The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders
Abstract
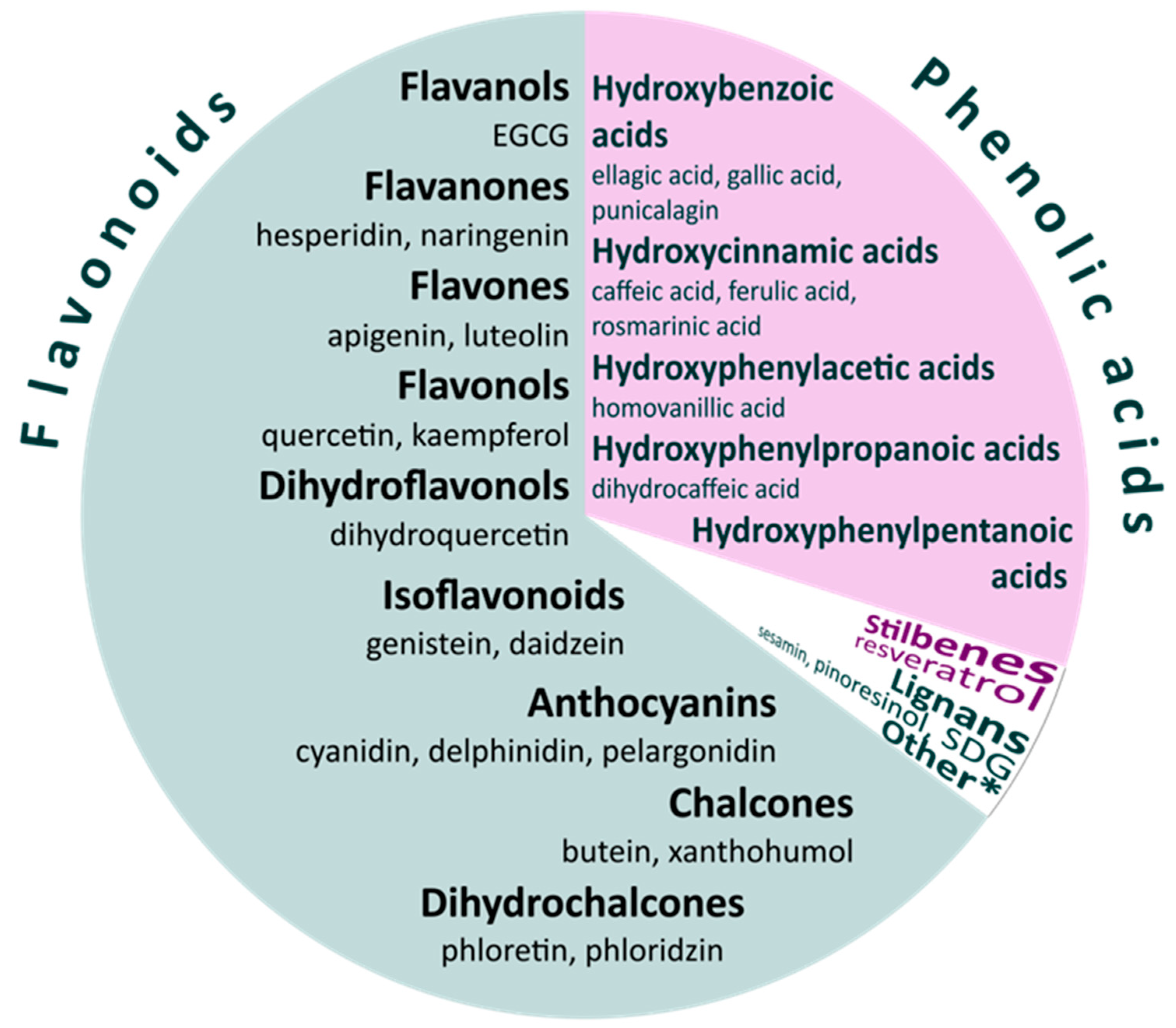
:1. Introduction
2. The Role of Dietary Polyphenols in Early-Pregnancy Events
3. The Role of Dietary Polyphenols in Pregnancy-Related Pathologies
3.1. Preeclampsia
3.2. Gestational Diabetes Mellitus
4. Potential Harmful Effects of Dietary Polyphenols in Pregnancy
5. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, P.; Santos, C.N. Worldwide (Poly)Phenol Intake: Assessment Methods and Identified Gaps. Eur. J. Nutr. 2017, 56, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Probst, Y.; Guan, V.; Kent, K. A Systematic Review of Food Composition Tools Used for Determining Dietary Polyphenol Intake in Estimated Intake Studies. Food Chem. 2018, 238, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Phenol-Explorer Database on Polyphenol Content in Foods. Available online: Http://Phenol-Explorer.Eu/ (accessed on 20 October 2022).
