Review of the Role of Ferroptosis in Testicular Function
Abstract
1. Introduction
2. The Physiological Role of Iron in the Male Reproductive System
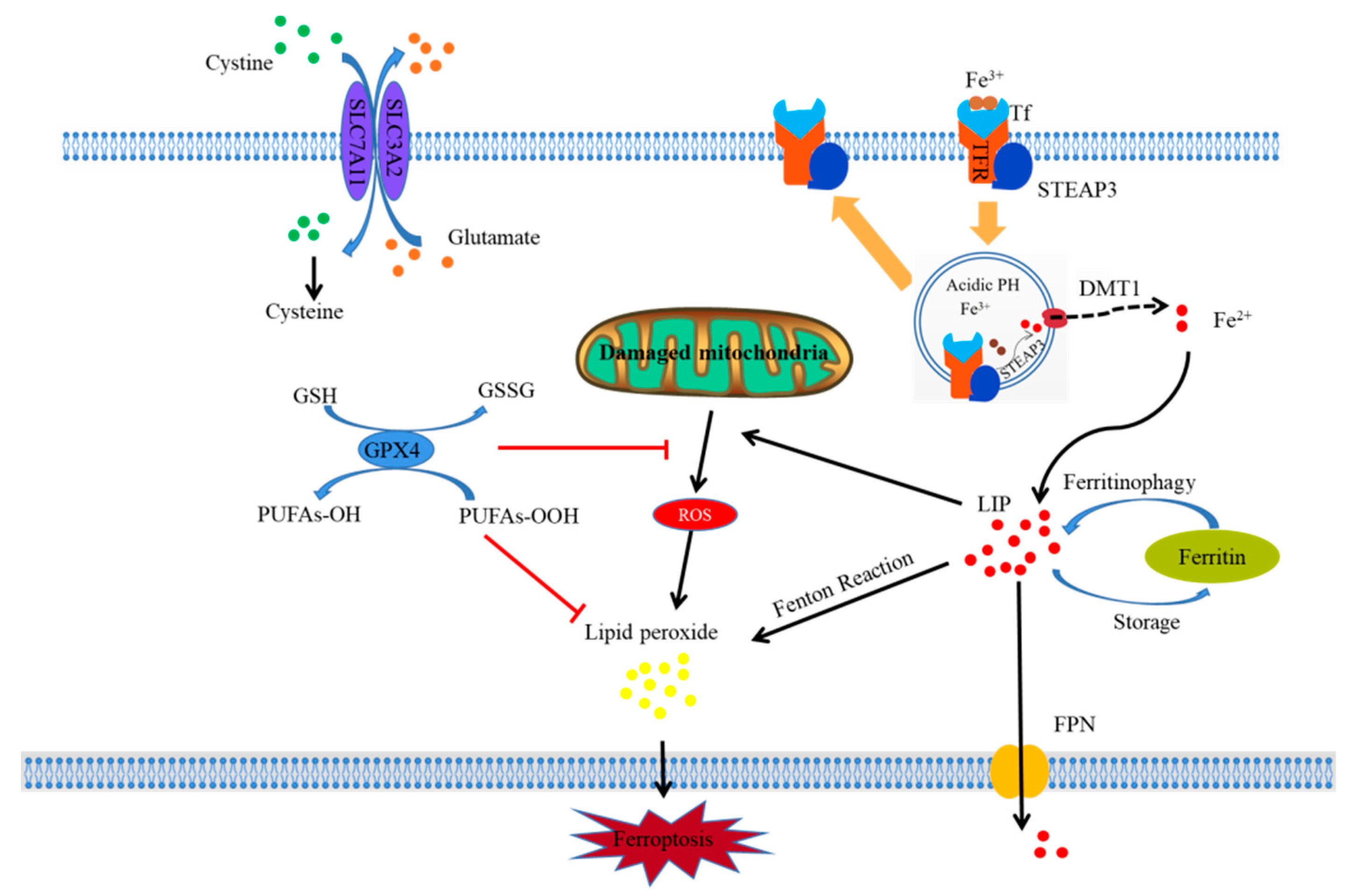
3. The Mechanism of Ferroptosis
3.1. Iron Overload and Ferroptosis
3.2. Lipid Peroxidation and Ferroptosis
3.3. Glutathione Peroxidase Depletion and Ferroptosis
3.4. Mitochondrial Damage and Ferroptosis
4. Research Progress on Ferroptosis in Testis Dysfunction
4.1. Ferroptosis in the Testis In Vivo
4.2. Ferroptosis of Testis-Related Cells In Vitro
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mancardi, D.; Mezzanotte, M.; Arrigo, E.; Barinotti, A.; Roetto, A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants 2021, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, R.S.; Han, S.M.; Leeuwenburgh, C.; Xiao, R. Iron homeostasis and organismal aging. Ageing Res. Rev. 2021, 72, 101510. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Chen, L.; Gao, X.; Shen, S.; Sheng, W.; Min, J.; Wang, F. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci. Bull. 2021, 66, 1806–1816. [Google Scholar] [CrossRef]
- Xu, S.; Min, J.; Wang, F. Ferroptosis: An emerging player in immune cells. Sci. Bull. 2021, 66, 2257–2260. [Google Scholar] [CrossRef]
- Lo, J.O.; Benson, A.E.; Martens, K.L.; Hedges, M.A.; McMurry, H.S.; DeLoughery, T.; Aslan, J.E.; Shatzel, J.J. The role of oral iron in the treatment of adults with iron deficiency. Eur. J. Haematol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Liao, Y.R.; Chang, T.C.; Liew, Y.F.; Liu, C.Y. Effects of Iron Supplementation on Testicular Function and Spermatogenesis of Iron-Deficient Rats. Nutrients 2022, 14, 2063. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.M.; Moreira, A.C.; Sousa, M.; Mathur, P.P.; Oliveira, P.F.; Alves, M.G. Sertoli cell as a model in male reproductive toxicology: Advantages and disadvantages. J. Appl. Toxicol. 2015, 35, 870–883. [Google Scholar] [CrossRef]
- Yokonishi, T.; McKey, J.; Ide, S.; Capel, B. Sertoli cell ablation and replacement of the spermatogonial niche in mouse. Nat. Commun. 2020, 11, 40. [Google Scholar] [CrossRef]
- Vallés, A.S.; Tenconi, P.E.; Luquez, J.M.; Furland, N.E. The inhibition of microtubule dynamics instability alters lipid homeostasis in TM4 Sertoli cells. Toxicol. Appl. Pharmacol. 2021, 426, 115607. [Google Scholar] [CrossRef]
- Mehta, S.; Goyal, L.; Meena, M.L.; Gulati, S.; Sharma, N.; Harshvardhan, L.; Jain, G.; Mehta, S. Assessment of Pituitary Gonadal Axis and Sperm Parameters in Anemic Eugonadal Males Before and After Correction of Iron Deficiency Anemia. J. Assoc. Physicians India 2018, 66, 11–12. [Google Scholar]
