Analysis of White Mulberry Leaves and Dietary Supplements, ATR-FTIR Combined with Chemometrics for the Rapid Determination of 1-Deoxynojirimycin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Analysis
2.3. Antioxidant Activity
2.4. ATR-FTIR Spectroscopy
2.5. Chemometric Analysis
3. Results and Discussion
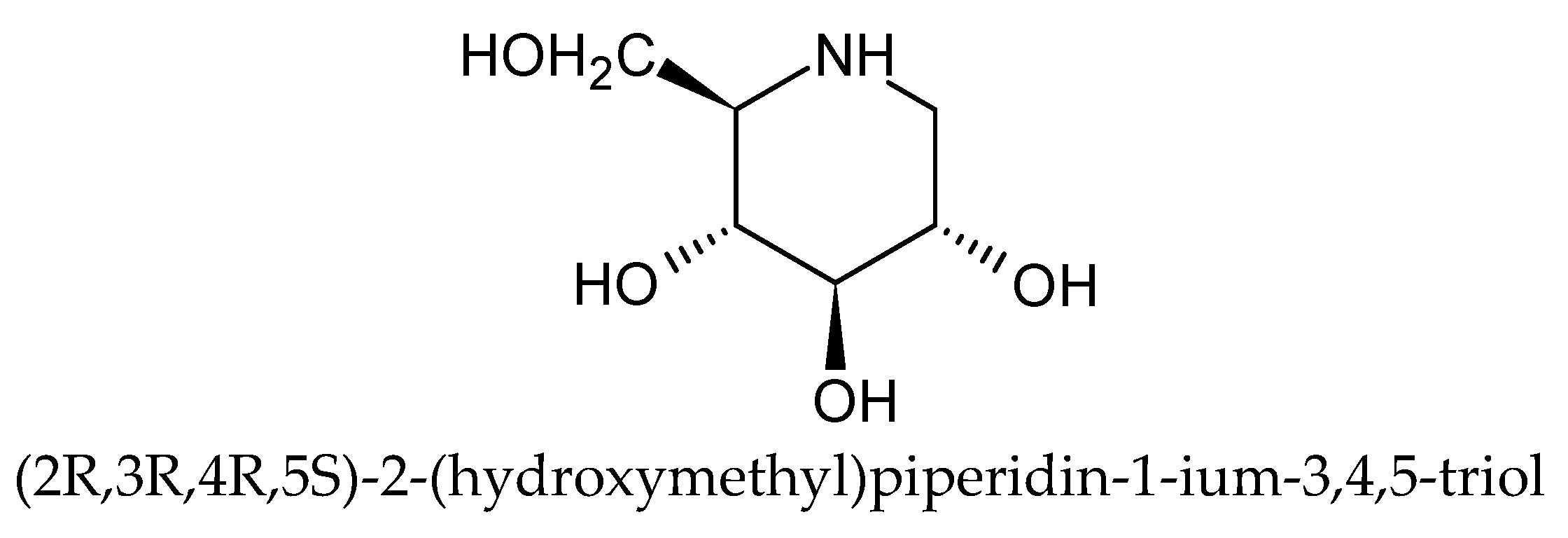
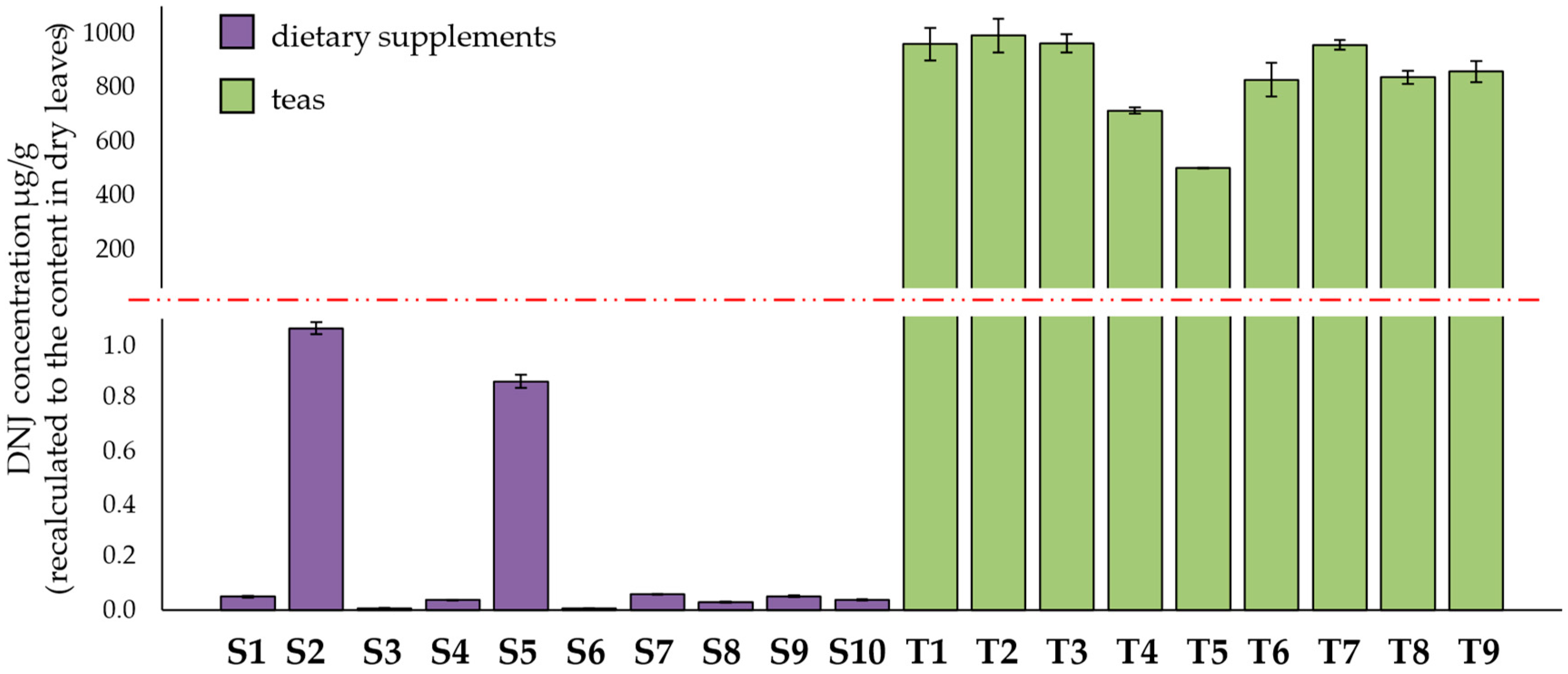
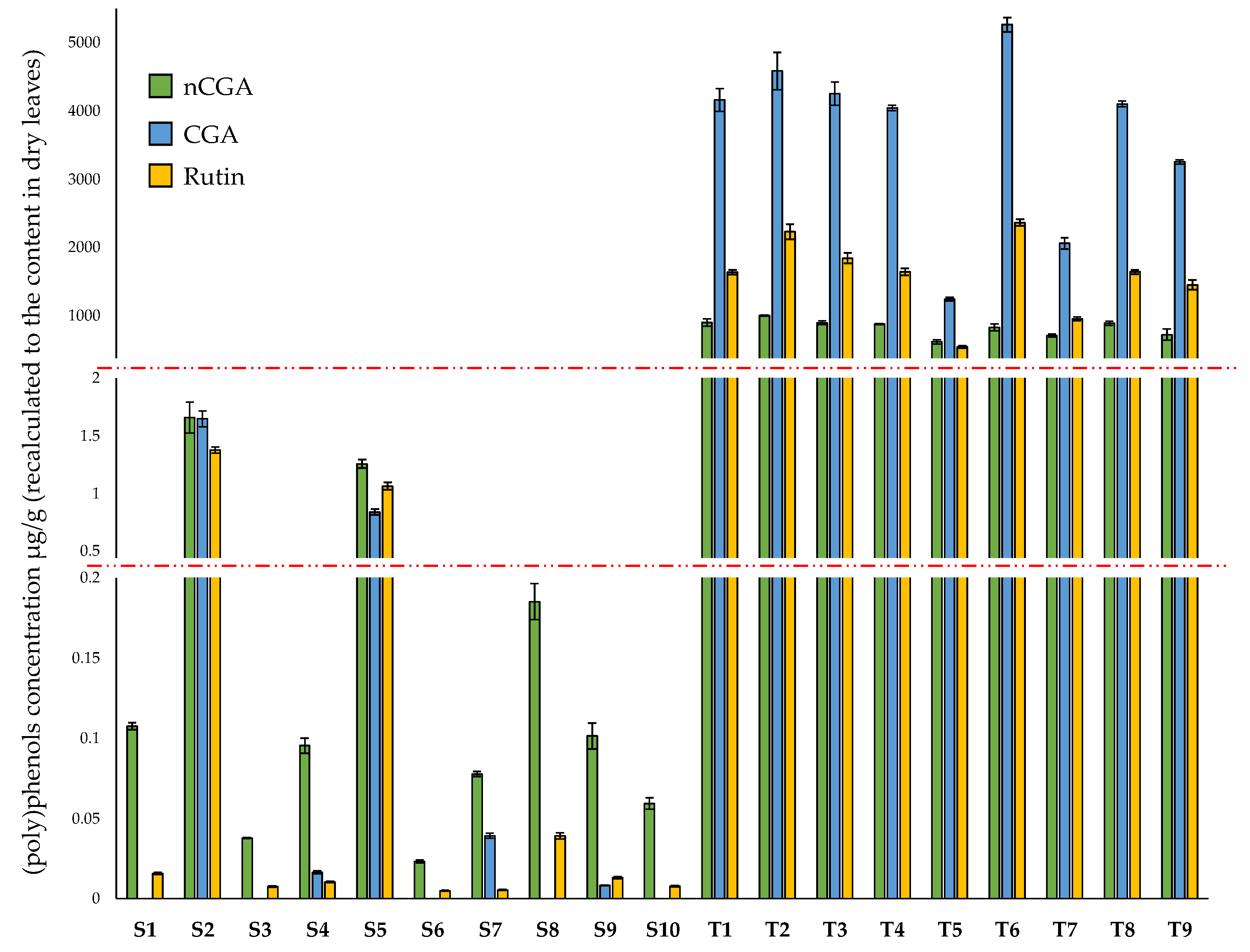
3.1. Determination of DNJ, CGA, nCGA, and Rutin
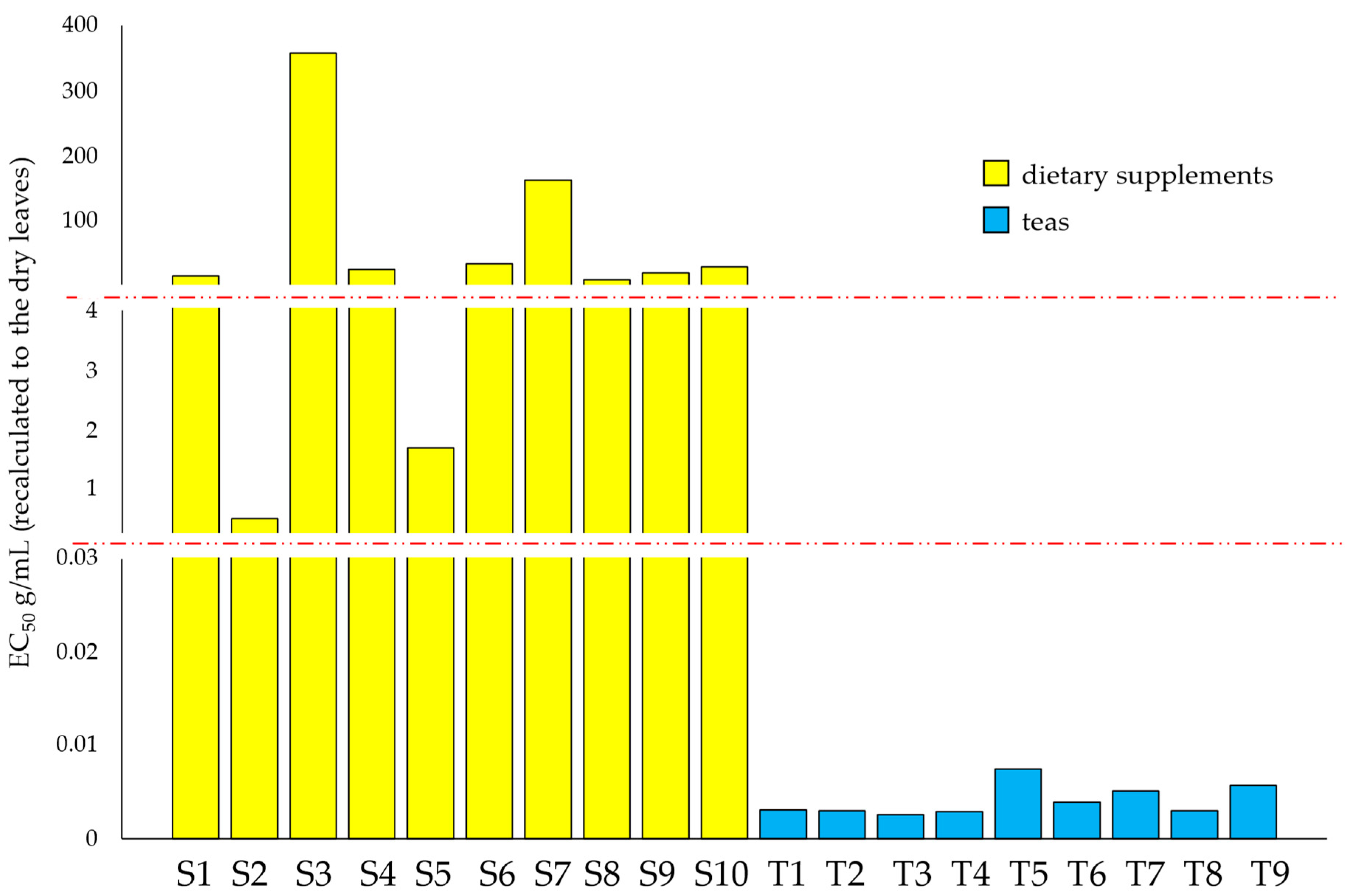
3.2. DPPH• Assay
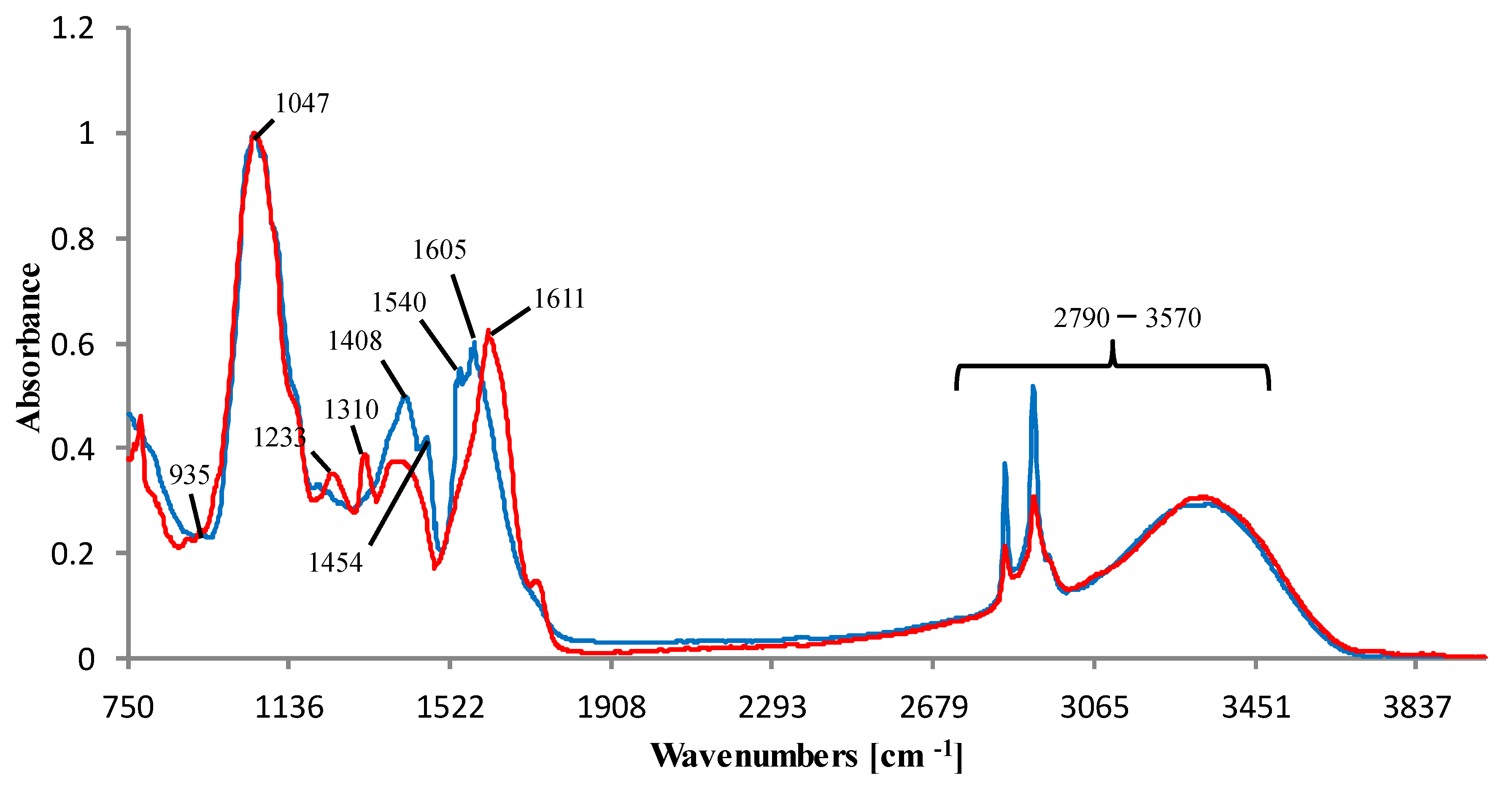
3.3. ATR-FTIR Spectra
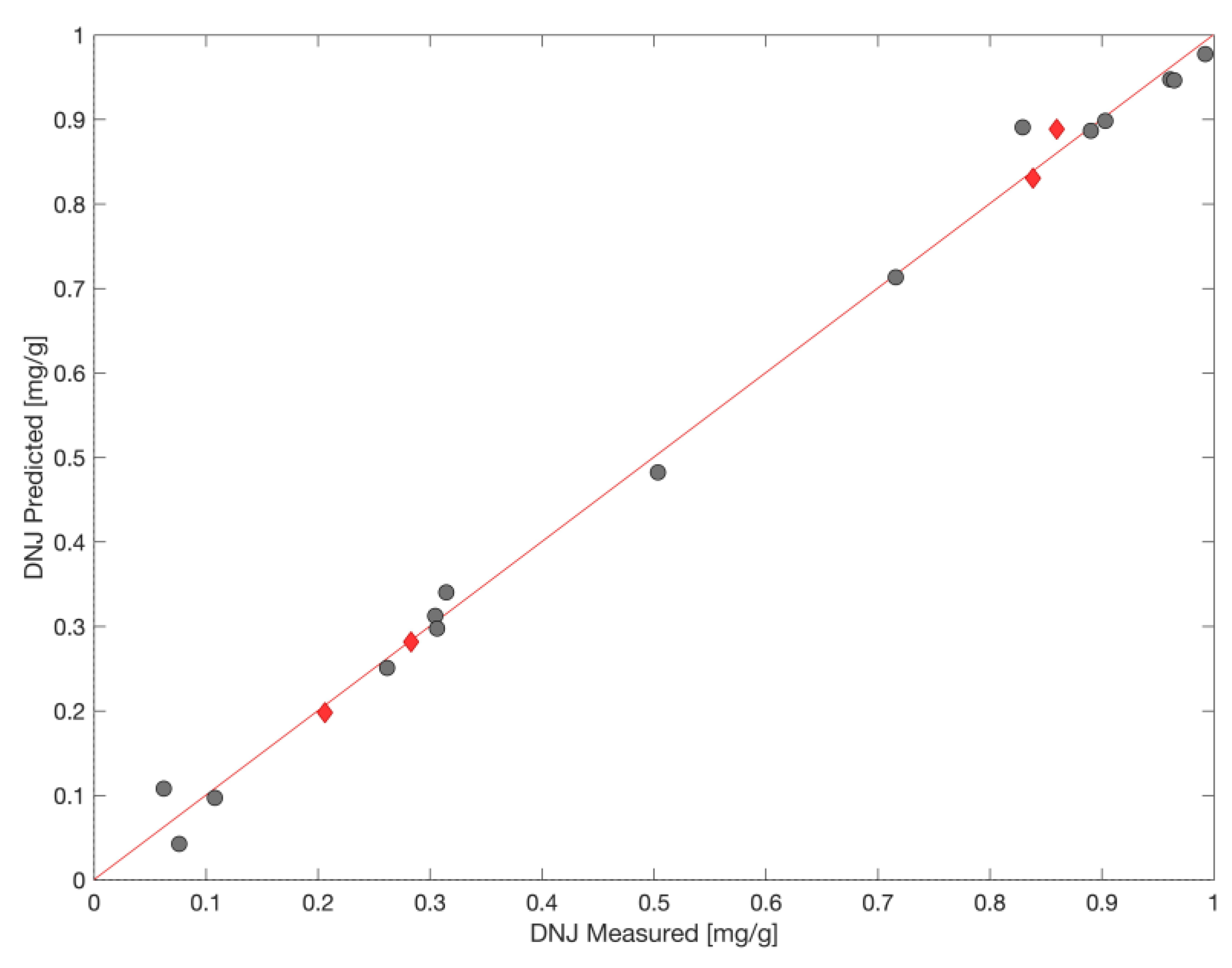
3.4. iPLS Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Ndjaboue, R.; Farhat, I.; Ferlatte, C.-A.; Ngueta, G.; Guay, D.; Delorme, S.; Ivers, N.; Shah, B.R.; Straus, S.; Yu, C.; et al. Predictive models of diabetes complications: Protocol for a scoping review. Syst. Rev. 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, X.; Zhou, D.; Jian, Y.; Jia, J.; Ge, F. Isolation of Chalcomoracin as a Potential α-Glycosidase Inhibitor from Mulberry Leaves and Its Binding Mechanism. Molecules 2022, 27, 5742. [Google Scholar] [CrossRef] [PubMed]
- Kwon, R.-H.; Thaku, N.; Timalsina, B.; Park, S.-E.; Choi, J.-S.; Jung, H.-A. Inhibition Mechanism of Components Isolated from Morus alba Branches on Diabetes and Diabetic Complications via Experimental and Molecular Docking Analyses. Antioxidants 2022, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Y.-J.; Liao, X.; Kuang, H.-M.; Li, J.-Y.; Zhang, S.-H. LC-MS Metabolite Profiling and the Hypoglycemic Activity of Morus alba L. Extracts. Molecules 2022, 27, 5360. [Google Scholar] [CrossRef]