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Martel, F.; Monteiro, R.; Calhau, C. Effect of Polyphenols on the Intestinal and Placental Transport of Some Bioactive Compounds. Nutr. Res. Rev. 2010, 23, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols—Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Vera-Ramírez, L.; Forbes-Hernández, T.J.; Esteban-Muñoz, A.; Giampieri, F.; Bullón, P.; Battino, M.; Sánchez-González, C.; et al. An oleuropein rich-olive (Olea europaea L.) leaf extract reduces β-amyloid and tau proteotoxicity through regulation of oxidative- and heat shock-stress responses in Caenorhabditis elegans. Food Chem. Toxicol. 2022, 162, 112914. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef]
- Ullah, H.; De Filippis, A.; Santarcangelo, C.; Daglia, M. Epigenetic regulation by polyphenols in diabetes and related complications. Med. J. Nutr. Metab. 2020, 13, 289–310. [Google Scholar] [CrossRef]
- Micek, A.; Owczarek, M.; Jurek, J.; Guerrera, I.; Torrisi, S.A.; Grosso, G.; Alshatwi, A.A.; Godos, J. Anthocyanin-rich fruits and mental health outcomes in an Italian cohort. J. Berry Res. 2022, Pre-press, 1–14. [Google Scholar] [CrossRef]
- Miyazawa, T.; Abe, C.; Carpentero Burdeos, G.; Matsumoto, A.; Toda, M. Food Antioxidants and Aging: Theory, Current Evidence and Perspectives. Nutraceuticals 2022, 2, 181–204. [Google Scholar] [CrossRef]
- Hinojosa-Nogueira, D.; Pérez-Burillo, S.; García-Rincón, I.; Rufián-Henares, J.A.; Pastoriza, S. A Useful and Simple Tool to Evaluate and Compare the Intake of Total Dietary Polyphenols in Different Populations. Public Health Nutr. 2021, 24, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Chan, K.P.; Rogers, M.S.; Choy, K.W.; Pang, C.P. Pharmacokinetic Studies of Green Tea Catechins in Maternal Plasma and Fetuses in Rats. J. Pharm. Sci. 2006, 95, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Arola-Arnal, A.; Oms-Oliu, G.; Crescenti, A.; del Bas, J.M.; Ras, M.R.; Arola, L.; Caimari, A. Distribution of Grape Seed Flavanols and Their Metabolites in Pregnant Rats and Their Fetuses. Mol. Nutr. Food Res. 2013, 57, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Middleton Jr., E.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells:Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and Function of the Normal Human Placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The Forgotten Organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.-P.; Skepper, J.N.; Burton, G.J. Onset of Maternal Arterial Blood Flow and Placental Oxidative Stress. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Jauniaux, E.; Gulbis, B.; Burton, G.J. The Human First Trimester Gestational Sac Limits Rather than Facilitates Oxygen Transfer to the Foetus—A Review. Placenta 2003, 24, S86–S93. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.-J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. Biomed Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef]
- Genbacev, O.; Joslin, R.; Damsky, C.H.; Polliotti, B.M.; Fisher, S.J. Hypoxia Alters Early Gestation Human Cytotrophoblast Differentiation/Invasion in Vitro and Models the Placental Defects That Occur in Preeclampsia. J. Clin. Investig. 1996, 97, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of Human Placental Development by Oxygen Tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Yockell-Lelièvre, J.; Ferraro, Z.M.; Arnason, J.T.; Ferrier, J.; Gruslin, A. The Effects of Dietary Polyphenols on Reproductive Health and Early Development. Hum. Reprod. Update 2015, 21, 228–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanović, M.; Stefanoska, I.; Radojcić, L.; Vićovac, L. Interleukin-8 (CXCL8) Stimulates Trophoblast Cell Migration and Invasion by Increasing Levels of Matrix Metalloproteinase (MMP)2 and MMP9 and Integrins Alpha5 and Beta1. Reproduction 2010, 139, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolundžić, N.; Bojić-Trbojević, Ž.; Kovačević, T.; Stefanoska, I.; Kadoya, T.; Vićovac, L. Galectin-1 Is Part of Human Trophoblast Invasion Machinery—A Functional Study In Vitro. PLoS ONE 2011, 6, e28514. [Google Scholar] [CrossRef] [Green Version]
- Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Vilotić, A.; Kolundžić, N.; Stefanoska, I.; Zetterberg, F.; Nilsson, U.J.; Leffler, H.; Vićovac, L. Human Trophoblast Requires Galectin-3 for Cell Migration and Invasion. Sci. Rep. 2019, 9, 2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Tanihara, F.; Do, L.; Sato, Y.; Taniguchi, M.; Takagi, M.; Van Nguyen, T.; Otoi, T. Chlorogenic Acid Supplementation during in Vitro Maturation Improves Maturation, Fertilization and Developmental Competence of Porcine Oocytes. Reprod. Domest. Anim. 2017, 52, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Jamali, N.; Mostafavi-Pour, Z.; Zal, F.; Kasraeian, M.; Poordast, T.; Ramezani, F.; Zare, R. Combination Effect of Caffeine and Caffeic Acid Treatment on the Oxidant Status of Ectopic Endometrial Cells Separated from Patients with Endometriosis. Iran. J. Med. Sci. 2019, 44, 315–324. [Google Scholar] [CrossRef]
- Cao, X.; Xia, Y.; Zeng, M.; Wang, W.; He, Y.; Liu, J. Caffeic Acid Inhibits the Formation of Advanced Glycation End Products (AGEs) and Mitigates the AGEs-Induced Oxidative Stress and Inflammation Reaction in Human Umbilical Vein Endothelial Cells (HUVECs). Chem. Biodivers. 2019, 16, e1900174. [Google Scholar] [CrossRef]
- Kostić, S.; Vilotić, A.; Pirković, A.; Dekanski, D.; Borozan, S.; Nacka-Aleksić, M.; Vrzić-Petronijević, S.; Jovanović Krivokuća, M. Caffeic Acid Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress and Genotoxicity. Food Chem. Toxicol. 2022, 163, 112993. [Google Scholar] [CrossRef]
