A High-Fiber Diet or Dietary Supplementation of Acetate Attenuate Hyperoxia-Induced Acute Lung Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Groups
2.2. HFD and Acetate Supplementation
2.3. Hyperoxia Exposure
2.4. Wet/Dry Lung-Weight and Lung Weight/Body Weight Ratios
2.5. Protein and Cytokine Levels in Bronchoalveolar Lavage Fluid (BALF)
2.6. Malondialdehyde (MDA), Glutathione, and H2O2 Contents
2.7. Immunohistochemical Staining for Myeloperoxidase
2.8. Western Blotting
2.9. Gut Microbiome Analysis
2.10. Hyperoxia Survival Study
2.11. Data Analysis
3. Results
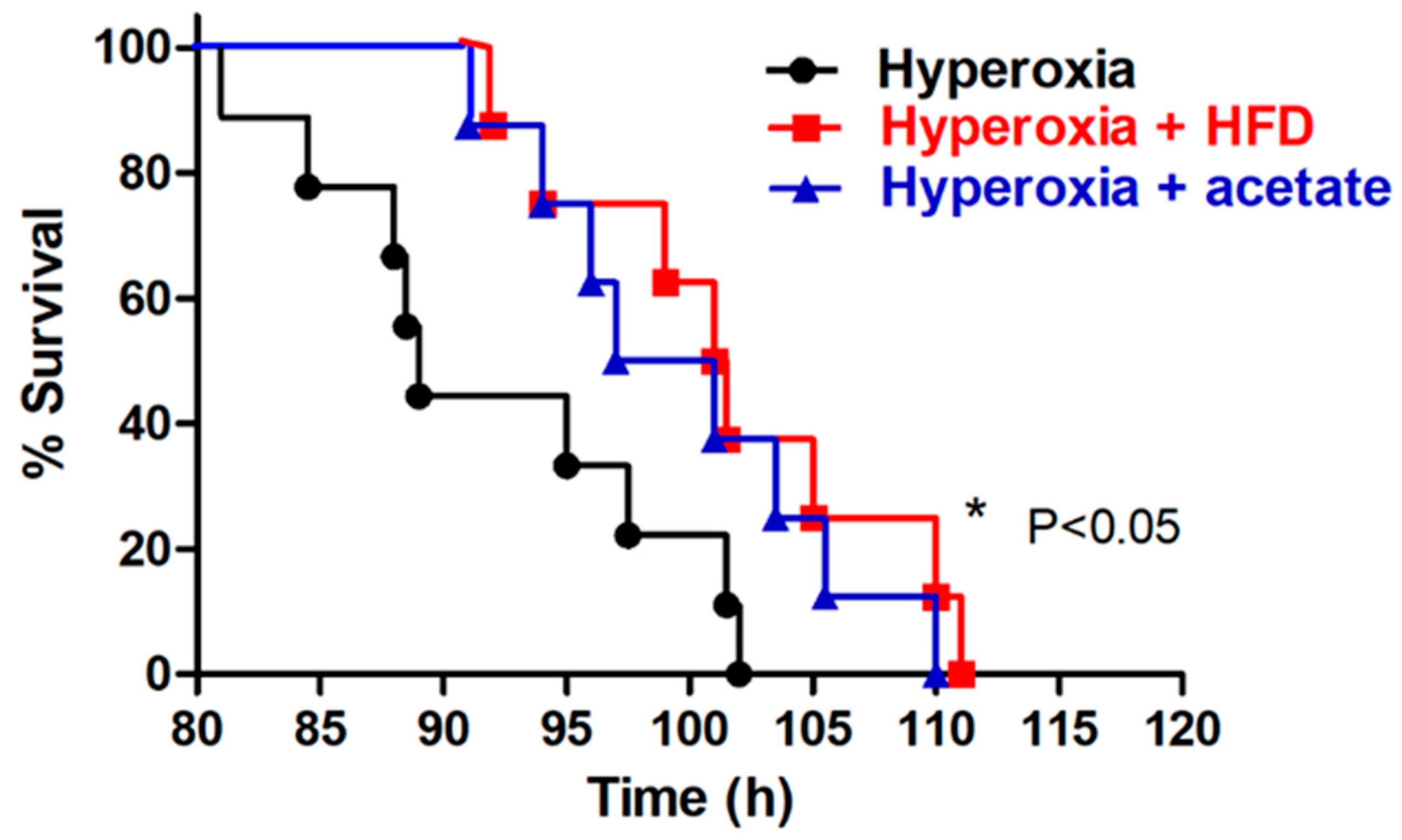
3.1. HFD or Acetate Supplementation Prolong Survival during Exposure to Hyperoxia
3.2. HFD or Acetate Supplementation Corrected Gut Microbiota Dysbiosis in Mice Exposed to Hyperoxia
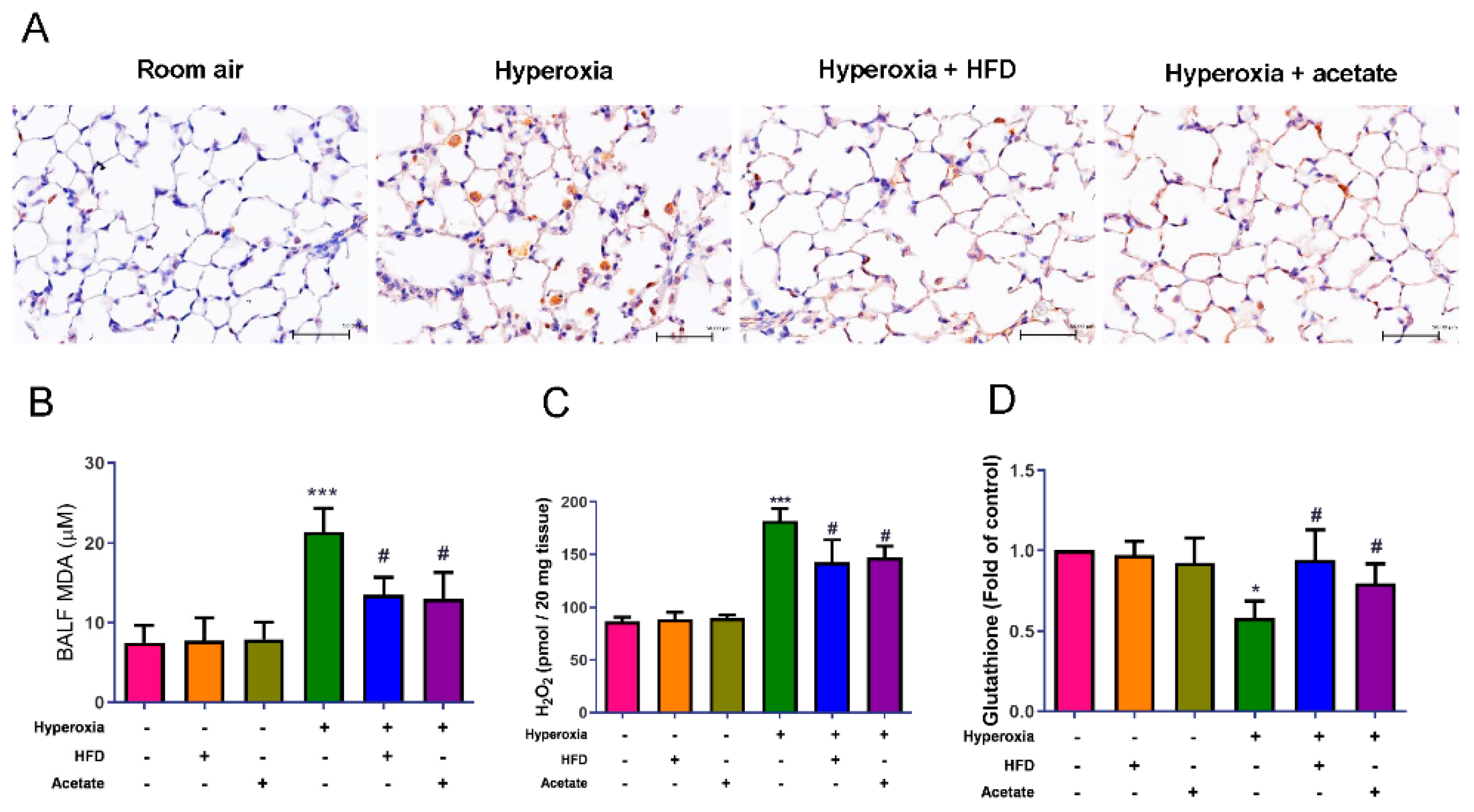
3.3. HFD and Acetate Supplementation Attenuate the Severity of HALI
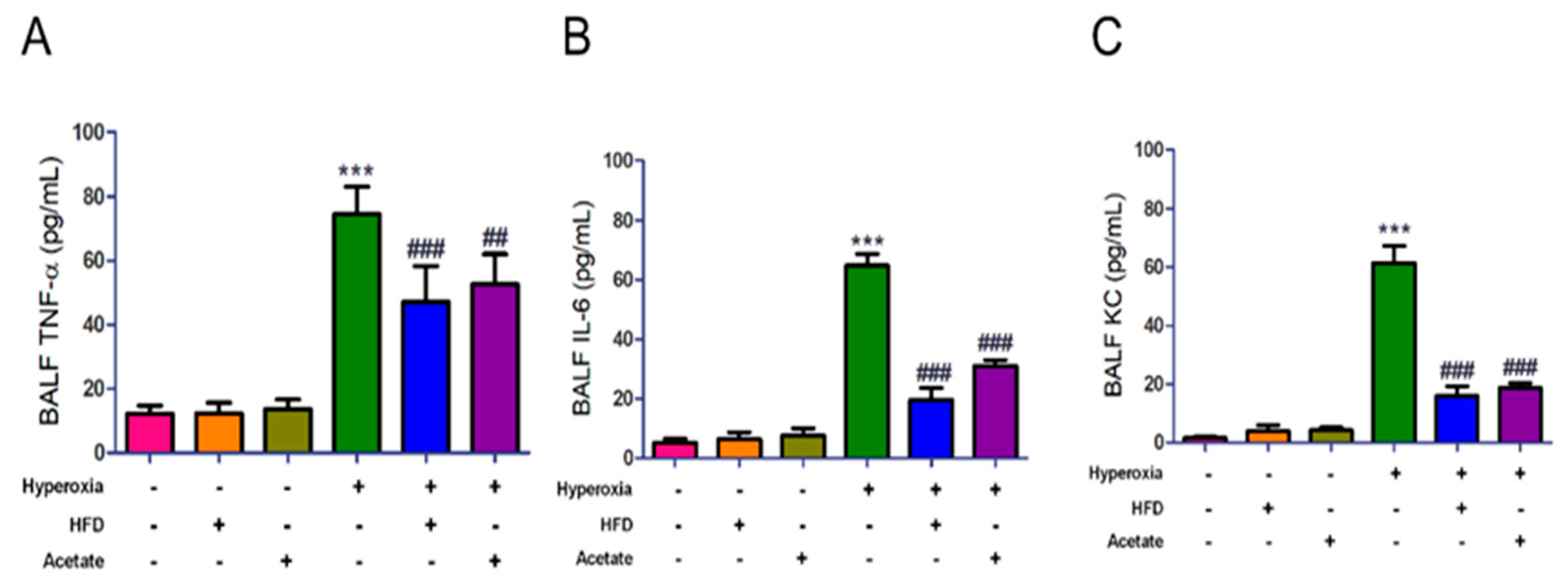
3.4. HFD and Acetate Supplementation Attenuate Hyperoxia-Induced Increases in the Levels of Inflammatory Mediators in BALF
3.5. HFD and Acetate Supplementation Reduce Hyperoxia-Induced Oxidative Stress in the Lungs
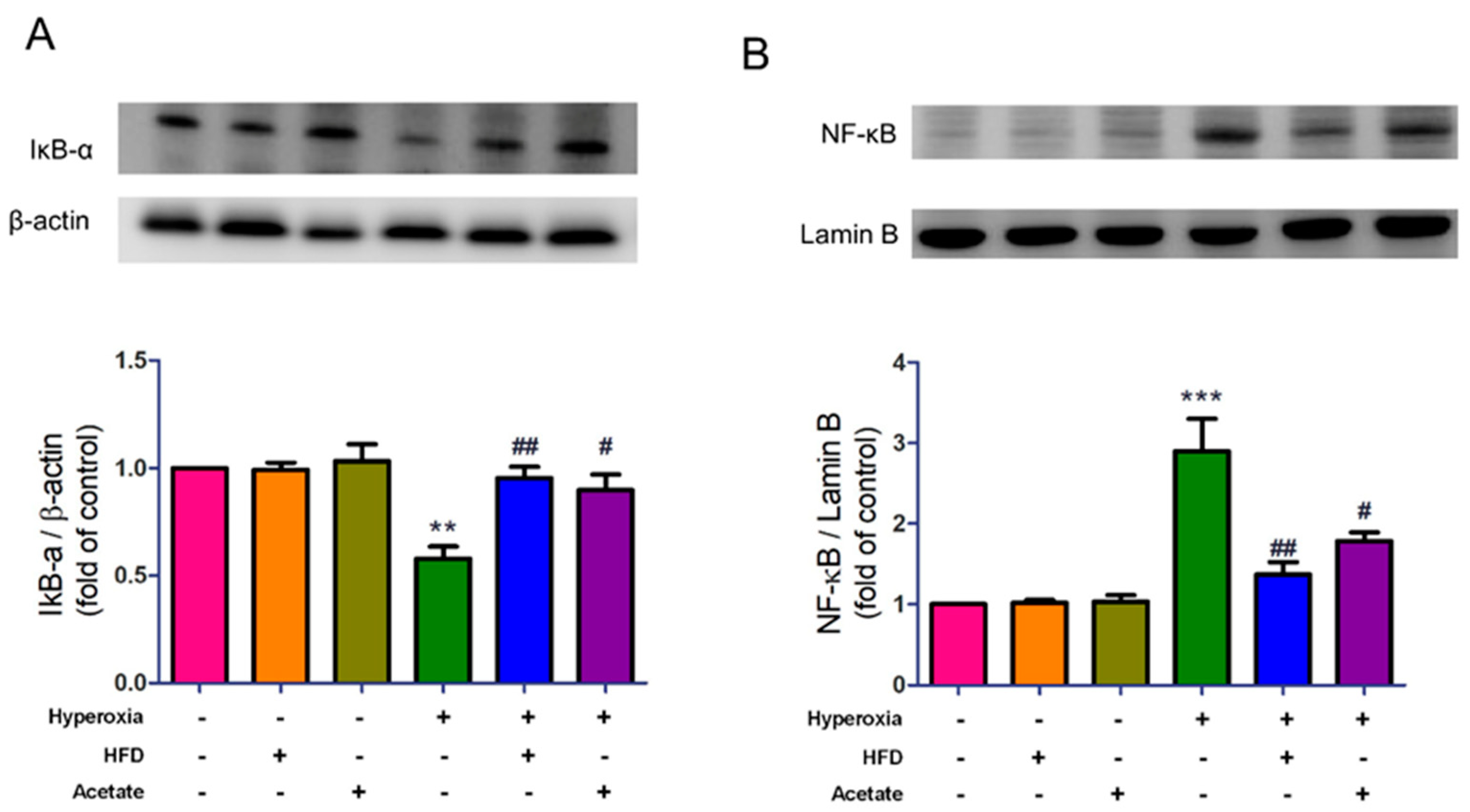