- Alleyne, M.; Horne, M.K.; Miller, J.L. Individualized treatment for iron-deficiency anemia in adults. Am. J. Med. 2008, 121, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X.; He, C.; Guo, X.; Cai, H.; Aierken, A.; Hua, J.; Peng, S. Effects of Ferroptosis on Male Reproduction. Int. J. Mol. Sci. 2022, 23, 7139. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.L.; Lee, Y.C.; Shih, C.K.; Hsieh, R.H.; Chen, S.H.; Chang, J.S. Alteration in iron efflux affects male sex hormone testosterone biosynthesis in a diet-induced obese rat model. Food Funct. 2019, 10, 4113–4123. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Costa, C.; Bassaizteguy, V.; Santos, M.; Cardozo, R.; Montes, J.; Settineri, R.; Nicolson, G.L. Incubation of human sperm with micelles made from glycerophospholipid mixtures increases sperm motility and resistance to oxidative stress. PLoS ONE 2018, 13, e0197897. [Google Scholar] [CrossRef]
- Chuai, Y.; Gao, F.; Li, B.; Zhao, L.; Qian, L.; Cao, F.; Wang, L.; Sun, X.; Cui, J.; Cai, J. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem. J. 2012, 442, 49–56. [Google Scholar] [CrossRef]
- Mojica-Villegas, M.A.; Izquierdo-Vega, J.A.; Chamorro-Cevallos, G.; Sanchez-Gutierrez, M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients 2014, 6, 489–503. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, R.; Li, H.; Yu, H.; Ren, K.; Jia, S.; Li, Y.; Wang, Q. Insight into the Double-Edged Role of Ferroptosis in Disease. Biomolecules 2021, 11, 1790. [Google Scholar] [CrossRef]
- Cheng, Y.; Zak, O.; Aisen, P.; Harrison, S.C.; Walz, T. Structure of the human transferrin receptor-transferrin complex. Cell 2004, 116, 565–576. [Google Scholar] [CrossRef]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202. [Google Scholar] [CrossRef]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Maccarinelli, F.; Regoni, M.; Carmona, F.; Poli, M.; Meyron-Holtz, E.G.; Arosio, P. Mitochondrial ferritin deficiency reduces male fertility in mice. Reprod. Fertil. Dev. 2017, 29, 2005–2010. [Google Scholar] [CrossRef][Green Version]
- Asano, T.; Komatsu, M.; Yamaguchi-Iwai, Y.; Ishikawa, F.; Mizushima, N.; Iwai, K. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol. Cell. Biol. 2011, 31, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Li, Q.S.; Jia, Y.J. Ferroptosis: A critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen. Res. 2023, 18, 506–512. [Google Scholar] [CrossRef] [PubMed]
- D’Herde, K.; Krysko, D.V. Ferroptosis: Oxidized PEs trigger death. Nat. Chem. Biol. 2017, 13, 4–5. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, S.; Deng, H.; Yang, W.; Rao, L.; Tian, R.; Liu, Y.; Yu, G.; Zhou, Z.; Song, J.; et al. Endogenous Labile Iron Pool-Mediated Free Radical Generation for Cancer Chemodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 15320–15330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, J.Y.; Liu, X.S.; Chen, H.Z.; Ai, Y.L.; Cheng, K.; Sun, R.Y.; Zhou, D.; Han, J.; Wu, Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018, 28, 1171–1185. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc− cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Oh, S.J.; Ikeda, M.; Ide, T.; Hur, K.Y.; Lee, M.S. Mitochondrial event as an ultimate step in ferroptosis. Cell Death Discov. 2022, 8, 414. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Santos, M.A.S.; Fardilha, M. Fighting Bisphenol A-Induced Male Infertility: The Power of Antioxidants. Antioxidants 2021, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, M.Y.; Jiang, H.B.; Guo, C.R.; Zhu, X.D.; Yao, X.Q.; Zeng, W.W.; Zhao, Y.; Chi, L.K. Bisphenol A induces testicular oxidative stress in mice leading to ferroptosis. Asian J. Androl. 2022, 23. [Google Scholar] [CrossRef]
- Hu, D.; Tian, L.; Li, X.; Chen, Y.; Xu, Z.; Ge, R.S.; Wang, Y. Tetramethyl bisphenol a inhibits leydig cell function in late puberty by inducing ferroptosis. Ecotoxicol. Environ. Saf. 2022, 236, 113515. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard. Mater. 2022, 426, 127807. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, L.; Sun, X.; Li, J.; Wang, N.; Liu, X.; Yao, X.; Zhang, C.; Deng, H.; Wang, S.; et al. DEHP induces ferroptosis in testes via p38α-lipid ROS circulation and destroys the BTB integrity. Food Chem. Toxicol. 2022, 164, 113046. [Google Scholar] [CrossRef]
- Xiong, L.; Bin, Z.; Young, J.L.; Wintergerst, K.; Cai, L. Exposure to low-dose cadmium induces testicular ferroptosis. Ecotoxicol. Environ. Saf. 2022, 234, 113373. [Google Scholar] [CrossRef]
- Meng, P.; Zhang, S.; Jiang, X.; Cheng, S.; Zhang, J.; Cao, X.; Qin, X.; Zou, Z.; Chen, C. Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol. Environ. Saf. 2020, 194, 110360. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Gao, J.; Li, H.; Wang, X.; Li, Y.; Sun, F. Inhibition of ferroptosis attenuates busulfan-induced oligospermia in mice. Toxicology 2020, 440, 152489. [Google Scholar] [CrossRef]
- Jing, S.; Liu, C.; Zheng, J.; Dong, Z.; Guo, N. Toxicity of zearalenone and its nutritional intervention by natural products. Food Funct. 2022, 13, 10374–10400. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Cui, H.; Ding, K.; Zhao, Y.; Ma, X.; Adetunji, A.O.; Min, L. Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals 2022, 12, 3026. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Wang, X.; Zhou, T.; Cheng, J.; Wang, C.; Sun, F.; Zhao, X. Inhibition of ferroptosis attenuates oligospermia in male Nrf2 knockout mice. Free Radic. Biol. Med. 2022, 193, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Khosravi, A.; Behnam, P.; Ramezani, F.; Eslami Farsani, B.; Aliaghaei, A.; Pirani, M.; Akaberi-Nasrabadi, S.; Abdi, S.; Abdollahifar, M.A. Non-apoptotic cell death such as pyroptosis, autophagy, necroptosis and ferroptosis acts as partners to induce testicular cell death after scrotal hyperthermia in mice. Andrologia 2022, 54, e14320. [Google Scholar] [CrossRef]