- Lazić, V.; Pirković, A.; Sredojević, D.; Marković, J.; Papan, J.; Ahrenkiel, S.P.; Janković-Častvan, I.; Dekanski, D.; Jovanović-Krivokuća, M.; Nedeljković, J.M. Surface-Modified ZrO2 Nanoparticles with Caffeic Acid: Characterization and in Vitro Evaluation of Biosafety for Placental Cells. Chem. Biol. Interact. 2021, 347, 109618. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, C.; Gao, J.; Shi, Y.; Li, H.; Yan, H.; Yan, S.; Zhang, Z. Resveratrol Induces SIRT1-Dependent Autophagy to Prevent H2O2-Induced Oxidative Stress and Apoptosis in HTR8/SVneo Cells. Placenta 2020, 91, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; An, P.; Dang, H.; Liu, Y.; Liu, R. Effects of Resveratrol on Autophagy and the Expression of Inflammasomes in a Placental Trophoblast Oxidative Stress Model. Life Sci. 2020, 256, 117890. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, S.; Wu, D.; Xu, Y.; Wang, S.; Jiang, Y.; Liu, F.; Jiang, Z.; Qu, H.; Yu, X.; et al. Resveratrol Promotes Trophoblast Invasion in Pre-eclampsia by Inducing Epithelial-mesenchymal Transition. J. Cell. Mol. Med. 2019, 23, 2702–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; Zeng, F.; Tang, G.; Lei, C.; Zou, X.; Liu, X.; Peng, B.; Qin, S.; Li, H. Expression of Galectin-3 and Apoptosis in Placental Villi from Patients with Missed Abortion during Early Pregnancy. Exp. Ther. Med. 2019, 17, 2623–2631. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, J.; Zhang, J.; Zhang, L.; Wang, T. Curcumin Protects Human Trophoblast HTR8/SVneo Cells from H2O2-Induced Oxidative Stress by Activating Nrf2 Signaling Pathway. Antioxidants 2020, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Srinivas, V.; Mallepogu, A.; Duttaroy, A.K. Curcumin Stimulates Angiogenesis through VEGF and Expression of HLA-G in First-trimester Human Placental Trophoblasts. Cell Biol. Int. 2020, 44, 1237–1251. [Google Scholar] [CrossRef] [Green Version]
- Karrar, S.A.; Hong, P.L. Preeclampsia; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes. JAMA 2017, 317, 2207. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-Eclampsia: Pathophysiology and Clinical Implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the Management of Cardiovascular Diseases during Pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef] [PubMed]
- Malha, L.; August, P. Safety of Antihypertensive Medications in Pregnancy: Living With Uncertainty. J. Am. Heart Assoc. 2019, 8, e013495. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Kujawski, R.; Mikołajczak, P.Ł.; Wielgus, K.; Klejewski, A.; Wolski, H.; Seremak-Mrozikiewicz, A. In Vitro and in Vivo Activities of Flavonoids—Apigenin, Baicalin, Chrysin, Scutellarin—In Regulation of Hypertension—A Review for Their Possible Effects in Pregnancy-Induced Hypertension. Herba Pol. 2019, 65, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Bogacz, A.; Mikołajczak, P.Ł.; Wolek, M.; Górska, A.; Szulc, M.; Ożarowski, M.; Kujawski, R.; Czerny, B.; Wolski, H.; Karpiński, T.M.; et al. Combined Effects of Methyldopa and Flavonoids on the Expression of Selected Factors Related to Inflammatory Processes and Vascular Diseases in Human Placenta Cells-An In Vitro Study. Molecules 2021, 26, 1259. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M.; Szulc, M.; Wielgus, K.; Kujawski, R.; Wolski, H.; Seremak-Mrozikiewicz, A. Plant Phenolics and Extracts in Animal Models of Preeclampsia and Clinical Trials-Review of Perspectives for Novel Therapies. Pharmaceuticals 2021, 14, 269. [Google Scholar] [CrossRef]
- Bai, D.-P.; Zhang, X.-F.; Zhang, G.-L.; Huang, Y.-F.; Gurunathan, S. Zinc Oxide Nanoparticles Induce Apoptosis and Autophagy in Human Ovarian Cancer Cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.-D.; Guo, J.-J.; Zhou, L.; Wang, N. Epigallocatechin Gallate Enhances Treatment Efficacy of Oral Nifedipine against Pregnancy-Induced Severe Pre-Eclampsia: A Double-Blind, Randomized and Placebo-Controlled Clinical Study. J. Clin. Pharm. Ther. 2018, 43, 21–25. [Google Scholar] [CrossRef]
- Moraloglu, O.; Engin-Ustun, Y.; Tonguç, E.; Var, T.; Tapisiz, O.L.; Ergün, H.; Guvenc, T.; Gacar, A. The Effect of Resveratrol on Blood Pressure in a Rat Model of Preeclampsia. J. Matern. Fetal. Neonatal Med. 2012, 25, 845–848. [Google Scholar] [CrossRef]
- Gong, P.; Liu, M.; Hong, G.; Li, Y.; Xue, P.; Zheng, M.; Wu, M.; Shen, L.; Yang, M.; Diao, Z.; et al. Curcumin Improves LPS-Induced Preeclampsia-like Phenotype in Rat by Inhibiting the TLR4 Signaling Pathway. Placenta 2016, 41, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Miao, H.; Li, X.; Hu, Y.; Sun, H.; Hou, Y. Curcumin Inhibits Placental Inflammation to Ameliorate LPS-Induced Adverse Pregnancy Outcomes in Mice via Upregulation of Phosphorylated Akt. Inflamm. Res. 2017, 66, 177–185. [Google Scholar] [CrossRef]
- Li, Q.; Yin, L.; Si, Y.; Zhang, C.; Meng, Y.; Yang, W. The Bioflavonoid Quercetin Improves Pathophysiology in a Rat Model of Preeclampsia. Biomed. Pharmacother. 2020, 127, 110122. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, S.; Song, H. Quercetin Attenuates Reduced Uterine Perfusion Pressure -Induced Hypertension in Pregnant Rats through Regulation of Endothelin-1 and Endothelin-1 Type A Receptor. Lipids Health Dis. 2020, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Song, L.; Shi, X.; Zhao, N.; Ma, Y. Ameliorative Effects of Pre-Eclampsia by Quercetin Supplement to Aspirin in a Rat Model Induced by L-NAME. Biomed. Pharmacother. 2019, 116, 108969. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, M.; Yang, X.; Yang, Z.; Li, L.; Mei, J. Supplementing Punicalagin Reduces Oxidative Stress Markers and Restores Angiogenic Balance in a Rat Model of Pregnancy-Induced Hypertension. Korean J. Physiol. Pharmacol. 2018, 22, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jia, Y.; Yang, X.; Liang, B.; Gao, H.; Yang, T. A Potential Role of Baicalin to Inhibit Apoptosis and Protect against Acute Liver and Kidney Injury in Rat Preeclampsia Model. Biomed. Pharmacother. 2018, 108, 1546–1552. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Chen, D.; Ding, J.; Zhang, Y.; Song, L. Quercetin Supplement to Aspirin Attenuates Lipopolysaccharide-Induced Pre-Eclampsia-Like Impairments in Rats Through the NLRP3 Inflammasome. Drugs R D 2022, 22, 271–279. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Chen, L.; Chen, S. The Natural Compound Puerarin Alleviates Inflammation and Apoptosis in Experimental Cell and Rat Preeclampsia Models. Int. Immunopharmacol. 2021, 99, 108001. [Google Scholar] [CrossRef]
