vaccinated | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Partially vaccinated | 2.91 (2.30–3.71) | <0.001 | 2.21 (1.70–2.88) | <0.001 | 2.20 (1.41–3.42) | <0.001 | 1.44 (0.84–2.47) | 0.18 | 5.82 (3.36–10.08) | <0.001 | 3.40 (1.89–6.10) | <0.001 | n/a | n/a | 3.39 (2.10–5.59) | n/a |
| Fully vaccinated | 4.04 (3.15–5.19) | <0.001 | 2.70 (2.04–3.57) | <0.001 | 2.00 (1.33–3.01) | <0.001 | 1.27 (0.76–2.15) | 0.36 | 7.51 (4.37–12.90) | <0.001 | 4.33 (2.42–7.74) | <0.001 | n/a | n/a | n/a | n/a |
| Female empowerment | ||||||||||||||||
| Very low female empowerment | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Low female empowerment | 1.08 (0.94–1.25) | 0.13 | 1.11 (0.96–1.29) | 0.16 | 0.85 (0.64–1.12) | 0.24 | 0.86 (0.65–1.13) | 0.28 | 1.05 (0.82–1.34) | 0.69 | 1.02 (0.79–1.31) | 0.91 | 1.33 (1.05–1.70) | 0.02 | 1.48 (1.15–1.92) | <0.001 |
| Medium female empowerment | 1.03 (0.87–1.21) | 0.75 | 1.08 (0.91–1.29) | 0.36 | 0.87 (0.63–1.20) | 0.41 | 0.91 (0.66–1.27) | 0.59 | 1.02 (0.77–1.34) | 0.92 | 1.01 (0.75–1.36) | 0.96 | 1.20 (0.91–1.58) | 0.19 | 1.39 (1.04–1.87) | 0.03 |
| High female empowerment | 0.84 (0.63–1.13) | 0.25 | 0.90 (0.66–1.21) | 0.48 | 1.05 (0.58–1.89) | 0.88 | 1.12 (0.61–2.06) | 0.71 | 0.88 (0.54–1.43) | 0.59 | 1.02 (0.60–1.72) | 0.95 | 0.65 (0.39–1.09) | 0.10 | 0.64 (0.37–1.10) | 0.10 |
| Wealth index | ||||||||||||||||
| First wealth percentile | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Second wealth percentile | 1.34 (1.13–1.59) | <0.001 | 1.28 (1.07–1.53) | <0.001 | 1.40 (1.01–1.94) | 0.04 | 1.39 (1.00–1.95) | 0.05 | 1.32 (0.98–1.76) | 0.06 | 1.25 (0.92–1.70) | 0.15 | 1.43 (1.07–1.92) | 0.02 | 1.22 (0.89–1.67) | 0.21 |
| Middle wealth percentile | 1.30 (1.08–1.55) | 0.04 | 1.24 (1.03–1.50) | 0.03 | 1.49 (1.07–2.08) | 0.02 | 1.44 (1.02–2.03) | 0.04 | 1.10 (0.81–1.49) | 0.53 | 1.06 (0.76–1.46) | 0.74 | 1.38 (1.01–1.89) | 0.04 | 1.20 (0.85–1.67) | 0.30 |
| Fourth wealth percentile | 1.45 (1.20–1.75) | <0.001 | 1.43 (1.17–1.76) | <0.001 | 1.53 (1.07–2.20) | 0.02 | 1.44 (0.98–2.13) | 0.06 | 1.38 (1.00–1.89) | 0.05 | 1.40 (0.99–1.99) | 0.06 | 1.61 (1.16–2.22) | <0.001 | 1.43 (1.00–2.05) | 0.05 |
| Highest wealth percentile | 1.43 (1.19–1.72) | <0.001 | 1.52 (1.18–1.94) | <0.001 | 1.63 (1.14–2.34) | 0.01 | 1.74 (1.10–2.75) | 0.02 | 1.60 (1.15–2.22) | <0.001 | 1.88 (1.20–2.93) | 0.01 | 1.27 (0.93–1.73) | 0.13 | 1.07 (0.70–1.63) | 0.74 |
| Health status | ||||||||||||||||
| Not sick | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Sick | 1.47 (1.30–1.66) | <0.001 | 1.21 (1.06–1.38) | <0.001 | 1.09 (0.86–1.39) | 0.48 | 0.95 (0.73–1.24) | 0.71 | 1.85 (1.49–2.30) | <0.001 | 1.48 (1.17–1.87) | <0.001 | 1.47 (1.19–1.81) | <0.001 | 1.31 (1.05–1.64) | 0.02 |
| (b) MDD | ||||||||||||||||
| All ages | 6–11 months | 12–17 months | 18–23 months | |||||||||||||
| MINIMUM DIETARY DIVERSITY | UNADJUSTED | ADJUSTED | UNADJUSTED | ADJUSTED | UNADJUSTED | ADJUSTED | UNADJUSTED | ADJUSTED | ||||||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Vaccination status | ||||||||||||||||
| Not vaccinated | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Partially vaccinated | 4.28 (2.95–6.19) | <0.001 | 3.95 (2.69–5.80) | <0.001 | 2.86 (1.52–5.39) | <0.001 | 2.72 (1.34–5.49) | 0.01 | 5.67 (2.69–11.93) | <0.001 | 4.14 (1.90–9.00) | <0.001 | n/a | n/a | n/a | n/a |
| Fully vaccinated | 3.82 (2.63–5.56) | <0.001 | 3.63 (2.45–5.41) | <0.001 | 3.13 (1.69–5.78) | <0.001 | 2.86 (1.43–5.71) | <0.001 | 6.15 (2.94–12.85) | <0.001 | 4.65 (2.15–10.00) | <0.001 | n/a | n/a | n/a | n/a |
