Obesity, Fat Distribution and Risk of Cancer in Women and Men: A Mendelian Randomisation Study
Abstract
1. Introduction
2. Methods
3. Results
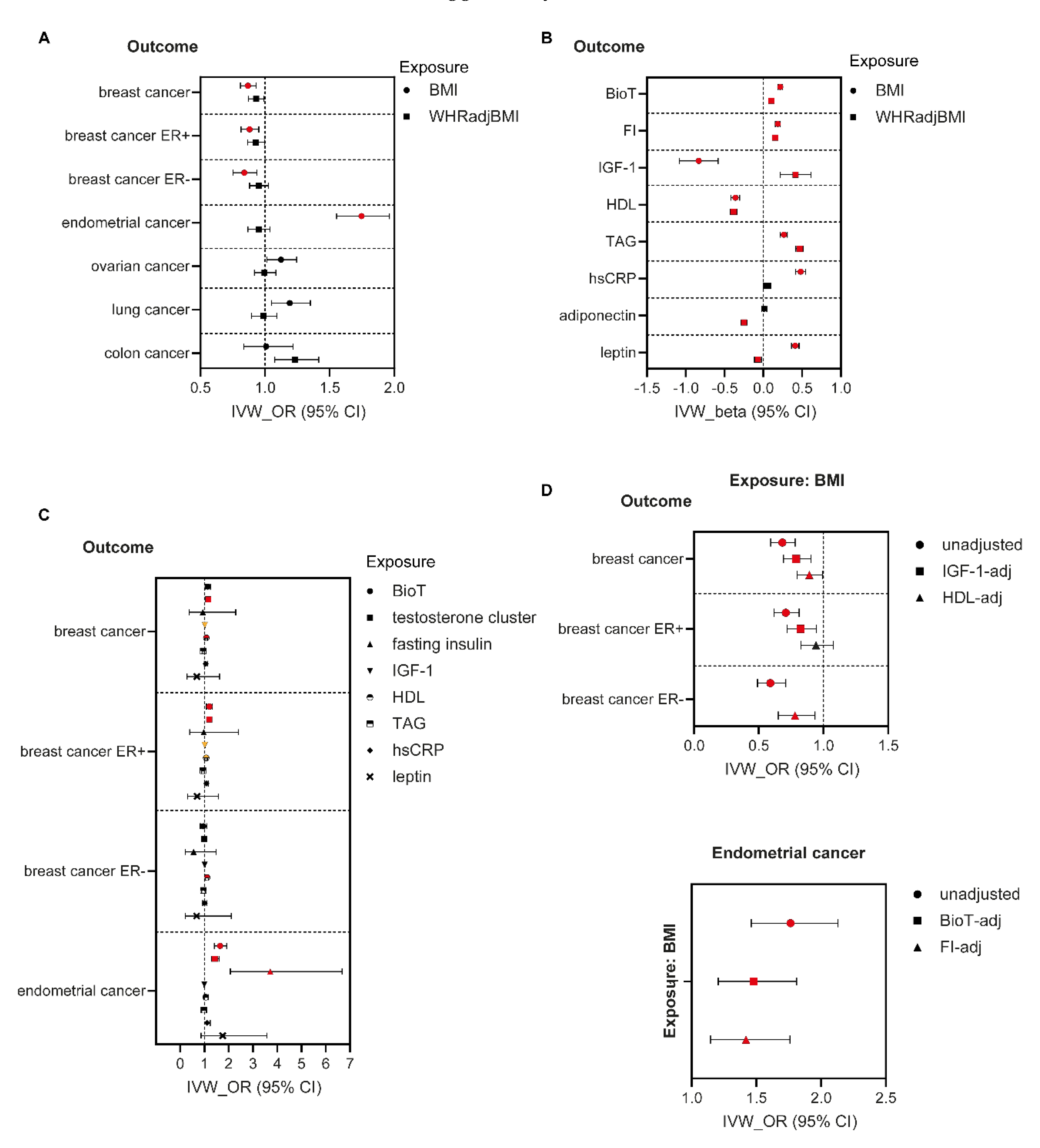
3.1. MR Analyses in Women
3.2. Mediation Analyses in Women
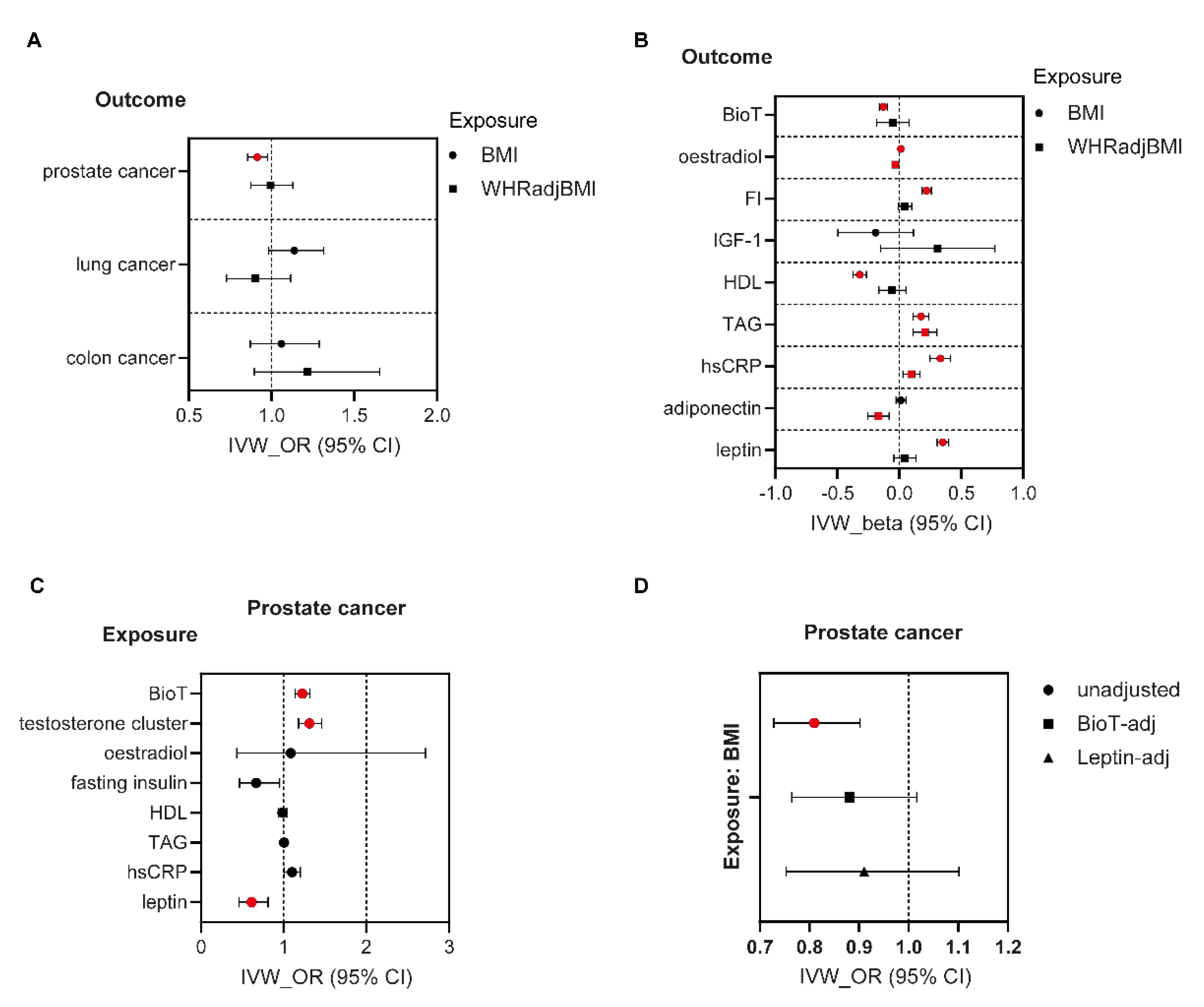
3.3. MR Analyses in Men
3.4. Mediation Analyses in Men
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | adipose tissue |
| BioT | bioavailable testosterone |
| BMI | body mass index |
| CI | confidence interval |
| CRC | colorectal cancer |
| CVD | cardiovascular disease |
| ER+ve | oestrogen receptor positive |
| ER-ve | oestrogen receptor negative |
| FI | fasting insulin |
| GWAS | genome-wide association study |
| hsCRP | high sensitivity C-reactive protein |
| HDL | high-density lipoprotein |
| IGF-1 | insulin-like growth factor-1 |
| IVW | inverse-variance weighted |
| MR | Mendelian randomisation |
| MR-PRESSO | MR Pleiotropy RESidual Sum and Outlier |
| MVMR | multivariable/multivariate Mendelian randomisation |
| OR | odds ratio |
| SHBG | sex hormone binding globulin |
| SNP | single nucleotide polymorphism |
| TAG | triglycerides |
| T2D | type 2 diabetes |
| UKBB | UK Biobank |
| WHR | waist-to-hip ratio |
| WHRadjBMI | BMI-adjusted waist-to-hip ratio |
References
- WHO. Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 October 2022).