- Gao, T.; Chen, J.; Xu, F.; Wang, Y.; Zhao, P.; Ding, Y.; Han, Y.; Yang, J.; Tao, Y. Mixed Mulberry Fruit and Mulberry Leaf Fermented Alcoholic Beverages: Assessment of Chemical Composition, Antioxidant Capacity In Vitro and Sensory Evaluation. Foods 2022, 11, 3125. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2021, 372, 131335. [Google Scholar] [CrossRef]
- Lange, E.; Kęszycka, P.K.; Pałkowska-Goździk, E.; Billing-Marczak, K. Comparison of Glycemic Response to Carbohydrate Meals without or with a Plant-Based Formula of Kidney Bean Extract, White Mulberry Leaf Extract, and Green Coffee Extract in Individuals with Abdominal Obesity. Int. J. Environ. Res. Public Heal. 2022, 19, 12117. [Google Scholar] [CrossRef]
- Lv, Q.; Lin, J.; Wu, X.; Pu, H.; Guan, Y.; Xiao, P.; He, C.; Jiang, B. Novel active compounds and the anti-diabetic mechanism of mulberry leaves. Front. Pharmacol. 2022, 13, 986931. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, W.; Wu, H.; Liang, Z. An overview of the biological production of 1-deoxynojirimycin: Current status and future perspective. Appl. Microbiol. Biotechnol. 2019, 103, 9335–9344. [Google Scholar] [CrossRef]
- Tsuduki, T.; Kikuchi, I.; Kimura, T.; Nakagawa, K.; Miyazawa, T. Intake of mulberry 1-deoxynojirimycin prevents diet-induced obesity through increases in adiponectin in mice. Food Chem. 2013, 139, 16–23. [Google Scholar] [CrossRef]
- Wang, G.Q.; Zhu, L.; Ma, M.L.; Chen, X.C.; Gao, Y.; Yu, T.Y.; Yang, G.S.; Pang, W.J. Mulberry 1-Deoxynojirimycin Inhibits Adipogenesis by Repression of the ERK/PPARγ Signaling Pathway in Porcine Intramuscular Adipocytes. J. Agric. Food Chem. 2015, 63, 6020–6212. [Google Scholar] [CrossRef]