3.6. HFD and Acetate Supplementation Attenuate Hyperoxia-Induced NF-κB Activation in the Lungs
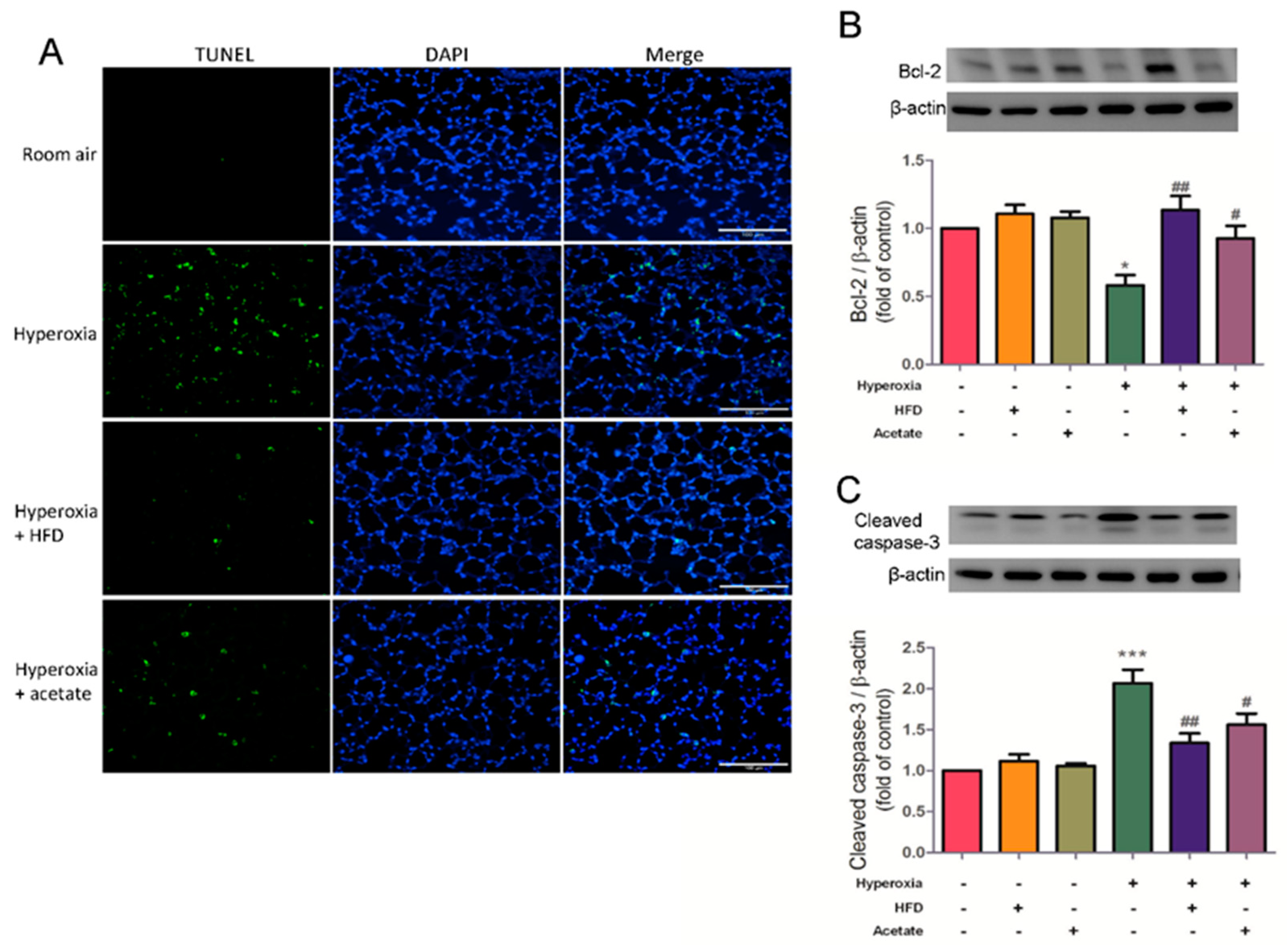
3.7. HFD and Aetate Supplementation Suppress Hyperoxia-Induced Apoptosis in the Lungs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kallet, R.H.; Matthay, M.A. Hyperoxic acute lung injury. Respir. Care 2013, 58, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.; Muralidhar, M.; Espey, M.G.; Degenhardt, K.; Mantell, L.L. Hyperoxia sensing: From molecular mechanisms to significance in disease. J. Immunotoxicol. 2010, 7, 239–254. [Google Scholar] [CrossRef]
- Pao, H.P.; Liao, W.I.; Tang, S.E.; Wu, S.Y.; Huang, K.L.; Chu, S.J. Suppression of Endoplasmic Reticulum Stress by 4-PBA Protects against Hyperoxia-Induced Acute Lung Injury via Up-Regulating Claudin-4 Expression. Front. Immunol. 2021, 12, 674316. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.C.; Huang, K.L.; Li, M.H.; Hsu, C.W.; Tsai, S.H.; Chu, S.J.; Chang, D.M. Glutamine attenuates hyperoxia-induced acute lung injury in mice. Clin. Exp. Pharmacol. Physiol. 2010, 37, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal. Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e4114. [Google Scholar] [CrossRef]