| Female empowerment | ||||||||||||||||
| Very low female empowerment | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Low female empowerment | 1.02 (0.87–1.18) | 0.83 | 0.98 (0.84–1.14) | 0.80 | 0.79 (0.61–1.03) | 0.08 | 0.80 (0.61–1.05) | 0.10 | 0.96 (0.75–1.24) | 0.75 | 0.94 (0.73–1.20) | 0.69 | 1.24 (0.96–1.59) | 0.11 | 1.38 (1.10–1.80) | 0.02 |
| Medium female empowerment | 1.12 (0.94–1.34) | 0.21 | 0.97 (0.82–1.15) | 0.76 | 0.95 (0.70–1.28) | 0.73 | 1.03 (0.75–1.41) | 0.85 | 0.71 (0.52–0.96) | 0.02 | 0.80 (0.58–1.10) | 0.18 | 1.33 (0.99–1.77) | 0.05 | 1.62 (1.20–2.20) | <0.001 |
| High female empowerment | 0.92 (0.66–1.29) | 0.62 | 0.73 (0.53–1.02) | 0.06 | 1.21 (0.71–2.04) | 0.48 | 1.50 (0.87–2.58) | 0.14 | 0.64 (0.37–1.11) | 0.11 | 0.85 (0.48–1.50) | 0.59 | 0.44 (0.22–0.86) | 0.02 | 0.52 (0.30–1.04) | 0.06 |
| Wealth index | ||||||||||||||||
| First wealth percentile | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Second wealth percentile | 1.43 (1.18–1.73) | <0.001 | 1.34 (1.18–1.53) | <0.001 | 1.17 (0.83–1.66) | 0.36 | 1.16 (0.81–1.65) | 0.42 | 1.84 (1.33–2.54) | <0.001 | 1.67 (1.20–2.30) | <0.001 | 1.29 (0.94–1.78) | 0.12 | 1.21 (0.90–1.69) | 0.27 |
| Middle wealth percentile | 1.54 (1.27–1.87) | <0.001 | 1.43 (1.17–1.75) | <0.001 | 1.41 (1.00–1.98) | 0.05 | 1.34 (0.94–1.91) | 0.10 | 1.47 (1.04–2.08) | 0.03 | 1.32 (0.92–1.90) | 0.14 | 1.77 (1.27–2.47) | <0.001 | 1.69 (1.20–2.39) | <0.001 |
| Fourth wealth percentile | 2.03 (1.66–2.47) | <0.001 | 1.80 (1.45–2.22) | <0.001 | 2.04 (1.44–2.89) | <0.001 | 1.86 (1.28–2.71) | <0.001 | 2.27 (1.61–3.19) | <0.001 | 1.93 (1.34–2.80) | <0.001 | 1.78 (1.27–2.50) | <0.001 | 1.66 (1.10–2.41) | 0.01 |
| Highest wealth percentile | 2.51 (2.06–3.04) | <0.001 | 2.03 (1.58–2.60) | <0.001 | 2.17 (1.54–3.06) | <0.001 | 1.90 (1.23–2.92) | <0.001 | 3.34 (2.36–4.71) | <0.001 | 2.73 (1.74–4.30) | <0.001 | 2.20 (1.59–3.05) | <0.001 | 1.80 (1.20–2.77) | 0.01 |
| Health status | ||||||||||||||||
| Not sick | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Sick | 1.34 (1.18–1.53) | <0.001 | 1.22 (1.06–1.41) | 0.01 | 1.13 (0.89–1.44) | 0.32 | 1.06 (0.82–1.37) | 0.64 | 1.62 (1.28–2.06) | <0.001 | 1.46 (1.14–1.90) | <0.001 | 1.36 (1.09–1.69) | 0.01 | 1.28 (1.00–1.61) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpa, G.; Berrang-Ford, L.; Galazoula, M.; Kakwangire, P.; Namanya, D.B.; Tushemerirwe, F.; Ahumuza, L.; Cade, J.E. Identifying Predictors for Minimum Dietary Diversity and Minimum Meal Frequency in Children Aged 6–23 Months in Uganda. Nutrients 2022, 14, 5208. https://doi.org/10.3390/nu14245208
Scarpa G, Berrang-Ford L, Galazoula M, Kakwangire P, Namanya DB, Tushemerirwe F, Ahumuza L, Cade JE. Identifying Predictors for Minimum Dietary Diversity and Minimum Meal Frequency in Children Aged 6–23 Months in Uganda. Nutrients. 2022; 14(24):5208. https://doi.org/10.3390/nu14245208
Chicago/Turabian StyleScarpa, Giulia, Lea Berrang-Ford, Maria Galazoula, Paul Kakwangire, Didacus B. Namanya, Florence Tushemerirwe, Laura Ahumuza, and Janet E. Cade. 2022. "Identifying Predictors for Minimum Dietary Diversity and Minimum Meal Frequency in Children Aged 6–23 Months in Uganda" Nutrients 14, no. 24: 5208. https://doi.org/10.3390/nu14245208
APA StyleScarpa, G., Berrang-Ford, L., Galazoula, M., Kakwangire, P., Namanya, D. B., Tushemerirwe, F., Ahumuza, L., & Cade, J. E. (2022). Identifying Predictors for Minimum Dietary Diversity and Minimum Meal Frequency in Children Aged 6–23 Months in Uganda. Nutrients, 14(24), 5208. https://doi.org/10.3390/nu14245208