- Chen, Z.; Du, X.; Yang, Y.; Cui, X.; Zhang, Z.; Li, Y. Comparative study of chemical composition and active components against α -glucosidase of various medicinal parts of Morus alba L. Biomed. Chromatogr. 2018, 32, e4328. [Google Scholar] [CrossRef] [PubMed]
- Fongsodsri, K.; Thaipitakwong, T.; Rujimongkon, K.; Kanjanapruthipong, T.; Ampawong, S.; Reamtong, O.; Aramwit, P. Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients 2022, 14, 4538. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.W.; Park, M.; Lee, H.J. Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice. Life 2022, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Takasu, S.; Parida, I.S.; Onose, S.; Ito, J.; Ikeda, R.; Yamagishi, K.; Higuchi, O.; Tanaka, F.; Kimura, T.; Miyazawa, T.; et al. Evaluation of the anti-hyperglycemic effect and safety of microorganism 1-deoxynojirimycin. PLoS ONE 2018, 13, e0199057. [Google Scholar] [CrossRef]
- Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules 2016, 21, 1600. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, L.; Saviane, A.; Montà, A.; Paglia, G.; Pellati, F.; Benvenuti, S.; Bertelli, D.; Cappellozza, S. Determination of 1-Deoxynojirimycin (1-DNJ) in Leaves of Italian or Italy-Adapted Cultivars of Mulberry (Morus sp.pl.) by HPLC-MS. Plants 2021, 10, 1553. [Google Scholar] [CrossRef]
- Zamoner, L.O.B.; Aragão-Leoneti, V.; Carvalho, I. Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities. Pharmaceuticals 2019, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Jokiaho, A.J.; Winchester, M.; Donovan, C.M. N-Hydroxyethyl-1-Deoxynojirimycin (Miglitol) Restores the Counterregulatory Response to Hypoglycemia Following Antecedent Hypoglycemia. Diabetes 2022, 71, 1063–1072. [Google Scholar] [CrossRef]