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lega, I.C.; Lipscombe, L.L. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr. Rev. 2020, 41, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet. Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Kuang, X.H.; Liu, K.; Li, J. The global burden and temporal trend of cancer attributable to high body mass index: Estimates from the Global Burden of Disease Study 2019. Front. Nutr. 2022, 9, 918330. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Pinnick, K.E. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat. Rev. Endocrinol. 2015, 11, 90–100. [Google Scholar] [CrossRef]
- Manolopoulos, K.N.; Karpe, F.; Frayn, K.N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. 2010, 34, 949–959. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Despres, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet. Diabetes. Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef]
- Font-Burgada, J.; Sun, B.; Karin, M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016, 23, 48–62. [Google Scholar] [CrossRef]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- WCRF. Worldwide Cancer Data: Global Cancer Statistics for the Most Common Cancers in the World. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 1 October 2022).
- CDC. Obesity and Cancer. Available online: https://www.cdc.gov/cancer/obesity/index.htm (accessed on 1 October 2022).
- Winkler, T.W.; Gunther, F.; Hollerer, S.; Zimmermann, M.; Loos, R.J.; Kutalik, Z.; Heid, I.M. A joint view on genetic variants for adiposity differentiates subtypes with distinct metabolic implications. Nat. Commun. 2018, 9, 1946. [Google Scholar] [CrossRef]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef]
- Ruth, K.S.; Day, F.R.; Tyrrell, J.; Thompson, D.J.; Wood, A.R.; Mahajan, A.; Beaumont, R.N.; Wittemans, L.; Martin, S.; Busch, A.S.; et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020, 26, 252–258. [Google Scholar] [CrossRef]
- Lagou, V.; Magi, R.; Hottenga, J.J.; Grallert, H.; Perry, J.R.B.; Bouatia-Naji, N.; Marullo, L.; Rybin, D.; Jansen, R.; Min, J.L.; et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat. Commun. 2021, 12, 24. [Google Scholar] [CrossRef]
- Kilpelainen, T.O.; Carli, J.F.; Skowronski, A.A.; Sun, Q.; Kriebel, J.; Feitosa, M.F.; Hedman, A.K.; Drong, A.W.; Hayes, J.E.; Zhao, J.; et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat. Commun. 2016, 7, 10494. [Google Scholar] [CrossRef]
- Dastani, Z.; Hivert, M.F.; Timpson, N.; Perry, J.R.; Yuan, X.; Scott, R.A.; Henneman, P.; Heid, I.M.; Kizer, J.R.; Lyytikainen, L.P.; et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: A multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012, 8, e1002607. [Google Scholar] [CrossRef]
- Wang, W.; Tesfay, E.B.; van Klinken, J.B.; Willems van Dijk, K.; Bartke, A.; van Heemst, D.; Noordam, R. Clustered Mendelian randomization analyses identify distinct and opposing pathways in the association between genetically influenced insulin-like growth factor-1 and type 2 diabetes mellitus. Int. J. Epidemiol. 2022. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Loh, P.R.; Tucker, G.; Bulik-Sullivan, B.K.; Vilhjalmsson, B.J.; Finucane, H.K.; Salem, R.M.; Chasman, D.I.; Ridker, P.M.; Neale, B.M.; Berger, B.; et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 2015, 47, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Tabuso, M.; Homer-Vanniasinkam, S.; Adya, R.; Arasaradnam, R.P. Role of tissue microenvironment resident adipocytes in colon cancer. World J. Gastroenterol. 2017, 23, 5829–5835. [Google Scholar] [CrossRef]