- Ding, J.; Kang, Y.; Fan, Y.; Chen, Q. Efficacy of Resveratrol to Supplement Oral Nifedipine Treatment in Pregnancy-Induced Preeclampsia. Endocr. Connect. 2017, 6, 595–600. [Google Scholar] [CrossRef]
- Ju, Y.; Feng, Y.; Yang, Y.; Hou, X.; Zhang, X.; Zhu, X.; Wang, Y.; Yang, M. Combining Curcumin and Aspirin Ameliorates Preeclampsia-like Symptoms by Inhibiting the Placental TLR4/NF-ΚB Signaling Pathway in Rats. J. Obstet. Gynaecol. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Osuga, Y.; Yoshino, O.; Hirota, Y.; Ruimeng, X.; Hirata, T.; Takeda, S.; Yano, T.; Tsutsumi, O.; Taketani, Y. Elevated Serum Soluble Vascular Endothelial Growth Factor Receptor 1 (SVEGFR-1) Levels in Women with Preeclampsia. J. Clin. Endocrinol. Metab. 2003, 88, 2348–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess Placental Soluble Fms-like Tyrosine Kinase 1 (SFlt1) May Contribute to Endothelial Dysfunction, Hypertension, and Proteinuria in Preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatsaris, V.; Goffin, F.; Munaut, C.; Brichant, J.-F.; Pignon, M.-R.; Noel, A.; Schaaps, J.-P.; Cabrol, D.; Frankenne, F.; Foidart, J.-M. Overexpression of the Soluble Vascular Endothelial Growth Factor Receptor in Preeclamptic Patients: Pathophysiological Consequences. J. Clin. Endocrinol. Metab. 2003, 88, 5555–5563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.-H.; Yuan, H.-T.; Libermann, T.A.; et al. Soluble Endoglin Contributes to the Pathogenesis of Preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble Endoglin and Other Circulating Antiangiogenic Factors in Preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Nova, A.; Sibai, B.M.; Barton, J.R.; Mercer, B.M.; Mitchell, M.D. Maternal Plasma Level of Endothelin Is Increased in Preeclampsia. Am. J. Obstet. Gynecol. 1991, 165, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Miyamoto, A.; Yamamoto, H.; Ohshika, H.; Kudo, R. The Relationship between Serum Nitrate and Endothelin-1 Concentrations in Preeclampsia. Life Sci. 2000, 67, 1447–1454. [Google Scholar] [CrossRef]
- Polliotti, B.M.; Fry, A.G.; Saller, D.N.; Mooney, R.A.; Cox, C.; Miller, R.K. Second-Trimester Maternal Serum Placental Growth Factor and Vascular Endothelial Growth Factor for Predicting Severe, Early-Onset Preeclampsia. Obstet. Gynecol. 2003, 101, 1266–1274. [Google Scholar] [CrossRef]
- Taylor, R.N.; Grimwood, J.; Taylor, R.S.; McMaster, M.T.; Fisher, S.J.; North, R.A. Longitudinal Serum Concentrations of Placental Growth Factor: Evidence for Abnormal Placental Angiogenesis in Pathologic Pregnancies. Am. J. Obstet. Gynecol. 2003, 188, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Rajakumar, A. Preeclampsia and Soluble Fms-Like Tyrosine Kinase 1. J. Clin. Endocrinol. Metab. 2009, 94, 2252–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, N.P.; Wrana, J.L.; Letarte, M. Endoglin Is an Accessory Protein That Interacts with the Signaling Receptor Complex of Multiple Members of the Transforming Growth Factor-Beta Superfamily. J. Biol. Chem. 1999, 274, 584–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A Novel Potent Vasoconstrictor Peptide Produced by Vascular Endothelial Cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorin, E.; Webb, D.J. Endothelium-Derived Endothelin-1. Pflugers Arch. 2010, 459, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Erez, O.; Wildman, D.E.; Tarca, A.L.; Edwin, S.S.; Abbas, A.; Hotra, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.S.; et al. Severe Preeclampsia Is Characterized by Increased Placental Expression of Galectin-1. J. Matern. Neonatal Med. 2008, 21, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the SFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Zheng, L.; Huang, J.; Su, Y.; Wang, F.; Kong, H.; Xin, H. Vitexin Ameliorates Preeclampsia Phenotypes by Inhibiting TFPI-2 and HIF-1α/VEGF in a l-NAME Induced Rat Model. Drug Dev. Res. 2019, 80, 1120–1127. [Google Scholar] [CrossRef]
- Cudmore, M.J.; Ramma, W.; Cai, M.; Fujisawa, T.; Ahmad, S.; Al-Ani, B.; Ahmed, A. Resveratrol Inhibits the Release of Soluble Fms-like Tyrosine Kinase (SFlt-1) from Human Placenta. Am. J. Obstet. Gynecol. 2012, 206, 253.e10–253.e15. [Google Scholar] [CrossRef]
- Hannan, N.J.; Brownfoot, F.C.; Cannon, P.; Deo, M.; Beard, S.; Nguyen, T.V.; Palmer, K.R.; Tong, S.; Kaitu’u-Lino, T.J. Resveratrol Inhibits Release of Soluble Fms-like Tyrosine Kinase (SFlt-1) and Soluble Endoglin and Improves Vascular Dysfunction—Implications as a Preeclampsia Treatment. Sci. Rep. 2017, 7, 1819. [Google Scholar] [CrossRef]
- Bueno-Pereira, T.O.; Bertozzi-Matheus, M.; Zampieri, G.M.; Abbade, J.F.; Cavalli, R.C.; Nunes, P.R.; Sandrim, V.C. Markers of Endothelial Dysfunction Are Attenuated by Resveratrol in Preeclampsia. Antioxidants 2022, 11, 2111. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulo, M.S.; Abdo, N.M.; Bettencourt-Silva, R.; Al-Rifai, R.H. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front. Endocrinol. 2021, 12, 691033. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal Obesity, Diabetes during Pregnancy and Epigenetic Mechanisms That Influence the Developmental Origins of Cardiometabolic Disease in the Offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; England, J.L.; Sharma, J.A.; Njoroge, T. Gestational Diabetes Mellitus and Risk of Childhood Overweight and Obesity in Offspring: A Systematic Review. Exp. Diabetes Res. 2011, 2011, 541308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Åberg, A.; Westbom, L.; Källén, B. Congenital Malformations among Infants Whose Mothers Had Gestational Diabetes or Preexisting Diabetes. Early Hum. Dev. 2001, 61, 85–95. [Google Scholar] [CrossRef]