- Noël, S.; Wilke, M.; Bot, A.G.M.; De Jonge, H.R.; Becq, F. Parallel Improvement of Sodium and Chloride Transport Defects by Miglustat (n-Butyldeoxynojyrimicin) in Cystic Fibrosis Epithelial Cells. J. Pharmacol. Exp. Ther. 2008, 325, 1016–1023. [Google Scholar] [CrossRef]
- Jennemann, R.; Volz, M.; Bestvater, F.; Schmidt, C.; Richter, K.; Kaden, S.; Müthing, J.; Gröne, H.-J.; Sandhoff, R. Blockade of Glycosphingolipid Synthesis Inhibits Cell Cycle and Spheroid Growth of Colon Cancer Cells In Vitro and Experimental Colon Cancer Incidence In Vivo. Int. J. Mol. Sci. 2021, 22, 10539. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.; Bonotto, R.M.; Alves, L.N.; Kazungu, Y.; Poggianella, M.; Martinez-Orellana, P.; Skoko, N.; Polez, S.; Marcello, A. Inhibitors of Protein Glycosylation Are Active against the Coronavirus Severe Acute Respiratory Syndrome Coronavirus SARS-CoV-2. Viruses 2021, 13, 808. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.H.M.; Wang, Y.; Foppen, E.; Ottenhoff, R.; van Roomen, C.; Parlevliet, E.T.; van Eijk, M.; Verhoek, M.; Boot, R.; Marques, A.R.; et al. The Iminosugar AMP-DNM Improves Satiety and Activates Brown Adipose Tissue Through GLP1. Diabetes 2019, 68, 2223–2234. [Google Scholar] [CrossRef] [Green Version]
- Bijl, N.; Scheij, S.; Houten, S.; Boot, R.G.; Groen, A.K.; Aerts, J.M.F.G. The Glucosylceramide Synthase InhibitorN-(5-Adamantane-1-yl-methoxy-pentyl)-deoxynojirimycin Induces Sterol Regulatory Element-Binding Protein-Regulated Gene Expression and Cholesterol Synthesis in HepG2 Cells. J. Pharmacol. Exp. Ther. 2008, 326, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Fortune Business Insights. Dietary Supplements Market Size, Share & COVID-19 Impact Analysis, By Type (Vitamins, Minerals, Enzymes, Fatty Acids, Proteins, and Others), Form (Tablets, Capsules, Liquids, and Powders), and Regional Forecasts, 2021–2028. Available online: https://www.fortunebusinessinsights.com/dietary-supplements-market-102082 (accessed on 15 October 2022).
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbach, M.; Marmouzi, I.; El Jemli, M.; Bouklouze, A.; Heyden, Y.V. Recent advances in untargeted and targeted approaches applied in herbal-extracts and essential-oils fingerprinting—A review. J. Pharm. Biomed. Anal. 2020, 177, 112849. [Google Scholar] [CrossRef] [PubMed]
- Durak, T.; Depciuch, J. Effect of plant sample preparation and measuring methods on ATR-FTIR spectra results. Environ. Exp. Bot. 2020, 169, 103915. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Q2(R2) Validation of Analytical Procedures; European Medicines Agency: Amsterdam, The Netherlands, 2022.
- Kim, J.-W.; Kim, S.-U.; Lee, H.S.; Kim, I.; Ahn, M.Y.; Ryu, K.S. Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2003, 1002, 93–99. [Google Scholar] [CrossRef]
- Nørgaard, L.; Saudland, A.; Wagner, J.; Nielsen, J.P.; Munck, L.; Engelsen, S.B. Interval Partial Least-Squares Regression (iPLS): A Comparative Chemometric Study with an Example from Near-Infrared Spectroscopy. Appl. Spectrosc. 2000, 54, 413–419. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Jiang, L.; Zhang, J.-G.; Deng, W.; Wang, H.-L.; Wei, Z.-J. Quantitative determination of 1-deoxynojirimycin in mulberry leaves from 132 varieties. Ind. Crop. Prod. 2013, 49, 782–784. [Google Scholar] [CrossRef]