- Crudele, L.; Piccinin, E.; Moschetta, A. Visceral Adiposity and Cancer: Role in Pathogenesis and Prognosis. Nutrients 2021, 13, 2101. [Google Scholar] [CrossRef]
- Gao, C.; Patel, C.J.; Michailidou, K.; Peters, U.; Gong, J.; Schildkraut, J.; Schumacher, F.R.; Zheng, W.; Boffetta, P.; Stucker, I.; et al. Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int. J. Epidemiol. 2016, 45, 896–908. [Google Scholar] [CrossRef]
- Murphy, N.; Knuppel, A.; Papadimitriou, N.; Martin, R.M.; Tsilidis, K.K.; Smith-Byrne, K.; Fensom, G.; Perez-Cornago, A.; Travis, R.C.; Key, T.J.; et al. Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, and breast cancer risk: Observational and Mendelian randomization analyses with approximately 430 000 women. Ann. Oncol. 2020, 31, 641–649. [Google Scholar] [CrossRef]
- Beeghly-Fadiel, A.; Khankari, N.K.; Delahanty, R.J.; Shu, X.O.; Lu, Y.; Schmidt, M.K.; Bolla, M.K.; Michailidou, K.; Wang, Q.; Dennis, J.; et al. A Mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int. J. Epidemiol. 2020, 49, 1117–1131. [Google Scholar] [CrossRef]
- Johnson, K.E.; Siewert, K.M.; Klarin, D.; Damrauer, S.M.; Program, V.A.M.V.; Chang, K.M.; Tsao, P.S.; Assimes, T.L.; Maxwell, K.N.; Voight, B.F. The relationship between circulating lipids and breast cancer risk: A Mendelian randomization study. PLoS Med. 2020, 17, e1003302. [Google Scholar] [CrossRef]
- Martin, L.J.; Melnichouk, O.; Huszti, E.; Connelly, P.W.; Greenberg, C.V.; Minkin, S.; Boyd, N.F. Serum lipids, lipoproteins, and risk of breast cancer: a nested case-control study using multiple time points. J. Natl. Cancer Inst. 2015, 107, djv032. [Google Scholar] [CrossRef]
- Rotheneder, M.; Kostner, G.M. Effects of low- and high-density lipoproteins on the proliferation of human breast cancer cells in vitro: Differences between hormone-dependent and hormone-independent cell lines. Int. J. Cancer 1989, 43, 875–879. [Google Scholar] [CrossRef]
- Tamimi, R.M.; Hankinson, S.E.; Chen, W.Y.; Rosner, B.; Colditz, G.A. Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch. Intern. Med. 2006, 166, 1483–1489. [Google Scholar] [CrossRef]
- Premenopausal Breast Cancer Collaborative, G.; Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O'Brien, K.M.; Adami, H.O.; Baglietto, L.; Bernstein, L.; et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef]
- Garcia-Estevez, L.; Cortes, J.; Perez, S.; Calvo, I.; Gallegos, I.; Moreno-Bueno, G. Obesity and Breast Cancer: A Paradoxical and Controversial Relationship Influenced by Menopausal Status. Front. Oncol. 2021, 11, 705911. [Google Scholar] [CrossRef]
- Civelek, M.; Wu, Y.; Pan, C.; Raulerson, C.K.; Ko, A.; He, A.; Tilford, C.; Saleem, N.K.; Stancakova, A.; Scott, L.J.; et al. Genetic Regulation of Adipose Gene Expression and Cardio-Metabolic Traits. Am. J. Hum. Genet. 2017, 100, 428–443. [Google Scholar] [CrossRef]
- Cohen, P.G. Aromatase, adiposity, aging and disease. The hypogonadal-metabolic-atherogenic-disease and aging connection. Med. Hypotheses 2001, 56, 702–708. [Google Scholar] [PubMed]