- Mirghani Dirar, A.; Doupis, J. Gestational Diabetes from A to Z. World J. Diabetes 2017, 8, 489–511. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal Changes in Glucose Metabolism during Pregnancy in Obese Women with Normal Glucose Tolerance and Gestational Diabetes Mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Sudharshana Murthy, K.; Bhandiwada, A.; Chandan, S.; Gowda, S.; Sindhusree, G. Evaluation of Oxidative Stress and Proinflammatory Cytokines in Gestational Diabetes Mellitus and Their Correlation with Pregnancy Outcome. Indian J. Endocrinol. Metab. 2018, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Mouzon, S.H.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef] [PubMed]
- LIN, H.; LI, S.; ZHANG, J.; LIN, S.; TAN, B.K.; HU, J. Functional Food Ingredients for Control of Gestational Diabetes Mellitus: A Review. Food Sci. Technol. 2022, 42, e03621. [Google Scholar] [CrossRef]
- Pham, N.M.; Van Do, V.; Lee, A.H. Polyphenol-Rich Foods and Risk of Gestational Diabetes: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2019, 73, 647–656. [Google Scholar] [CrossRef]
- Salinas-Roca, B.; Rubió-Piqué, L.; Montull-López, A. Polyphenol Intake in Pregnant Women on Gestational Diabetes Risk and Neurodevelopmental Disorders in Offspring: A Systematic Review. Nutrients 2022, 14, 3753. [Google Scholar] [CrossRef]
- Gao, Q.; Zhong, C.; Zhou, X.; Chen, R.; Xiong, T.; Hong, M.; Li, Q.; Kong, M.; Xiong, G.; Han, W.; et al. Inverse Association of Total Polyphenols and Flavonoids Intake and the Intake from Fruits with the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. Clin. Nutr. 2021, 40, 550–559. [Google Scholar] [CrossRef]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Al-kuraishy, H.M.; Al-Maiahy, T.J.; Al-Buhadily, A.K.; Saad, H.M.; Al-Gareeb, A.I.; Simal-Gandara, J. Plasminogen Activator Inhibitor 1 and Gestational Diabetes: The Causal Relationship. Diabetol. Metab. Syndr. 2022, 14, 127. [Google Scholar] [CrossRef]
- Basu, A.; Crew, J.; Ebersole, J.L.; Kinney, J.W.; Salazar, A.M.; Planinic, P.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Improve Serum Antioxidant and Adipokine Biomarkers and Lipid Peroxidation in Pregnant Women with Obesity and at Risk for Gestational Diabetes. Antioxidants 2021, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and Diabetes: From Animal to Human Studies. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Wan, J.; Li, H.; Ding, J.; Wang, Y.; Wang, X.; Li, M. Resveratrol Relieves Gestational Diabetes Mellitus in Mice through Activating AMPK. Reprod. Biol. Endocrinol. 2015, 13, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.; Chen, H. Resveratrol Ameliorates the Glucose Uptake and Lipid Metabolism in Gestational Diabetes Mellitus Mice and Insulin-Resistant Adipocytes via MiR-23a-3p/NOV Axis. Mol. Immunol. 2021, 137, 163–173. [Google Scholar] [CrossRef]
- Singh, C.K.; Kumar, A.; Hitchcock, D.B.; Fan, D.; Goodwin, R.; Lavoie, H.A.; Nagarkatti, P.; Dipette, D.J.; Singh, U.S. Resveratrol Prevents Embryonic Oxidative Stress and Apoptosis Associated with Diabetic Embryopathy and Improves Glucose and Lipid Profile of Diabetic Dam. Mol. Nutr. Food Res. 2011, 55, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Brawerman, G.M.; Kereliuk, S.M.; Brar, N.; Cole, L.K.; Seshadri, N.; Pereira, T.J.; Xiang, B.; Hunt, K.L.; Fonseca, M.A.; Hatch, G.M.; et al. Maternal Resveratrol Administration Protects against Gestational Diabetes-Induced Glucose Intolerance and Islet Dysfunction in the Rat Offspring. J. Physiol. 2019, 597, 4175–4192. [Google Scholar] [CrossRef]
- Du, S.; Lv, Y.; Li, N.; Huang, X.; Liu, X.; Li, H.; Wang, C.; Jia, Y.-F. Biological Investigations on Therapeutic Effect of Chitosan Encapsulated Nano Resveratrol against Gestational Diabetes Mellitus Rats Induced by Streptozotocin. Drug Deliv. 2020, 27, 953–963. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Xie, W.; Lu, X.; Liang, Y.; Li, M.; Wang, Z.; Huang, X.; Tang, M.; Pfaff, D.W.; et al. Maternal Diabetes Induces Autism-like Behavior by Hyperglycemia-Mediated Persistent Oxidative Stress and Suppression of Superoxide Dismutase 2. Proc. Natl. Acad. Sci. USA 2019, 116, 23743–23752. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.T.; Liong, S.; Lim, R.; Barker, G.; Lappas, M. Resveratrol Ameliorates the Chemical and Microbial Induction of Inflammation and Insulin Resistance in Human Placenta, Adipose Tissue and Skeletal Muscle. PLoS ONE 2017, 12, e0173373. [Google Scholar] [CrossRef]
- Malvasi, A.; Kosmas, I.; Mynbaev, O.A.; Sparic, R.; Gustapane, S.; Guido, M.; Tinelli, A. Can Trans Resveratrol plus D-Chiro-Inositol and Myo-Inositol Improve Maternal Metabolic Profile in Overweight Pregnant Patients? Clin. Ter. 2017, 168, e240–e247. [Google Scholar] [CrossRef]
- Lu, X.; Wu, F.; Jiang, M.; Sun, X.; Tian, G. Curcumin Ameliorates Gestational Diabetes in Mice Partly through Activating AMPK. Pharm. Biol. 2019, 57, 250–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wang, F.; Albert Reece, E.; Yang, P. Curcumin Ameliorates High Glucose-Induced Neural Tube Defects by Suppressing Cellular Stress and Apoptosis. Am. J. Obstet. Gynecol. 2015, 212, 802.e1–802.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen-Ngo, C.; Willcox, J.C.; Lappas, M. Anti-Inflammatory Effects of Phenolic Acids Punicalagin and Curcumin in Human Placenta and Adipose Tissue. Placenta 2020, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bolouki, A.; Zal, F.; Alaee, S. Ameliorative Effects of Quercetin on the Preimplantation Embryos Development in Diabetic Pregnant Mice. J. Obstet. Gynaecol. Res. 2020, 46, 736–744. [Google Scholar] [CrossRef]