- Wood, L.G.; Shivappa, N.; Berthon, B.S.; Gibson, P.G.; Hebert, J.R. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin. Exp. Allergy 2015, 45, 177–183. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, X.; Liu, J.; Sun, J.; Wang, X.; Fan, G.; Meng, X.; Zhang, J.; Zhang, Y. The regulatory roles of dietary fibers on host health via gut microbiota-derived short chain fatty acids. Curr. Opin. Pharmacol. 2022, 62, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Antunes, K.H.; Fachi, J.L.; de Paula, R.; da Silva, E.F.; Pral, L.P.; Dos Santos, A.A.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 3273. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.Y.; Wu, S.Y.; Pao, H.P.; Liao, W.I.; Chu, S.J. Acetate, a gut bacterial product, ameliorates ischemia-reperfusion induced acute lung injury in rats. Int. Immunopharmacol. 2022, 111, 109136. [Google Scholar] [CrossRef]
- Jang, Y.O.; Kim, O.H.; Kim, S.J.; Lee, S.H.; Yun, S.; Lim, S.E.; Yoo, H.J.; Shin, Y.; Lee, S.W. High-fiber diets attenuate emphysema development via modulation of gut microbiota and metabolism. Sci. Rep. 2021, 11, 7008. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.I.; Wu, S.Y.; Tsai, S.H.; Pao, H.P.; Huang, K.L.; Chu, S.J. 2-Methoxyestradiol Protects Against Lung Ischemia/Reperfusion Injury by Upregulating Annexin A1 Protein Expression. Front. Immunol. 2021, 12, 596376. [Google Scholar] [CrossRef]
- Wu, S.Y.; Tang, S.E.; Ko, F.C.; Wu, G.C.; Huang, K.L.; Chu, S.J. Valproic acid attenuates acute lung injury induced by ischemia-reperfusion in rats. Anesthesiology 2015, 122, 1327–1337. [Google Scholar] [CrossRef]
- Pao, H.P.; Liao, W.I.; Wu, S.Y.; Hung, K.Y.; Huang, K.L.; Chu, S.J. PG490-88, a derivative of triptolide, suppresses ischemia/reperfusion-induced lung damage by maintaining tight junction barriers and targeting multiple signaling pathways. Int. Immunopharmacol. 2019, 68, 17–29. [Google Scholar] [CrossRef]
- Gonzalez-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S150–S156. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.Y.; Ma, M.M.; Qi, Z.J.; Zhang, X.Q.; Li, Z.; Cao, G.H.; Li, J.; Zhu, W.W.; Wang, X.Z. Changes in intestinal microflora in rats with acute respiratory distress syndrome. World J. Gastroenterol. 2014, 20, 5849–5858. [Google Scholar] [CrossRef]