- Rose, D.P.; Vona-Davis, L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 2010, 66, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Gracia, C.R. Obesity and reproductive hormone levels in the transition to menopause. Menopause 2010, 17, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Folkerd, E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: A new hypothesis. Breast. Cancer Res. Treat. 2015, 149, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Verkouter, I.; Noordam, R.; Loh, N.Y.; van Dijk, K.W.; Zock, P.L.; Mook-Kanamori, D.O.; le Cessie, S.; Rosendaal, F.R.; Karpe, F.; Christodoulides, C.; et al. The Relation Between Adult Weight Gain, Adipocyte Volume, and the Metabolic Profile at Middle Age. J. Clin. Endocrinol. Metab. 2021, 106, e4438–e4447. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Ryden, M.; Frisen, J.; Bernard, S.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- van den Berg, M.M.; Winkels, R.M.; de Kruif, J.T.; van Laarhoven, H.W.; Visser, M.; de Vries, J.H.; de Vries, Y.C.; Kampman, E. Weight change during chemotherapy in breast cancer patients: A meta-analysis. BMC Cancer 2017, 17, 259. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Zain, M.M.; Norman, R.J. Impact of obesity on female fertility and fertility treatment. Womens Health (Lond.) 2008, 4, 183–194. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Ernster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Felix, A.S. Menopausal hormone therapy and risk of endometrial cancer. J. Steroid. Biochem. Mol. Biol. 2014, 142, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Pasupuleti, V.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Perez-Lopez, F.R. Insulin resistance and endometrial cancer risk: A systematic review and meta-analysis. Eur. J. Cancer 2015, 51, 2747–2758. [Google Scholar] [CrossRef]
- Bull, C.J.; Bell, J.A.; Murphy, N.; Sanderson, E.; Davey Smith, G.; Timpson, N.J.; Banbury, B.L.; Albanes, D.; Berndt, S.I.; Bezieau, S.; et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. 2020, 18, 396. [Google Scholar] [CrossRef]
- Murphy, N.; Carreras-Torres, R.; Song, M.; Chan, A.T.; Martin, R.M.; Papadimitriou, N.; Dimou, N.; Tsilidis, K.K.; Banbury, B.; Bradbury, K.E.; et al. Circulating Levels of Insulin-like Growth Factor 1 and Insulin-like Growth Factor Binding Protein 3 Associate With Risk of Colorectal Cancer Based on Serologic and Mendelian Randomization Analyses. Gastroenterology 2020, 158, 1300–1312.e20. [Google Scholar] [CrossRef]
- Watts, E.L.; Appleby, P.N.; Perez-Cornago, A.; Bueno-de-Mesquita, H.B.; Chan, J.M.; Chen, C.; Cohn, B.A.; Cook, M.B.; Flicker, L.; Freedman, N.D.; et al. Low Free Testosterone and Prostate Cancer Risk: A Collaborative Analysis of 20 Prospective Studies. Eur. Urol. 2018, 74, 585–594. [Google Scholar] [CrossRef]
- Burton, A.J.; Gilbert, R.; Tilling, K.; Langdon, R.; Donovan, J.L.; Holly, J.M.P.; Martin, R.M. Circulating adiponectin and leptin and risk of overall and aggressive prostate cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loh, N.Y.; Wang, W.; Noordam, R.; Christodoulides, C. Obesity, Fat Distribution and Risk of Cancer in Women and Men: A Mendelian Randomisation Study. Nutrients 2022, 14, 5259. https://doi.org/10.3390/nu14245259
Loh NY, Wang W, Noordam R, Christodoulides C. Obesity, Fat Distribution and Risk of Cancer in Women and Men: A Mendelian Randomisation Study. Nutrients. 2022; 14(24):5259. https://doi.org/10.3390/nu14245259
Chicago/Turabian StyleLoh, Nellie Y., Wenyi Wang, Raymond Noordam, and Constantinos Christodoulides. 2022. "Obesity, Fat Distribution and Risk of Cancer in Women and Men: A Mendelian Randomisation Study" Nutrients 14, no. 24: 5259. https://doi.org/10.3390/nu14245259
APA StyleLoh, N. Y., Wang, W., Noordam, R., & Christodoulides, C. (2022). Obesity, Fat Distribution and Risk of Cancer in Women and Men: A Mendelian Randomisation Study. Nutrients, 14(24), 5259. https://doi.org/10.3390/nu14245259