- Shaygannia, E.; Nasr-Esfahani, M.H.; Sotoodehnejadnematalahi, F.; Parivar, K. Is ferroptosis involved in ROS-induced testicular lesions in a varicocele rat model? Basic Clin. Androl. 2021, 31, 10. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Z.; Cui, H.; Wang, J.; Wang, Y.; Tang, Y.; Yang, W.; Zou, P.; Ling, X.; Han, F.; et al. Analysis by transcriptomics and metabolomics for the proliferation inhibition and dysfunction through redox imbalance-mediated DNA damage response and ferroptosis in male reproduction of mice and TM4 Sertoli cells exposed to PM2.5. Ecotoxicol. Environ. Saf. 2022, 238, 113569. [Google Scholar] [CrossRef]
- Mei, Z.; Du, L.; Liu, X.; Chen, X.; Tian, H.; Deng, Y.; Zhang, W. Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct. 2022, 13, 198–212. [Google Scholar] [CrossRef]
- Li, L.; Hao, Y.; Zhao, Y.; Wang, H.; Zhao, X.; Jiang, Y.; Gao, F. Ferroptosis is associated with oxygen-glucose deprivation/reoxygenation-induced Sertoli cell death. Int. J. Mol. Med. 2018, 41, 3051–3062. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: Toward the era of mesothelioma prevention. Free Radic. Biol. Med. 2019, 133, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Oresti, G.M.; Reyes, J.G.; Luquez, J.M.; Osses, N.; Furland, N.E.; Aveldano, M.I. Differentiation-related changes in lipid classes with long-chain and very long-chain polyenoic fatty acids in rat spermatogenic cells. J. Lipid Res. 2010, 51, 2909–2921. [Google Scholar] [CrossRef]
- Chung, J.Y.; Park, J.E.; Kim, Y.J.; Lee, S.J.; Yu, W.J.; Kim, J.M. Styrene Cytotoxicity in Testicular Leydig Cells In Vitro. Dev. Reprod. 2022, 26, 99–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chen, Y.; Song, W.; Huang, T.; Wang, Y.; Chen, Z.; Chen, F.; Liu, Y.; Wang, X.; Jiang, Y.; et al. Review of the Role of Ferroptosis in Testicular Function. Nutrients 2022, 14, 5268. https://doi.org/10.3390/nu14245268
Yang X, Chen Y, Song W, Huang T, Wang Y, Chen Z, Chen F, Liu Y, Wang X, Jiang Y, et al. Review of the Role of Ferroptosis in Testicular Function. Nutrients. 2022; 14(24):5268. https://doi.org/10.3390/nu14245268
Chicago/Turabian StyleYang, Xu, Yunhe Chen, Wenxi Song, Tingyu Huang, Youshuang Wang, Zhong Chen, Fengjuan Chen, Yu Liu, Xuebing Wang, Yibao Jiang, and et al. 2022. "Review of the Role of Ferroptosis in Testicular Function" Nutrients 14, no. 24: 5268. https://doi.org/10.3390/nu14245268
APA StyleYang, X., Chen, Y., Song, W., Huang, T., Wang, Y., Chen, Z., Chen, F., Liu, Y., Wang, X., Jiang, Y., & Zhang, C. (2022). Review of the Role of Ferroptosis in Testicular Function. Nutrients, 14(24), 5268. https://doi.org/10.3390/nu14245268