- Ashley, S.L.; Sjoding, M.W.; Popova, A.P.; Cui, T.X.; Hoostal, M.J.; Schmidt, T.M.; Branton, W.R.; Dieterle, M.G.; Falkowski, N.R.; Baker, J.M.; et al. Lung and gut microbiota are altered by hyperoxia and contribute to oxygen-induced lung injury in mice. Sci. Transl. Med. 2020, 12, eaau9959. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal. Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Raizada, M.K.; Joe, B.; Bryan, N.S.; Chang, E.B.; Dewhirst, F.E.; Borisy, G.G.; Galis, Z.S.; Henderson, W.; Jose, P.A.; Ketchum, C.J.; et al. Report of the National Heart, Lung, and Blood Institute Working Group on the Role of Microbiota in Blood Pressure Regulation: Current Status and Future Directions. Hypertension 2017, 70, 479–485. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Willett, W.C.; Camargo, C.A., Jr. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. Am. J. Epidemiol. 2010, 171, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Gao, J.; Zhang, Y.; Hou, W.; Han, T.; Sun, C. The Association of Dietary Fiber Intake in Three Meals with All-Cause and Disease-Specific Mortality among Adults: The U.S. National Health and Nutrition Examination Survey, 2003–2014. Nutrients 2022, 14, 2521. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, A.; Xie, K.; Yu, Y. Dietary Supplementation With High Fiber Alleviates Oxidative Stress and Inflammatory Responses Caused by Severe Sepsis in Mice without Altering Microbiome Diversity. Front. Physiol. 2018, 9, 1929. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S.; et al. Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. Int. J. Mol. Sci. 2019, 21, 31. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Gong, T.; Chen, W.; Mao, S.; Kong, Y.; Yu, J.; Sun, J. Anti-neuroinflammatory Effect of Short-Chain Fatty Acid Acetate against Alzheimer’s Disease via Upregulating GPR41 and Inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 2020, 68, 7152–7161. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef]
- Tang, P.S.; Mura, M.; Seth, R.; Liu, M. Acute lung injury and cell death: How many ways can cells die? Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L632–L641. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, M.Y.; Lee, J. Apple pectin, a dietary fiber, ameliorates myocardial injury by inhibiting apoptosis in a rat model of ischemia/reperfusion. Nutr. Res. Pract. 2014, 8, 391–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, H.S.; Lin, W.H.; Chen, P.R.; Wu, H.C.; Lee, P.H.; Chen, W.J. Effects of a high-fiber diet on hepatocyte apoptosis and liver regeneration after partial hepatectomy in rats with fatty liver. JPEN J. Parenter. Enteral. Nutr. 2005, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.-J.; Tang, S.-E.; Pao, H.-P.; Wu, S.-Y.; Liao, W.-I. A High-Fiber Diet or Dietary Supplementation of Acetate Attenuate Hyperoxia-Induced Acute Lung Injury. Nutrients 2022, 14, 5231. https://doi.org/10.3390/nu14245231
Chu S-J, Tang S-E, Pao H-P, Wu S-Y, Liao W-I. A High-Fiber Diet or Dietary Supplementation of Acetate Attenuate Hyperoxia-Induced Acute Lung Injury. Nutrients. 2022; 14(24):5231. https://doi.org/10.3390/nu14245231
Chicago/Turabian StyleChu, Shi-Jye, Shih-En Tang, Hsin-Ping Pao, Shu-Yu Wu, and Wen-I Liao. 2022. "A High-Fiber Diet or Dietary Supplementation of Acetate Attenuate Hyperoxia-Induced Acute Lung Injury" Nutrients 14, no. 24: 5231. https://doi.org/10.3390/nu14245231
APA StyleChu, S.-J., Tang, S.-E., Pao, H.-P., Wu, S.-Y., & Liao, W.-I. (2022). A High-Fiber Diet or Dietary Supplementation of Acetate Attenuate Hyperoxia-Induced Acute Lung Injury. Nutrients, 14(24), 5231. https://doi.org/10.3390/nu14245231