- Ji, T.; Li, J.; Su, S.-L.; Zhu, Z.-H.; Guo, S.; Qian, D.-W.; Duan, J.-A. Identification and Determination of the Polyhydroxylated Alkaloids Compounds with α-Glucosidase Inhibitor Activity in Mulberry Leaves of Different Origins. Molecules 2016, 21, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, N.; Jiang, Y.-W.; Kou, P.; Liu, Z.-M.; Efferth, T.; Li, Y.-Y.; Fu, Y.-J. Application of integrative cloud point extraction and concentration for the analysis of polyphenols and alkaloids in mulberry leaves. J. Pharm. Biomed. Anal. 2019, 167, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Razali, U.M.; Saikim, F.; Mahyudin, A.; Noor, N.M. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Thaipitakwong, T.; Supasyndh, O.; Rasmi, Y.; Aramwit, P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement. Ther. Med. 2019, 49, 102292. [Google Scholar] [CrossRef]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef]
- Chung, H.I.; Kim, J.; Kim, J.Y.; Kwon, O. Acute intake of mulberry leaf aqueous extract affects postprandial glucose response after maltose loading: Randomized double-blind placebo-controlled pilot study. J. Funct. Foods 2013, 5, 1502–1506. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Wan, Y.; Hao, J.-Y.; Hu, R.-Z.; Chen, C.; Yao, X.-H.; Zhao, W.-G.; Liu, Z.-Y.; Li, L. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crop. Prod. 2018, 122, 298–307. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Wesolowski, M.; Viapiana, A. Morus alba L. and Morus nigra L. Leaves as a Promising Food Source of Phenolic Compounds with Antioxidant Activity. Plant Foods Hum. Nutr. 2021, 76, 458–465. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Hernández, F.; Martínez, J.J. (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J. Funct. Foods 2015, 18, 1039–1046. [Google Scholar] [CrossRef]