- Cao, L.; Tan, C.; Meng, F.; Liu, P.; Reece, E.A.; Zhao, Z. Amelioration of Intracellular Stress and Reduction of Neural Tube Defects in Embryos of Diabetic Mice by Phytochemical Quercetin. Sci. Rep. 2016, 6, 21491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahabady, M.K.; Shamsi, M.M.; Ranjbar, R.; Tabandeh, M.R.; Khazaeel, K. Quercetin Improved Histological Structure and Upregulated Adiponectin and Adiponectin Receptors in the Placenta of Rats with Gestational Diabetes Mellitus. Placenta 2021, 106, 49–57. [Google Scholar] [CrossRef]
- Tan, C.; Meng, F.; Reece, E.A.; Zhao, Z. Modulation of Nuclear Factor-ΚB Signaling and Reduction of Neural Tube Defects by Quercetin-3-Glucoside in Embryos of Diabetic Mice. Am. J. Obstet. Gynecol. 2018, 219, 197.e1–197.e8. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Rosario, F.J.; Powell, T.L.; Jansson, T. Adiponectin Supplementation in Pregnant Mice Prevents the Adverse Effects of Maternal Obesity on Placental Function and Fetal Growth. Proc. Natl. Acad. Sci. USA 2015, 112, 12858–12863. [Google Scholar] [CrossRef] [Green Version]
- da Costa, P.C.T.; de Souza, E.L.; Lacerda, D.C.; Cruz Neto, J.P.R.; de Sales, L.C.S.; Silva Luis, C.C.; Pontes, P.B.; Cavalcanti Neto, M.P.; de Brito Alves, J.L. Evidence for Quercetin as a Dietary Supplement for the Treatment of Cardio-Metabolic Diseases in Pregnancy: A Review in Rodent Models. Foods 2022, 11, 2772. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Salomon, C.; Quak, S.; Lai, A.; Willcox, J.C.; Lappas, M. Nobiletin Exerts Anti-Diabetic and Anti-Inflammatory Effects in an in Vitro Human Model and in Vivo Murine Model of Gestational Diabetes. Clin. Sci. 2020, 134, 571–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.; Judy, W.V.; Wilson, D.; Rumberger, J.A.; Guthrie, N. Randomized, Double-Blind, Placebo-Controlled, Clinical Study on the Effect of Diabetinol® on Glycemic Control of Subjects with Impaired Fasting Glucose. Diabetes, Metab. Syndr. Obes. Targets Ther. 2015, 8, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roza, J.M.; Xian-Liu, Z.; Guthrie, N. Effect of Citrus Flavonoids and Tocotrienols on Serum Cholesterol Levels in Hypercholesterolemic Subjects. Altern. Ther. Health Med. 2007, 13, 44–48. [Google Scholar]
- Zhong, J.; Xu, C.; Reece, E.A.; Yang, P. The Green Tea Polyphenol EGCG Alleviates Maternal Diabetes–Induced Neural Tube Defects by Inhibiting DNA Hypermethylation. Am. J. Obstet. Gynecol. 2016, 215, 368.e1–368.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhao, H.; Wang, A. Oleuropein Alleviates Gestational Diabetes Mellitus by Activating AMPK Signaling. Endocr. Connect. 2021, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Zhou, F.M.; Wang, H.R. Mechanism of Pomegranate Ellagic Polyphenols Reducing Insulin Resistance on Gestational Diabetes Mellitus Rats. Am. J. Transl. Res. 2019, 11, 5487–5500. [Google Scholar] [PubMed]
- Huppertz, B.; Gauster, M.; Orendi, K.; König, J.; Moser, G. Oxygen as Modulator of Trophoblast Invasion. J. Anat. 2009, 215, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the Placenta: The Role of Reactive Oxygen Species Signaling. Biomed Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xu, P.; Zhu, F.; Liao, J.; Wu, Y.; Hu, M.; Fu, H.; Qiao, J.; Lin, L.; Huang, B.; et al. The Potent Antioxidant MitoQ Protects Against Preeclampsia During Late Gestation but Increases the Risk of Preeclampsia When Administered in Early Pregnancy. Antioxid. Redox Signal. 2021, 34, 118–136. [Google Scholar] [CrossRef]
- Onogi, A.; Naruse, K.; Sado, T.; Tsunemi, T.; Shigetomi, H.; Noguchi, T.; Yamada, Y.; Akasaki, M.; Oi, H.; Kobayashi, H. Hypoxia Inhibits Invasion of Extravillous Trophoblast Cells through Reduction of Matrix Metalloproteinase (MMP)-2 Activation in the Early First Trimester of Human Pregnancy. Placenta 2011, 32, 665–670. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Y.; Cao, Q.; Hou, Y.; Wang, C. Role of Integrin Switch and Transforming Growth Factor Beta 3 in Hypoxia-Induced Invasion Inhibition of Human Extravillous Trophoblast Cells1. Biol. Reprod. 2012, 87, 47. [Google Scholar] [CrossRef] [PubMed]
- Chard, T. Cytokines in Implantation. Hum. Reprod. Update 1995, 1, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.; King, A.; Critchley, H. Cytokine Control in Human Endometrium. Reproduction 2001, 121, 3–19. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Baek, S.J. Molecular Targets of Dietary Polyphenols with Anti-Inflammatory Properties. Yonsei Med. J. 2005, 46, 585. [Google Scholar] [CrossRef] [Green Version]
- Zielinsky, P.; Busato, S. Prenatal Effects of Maternal Consumption of Polyphenol-rich Foods in Late Pregnancy upon Fetal Ductus Arteriosus. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 256–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S.; et al. Targeting NF-ΚB Signaling Pathway in Cancer by Dietary Polyphenols. Crit. Rev. Food Sci. Nutr. 2020, 60, 2790–2800. [Google Scholar] [CrossRef] [PubMed]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Münch, G. Plant Polyphenols as Inhibitors of NF-ÎoB Induced Cytokine Production—a Potential Anti-Inflammatory Treatment for Alzheimer’s Disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Olcum, M.; Tastan, B.; Ercan, I.; Eltutan, I.B.; Genc, S. Inhibitory Effects of Phytochemicals on NLRP3 Inflammasome Activation: A Review. Phytomedicine 2020, 75, 153238. [Google Scholar] [CrossRef]