- Radojković, M.; Zeković, Z.; Mašković, P.; Vidović, S.; Mandić, A.; Mišan, A.; Đurović, S. Biological activities and chemical composition of Morus leaves extracts obtained by maceration and supercritical fluid extraction. J. Supercrit. Fluids 2016, 117, 50–58. [Google Scholar] [CrossRef]
- Przeor, M.; Flaczyk, E.; Kmiecik, D.; Buchowski, M.S.; Staniek, H.; Tomczak-Graczyk, A.; Kobus-Cisowska, J.; Gramza-Michałowska, A.; Foksowicz-Flaczyk, J. Functional Properties and Antioxidant Activity of Morus alba L. Leaves var. Zolwinska Wielkolistna (WML-P)—The Effect of Controlled Conditioning Process. Antioxidants 2020, 9, 668. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, C.-I. Pharmacological Properties of Morus nigra L. (Black Mulberry) as A Promising Nutraceutical Resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef] [Green Version]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Adiana, M.; Mazura, M. Study on Senna alata and its different extracts by Fourier transform infrared spectroscopy and two-dimensional correlation infrared spectroscopy. J. Mol. Struct. 2011, 991, 84–91. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Z.J.; Liao, W.; Li, J.; Gruget, P.; Kitts, D.D.; Lu, X. Determination of antioxidant capacity and phenolic content of chocolate by attenuated total reflectance-Fourier transformed-infrared spectroscopy. Food Chem. 2016, 202, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Biancolillo, A.; Marini, F.; D’Archivio, A.A. Geographical discrimination of red garlic (Allium sativum L.) using fast and non-invasive Attenuated Total Reflectance-Fourier Transformed Infrared (ATR-FTIR) spectroscopy combined with chemometrics. J. Food Compos. Anal. 2020, 86, 103351. [Google Scholar] [CrossRef]

| Parameter | DNJ | CGA | nCGA | Rutin |
|---|---|---|---|---|
| Range (µg/mL) | 3.14–157.14 | 10.00–140.00 | 5.00–80.00 | 5.00–50.00 |
| Regression equation | y = 88.68x − 0.06 | y = 44.56x − 0.17 | y = 52.56x − 0.10 | y = 24.48x − 0.02 |
| Regression coefficient (R2) | 0.999 ± 0.001 | 0.995 ± 0.002 | 0.999 ± 0.001 | 0.999 ± 0.001 |
| Recovery (%) | 90.4 ± 1.7 | 96.2 ± 1.2 | 94.1 ± 0.6 | 93.8 ± 1.1 |
| Precision (%RSD) | 7.5 | 5.6 | 6.7 | 6.2 |
| LOD (ng/mL) | 9.1 | 3.52 | 2.82 | 4.07 |
| LOQ (ng/mL) | 27.7 | 10.67 | 8.55 | 12.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkowiak-Bródka, A.; Piekuś-Słomka, N.; Wnuk, K.; Kupcewicz, B. Analysis of White Mulberry Leaves and Dietary Supplements, ATR-FTIR Combined with Chemometrics for the Rapid Determination of 1-Deoxynojirimycin. Nutrients 2022, 14, 5276. https://doi.org/10.3390/nu14245276
Walkowiak-Bródka A, Piekuś-Słomka N, Wnuk K, Kupcewicz B. Analysis of White Mulberry Leaves and Dietary Supplements, ATR-FTIR Combined with Chemometrics for the Rapid Determination of 1-Deoxynojirimycin. Nutrients. 2022; 14(24):5276. https://doi.org/10.3390/nu14245276
Chicago/Turabian StyleWalkowiak-Bródka, Agata, Natalia Piekuś-Słomka, Kacper Wnuk, and Bogumiła Kupcewicz. 2022. "Analysis of White Mulberry Leaves and Dietary Supplements, ATR-FTIR Combined with Chemometrics for the Rapid Determination of 1-Deoxynojirimycin" Nutrients 14, no. 24: 5276. https://doi.org/10.3390/nu14245276
APA StyleWalkowiak-Bródka, A., Piekuś-Słomka, N., Wnuk, K., & Kupcewicz, B. (2022). Analysis of White Mulberry Leaves and Dietary Supplements, ATR-FTIR Combined with Chemometrics for the Rapid Determination of 1-Deoxynojirimycin. Nutrients, 14(24), 5276. https://doi.org/10.3390/nu14245276