- Frolinger, T.; Pasinetti, G. Polyphenolic Compounds Ameliorate Stress-Induced Depression by Preventing NLRP3 Inflammasome Priming (P19-011-19). Curr. Dev. Nutr. 2019, 3 (Suppl. 1), nzz049.P19-011-19. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1–M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Micaelo, N.; González-Abuín, N.; Pinent, M.; Ardévol, A.; Blay, M. Procyanidin B 2 Inhibits Inflammasome-Mediated IL-1β Production in Lipopolysaccharide-Stimulated Macrophages. Mol. Nutr. Food Res. 2015, 59, 262–269. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.; Qian, F.; Wang, Y.; Granato, D. Green Tea Polyphenols and Epigallocatechin-3-Gallate Protect against Perfluorodecanoic Acid Induced Liver Damage and Inflammation in Mice by Inhibiting NLRP3 Inflammasome Activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-H. Impact of Genistein on Maturation of Mouse Oocytes, Fertilization, and Fetal Development. Reprod. Toxicol. 2009, 28, 52–58. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chan, W.-H. Injurious Effects of Curcumin on Maturation of Mouse Oocytes, Fertilization and Fetal Development via Apoptosis. Int. J. Mol. Sci. 2012, 13, 4655–4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.-J.; Lan, K.-C.; Kang, H.-Y.; Liu, Y.-C.; Hsuuw, Y.-D.; Chan, W.-H.; Huang, K.-E. Effect of Curcumin on in Vitro Early Post-Implantation Stages of Mouse Embryo Development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Hsieh, M.-S.; Hsuuw, Y.-D.; Huang, F.-J.; Chan, W.-H. Hazardous Effects of Curcumin on Mouse Embryonic Development through a Mitochondria-Dependent Apoptotic Signaling Pathway. Int. J. Mol. Sci. 2010, 11, 2839–2855. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, H.; Giribabu, N.; Karim, K.; Kassim, N.; Muniandy, S.; Kumar, K.E.; Salleh, N. Quercetin Interferes with the Fluid Volume and Receptivity Development of the Uterus in Rats during the Peri-Implantation Period. Reprod. Toxicol. 2017, 71, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pastén, R.; Martínez-Galero, E.; Chamorro-Cevallos, G. Quercetin and Naringenin Reduce Abnormal Development of Mouse Embryos Produced by Hydroxyurea. J. Pharm. Pharmacol. 2010, 62, 1003–1009. [Google Scholar] [CrossRef]
- Chan, W.-H. Ginkgolide B Induces Apoptosis and Developmental Injury in Mouse Embryonic Stem Cells and Blastocysts. Hum. Reprod. 2006, 21, 2985–2995. [Google Scholar] [CrossRef] [Green Version]
- Ganiger, S.; Malleshappa, H.N.; Krishnappa, H.; Rajashekhar, G.; Ramakrishna Rao, V.; Sullivan, F. A Two Generation Reproductive Toxicity Study with Curcumin, Turmeric Yellow, in Wistar Rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar] [CrossRef]
- Vijayalaxmi Genetic Effects of Turmeric and Curcumin in Mice and Rats. Mutat. Res. Toxicol. 1980, 79, 125–132. [CrossRef] [PubMed]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and Its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phyther. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandasana, H.; Bhatta, R.S.; Sethi, N.; Yadav, S.; Sinha, N. Curcumin Affords Protection against Valproic Acid Induced Teratogenicity by Curtailing Oxidative Stress and Inhibiting CYP2C9 Activity. RSC Adv. 2015, 5, 82756–82764. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K.; Ikemoto, Y.; Ozaki, R.; Nakagawa, K.; Nojiri, S.; Takeda, S.; Sugiyama, R. Influence of Resveratrol Supplementation on IVF–Embryo Transfer Cycle Outcomes. Reprod. Biomed. Online 2019, 39, 205–210. [Google Scholar] [CrossRef]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of Senescent Decidual Cells by Uterine Natural Killer Cells in Cycling Human Endometrium. Elife 2017, 6, e31274. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Pound, L.D.; Thorn, S.R.; Gillingham, M.B.; Thornburg, K.L.; Friedman, J.E.; Frias, A.E.; Grove, K.L. Beneficial and Cautionary Outcomes of Resveratrol Supplementation in Pregnant Nonhuman Primates. FASEB J. 2014, 28, 2466–2477. [Google Scholar] [CrossRef] [Green Version]
- Conover, E.A. Herbal Agents and Over-the-Counter Medications in Pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 237–251. [Google Scholar] [CrossRef]
- Pinn, G.; Pallett, L. Herbal Medicine in Pregnancy. Complement. Ther. Nurs. Midwifery 2002, 8, 77–80. [Google Scholar] [CrossRef]
- Amundsen, S.; Nordeng, H.; Nezvalová-Henriksen, K.; Stovner, L.J.; Spigset, O. Pharmacological Treatment of Migraine during Pregnancy and Breastfeeding. Nat. Rev. Neurol. 2015, 11, 209–219. [Google Scholar] [CrossRef]
- Ciganda, C.; Laborde, A. Herbal Infusions Used for Induced Abortion. J. Toxicol. Clin. Toxicol. 2003, 41, 235–239. [Google Scholar] [CrossRef]
- Bruno, L.O.; Simoes, R.S.; de Jesus Simoes, M.; Girão, M.J.B.C.; Grundmann, O. Pregnancy and Herbal Medicines: An Unnecessary Risk for Women’s Health-A Narrative Review. Phyther. Res. 2018, 32, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Zohrabi, D.; Parivar, K.; Sanati, M.H.; Hayati Roodbari, N. Effects of Crocin on The Pituitary-Gonadal Axis and Hypothalamic Kiss-1 Gene Expression in Female Wistar Rats. Int. J. Fertil. Steril. 2018, 12, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Tomaino, A.; Trombetta, D. Herbal Products in Pregnancy: Experimental Studies and Clinical Reports. Phyther. Res. 2014, 28, 1107–1116. [Google Scholar] [CrossRef]
- Zielinsky, P.; Manica, J.L.L.; Piccoli, A.L.; Nicoloso, L.H.S.; Barra, M.; Alievi, M.M.; Vian, I.; Zilio, A.; Pizzato, P.E.; Silva, J.S.; et al. Fetal Ductal Constriction Caused by Maternal Ingestion of Green Tea in Late Pregnancy: An Experimental Study. Prenat. Diagn. 2012, 32, 921–926. [Google Scholar] [CrossRef]
- Zielinsky, P.; Manica, J.L.; Piccoli Jr, A.; Areias, J.C.N.; Nicoloso, L.H.; Menezes, H.S.; Frajndlich, R.; Busato, A.K.; Petracco, R.; Hagemann, L.; et al. OP18.03: Experimental Study of the Role of Maternal Consumption of Green Tea, Mate Tea and Grape Juice on Fetal Ductal Constriction. Ultrasound Obstet. Gynecol. 2007, 30, 515. [Google Scholar] [CrossRef]
- Sridharan, S.; Archer, N.; Manning, N. Premature Constriction of the Fetal Ductus Arteriosus Following the Maternal Consumption of Camomile Herbal Tea. Ultrasound Obstet. Gynecol. 2009, 34, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Miyakoshi, K.; Yamada, M.; Kadohira, I.; Minegishi, K.; Yoshimura, Y. Functional Foods for the Fetus? Acta Obstet. Gynecol. Scand. 2011, 90, 1172–1173. [Google Scholar] [CrossRef]
- Kapadia, V.; Embers, D.; Wells, E.; Lemler, M.; Rosenfeld, C.R. Prenatal Closure of the Ductus Arteriosus and Maternal Ingestion of Anthocyanins. J. Perinatol. 2010, 30, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Rakha, S. Excessive Maternal Orange Intake—A Reversible Etiology of Fetal Premature Ductus Arteriosus Constriction: A Case Report. Fetal Diagn. Ther. 2017, 42, 158–160. [Google Scholar] [CrossRef]
- Zielinsky, P.; Martignoni, F.V.; Vian, I. Deleterious Effects of Maternal Ingestion of Cocoa upon Fetal Ductus Arteriosus in Late Pregnancy. Front. Pharmacol. 2014, 5, 281. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M.; Baierle, M.; Charão, M.F.; Bubols, G.B.; Gravina, F.S.; Zielinsky, P.; Arbo, M.D.; Cristina Garcia, S. Polyphenol-Rich Food General and on Pregnancy Effects: A Review. Drug Chem. Toxicol. 2017, 40, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Zielinsky, P.; Piccoli, A.L.; Manica, J.L.L.; Nicoloso, L.H.; Vian, I.; Bender, L.; Pizzato, P.; Pizzato, M.; Swarowsky, F.; Barbisan, C.; et al. Reversal of Fetal Ductal Constriction after Maternal Restriction of Polyphenol-Rich Foods: An Open Clinical Trial. J. Perinatol. 2012, 32, 574–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillam-Krakauer, M.; Mahajan, K. Patent Ductus Arteriosus; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Van Overmeire, B.; Chemtob, S. The Pharmacologic Closure of the Patent Ductus Arteriosus. Semin. Fetal Neonatal Med. 2005, 10, 177–184. [Google Scholar] [CrossRef]
- Tang, E.H.C.; Vanhoutte, P.M. Gene Expression Changes of Prostanoid Synthases in Endothelial Cells and Prostanoid Receptors in Vascular Smooth Muscle Cells Caused by Aging and Hypertension. Physiol. Genom. 2008, 32, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftin, C.D.; Trivedi, D.B.; Tiano, H.F.; Clark, J.A.; Lee, C.A.; Epstein, J.A.; Morham, S.G.; Breyer, M.D.; Nguyen, M.; Hawkins, B.M.; et al. Failure of Ductus Arteriosus Closure and Remodeling in Neonatal Mice Deficient in Cyclooxygenase-1 and Cyclooxygenase-2. Proc. Natl. Acad. Sci. USA 2001, 98, 1059–1064. [Google Scholar] [CrossRef]
- Bubols, G.B.; Zielinsky, P.; Piccoli, A.L.; Nicoloso, L.H.; Vian, I.; Moro, A.M.; Charão, M.F.; Brucker, N.; Bulcão, R.P.; Nascimento, S.N.; et al. Nitric Oxide and Reactive Species Are Modulated in the Polyphenol-Induced Ductus Arteriosus Constriction in Pregnant Sheep. Prenat. Diagn. 2014, 34, 1268–1276. [Google Scholar] [CrossRef]
- Pacheco-Romero, J.; Acosta Conchucos, O.; Huerta Canales, D.; Cabrera Ramos, S.; Vargas Chávez, M.; Mascaro Sánchez, P.; Huamán Guerrero, M.; Sandoval Paredes, J.; López Gabriel, R.; Mateus, J.; et al. Genetic Markers for Preeclampsia in Peruvian Women. Colomb. Med. 2020, 52, e2014437. [Google Scholar] [CrossRef]
- Palis, J. Ontogeny of Erythropoiesis. Curr. Opin. Hematol. 2008, 15, 155–161. [Google Scholar] [CrossRef]
- Dennery, P.A. Effects of Oxidative Stress on Embryonic Development. Birth Defects Res. Part C Embryo Today Rev. 2007, 81, 155–162. [Google Scholar] [CrossRef]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic Modifications by Dietary Phytochemicals: Implications for Personalized Nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Parasramka, M.A.; Ho, E.; Williams, D.E.; Dashwood, R.H. MicroRNAs, Diet, and Cancer: New Mechanistic Insights on the Epigenetic Actions of Phytochemicals. Mol. Carcinog. 2012, 51, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Chango, A.; Pogribny, I. Considering Maternal Dietary Modulators for Epigenetic Regulation and Programming of the Fetal Epigenome. Nutrients 2015, 7, 2748–2770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gicquel, C.; El-Osta, A.; Le Bouc, Y. Epigenetic Regulation and Fetal Programming. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 764. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Barker, D.J. Fetal Programming and Adult Health. Public Health Nutr. 2001, 4, 611–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhees, K.; Vonhögen, I.G.C.; van Schooten, F.J.; Godschalk, R.W.L. You Are What You Eat, and so Are Your Children: The Impact of Micronutrients on the Epigenetic Programming of Offspring. Cell. Mol. Life Sci. 2014, 71, 271–285. [Google Scholar] [CrossRef]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef] [Green Version]
- Branum, A.M.; Bailey, R.; Singer, B.J. Dietary Supplement Use and Folate Status during Pregnancy in the United States. J. Nutr. 2013, 143, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and Safety of Polyphenol Consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef] [Green Version]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is There Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Lean, S.C.; Jones, R.L.; Roberts, S.A.; Heazell, A.E.P. A Prospective Cohort Study Providing Insights for Markers of Adverse Pregnancy Outcome in Older Mothers. BMC Pregnancy Childbirth 2021, 21, 706. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. https://doi.org/10.3390/nu14245246
Nacka-Aleksić M, Pirković A, Vilotić A, Bojić-Trbojević Ž, Jovanović Krivokuća M, Giampieri F, Battino M, Dekanski D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients. 2022; 14(24):5246. https://doi.org/10.3390/nu14245246
Chicago/Turabian StyleNacka-Aleksić, Mirjana, Andrea Pirković, Aleksandra Vilotić, Žanka Bojić-Trbojević, Milica Jovanović Krivokuća, Francesca Giampieri, Maurizio Battino, and Dragana Dekanski. 2022. "The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders" Nutrients 14, no. 24: 5246. https://doi.org/10.3390/nu14245246




