Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome
Abstract
:1. Introduction
2. Methods
2.1. Short-Term IF and Long-Term IF Experimental Design
2.2. DSS-Induced Colitis and Experimental Design
2.3. Microbiome Sequencing of Faecal Samples
2.4. Bile Acids Analysis of Faecal Samples
2.5. Metabolomic Profiling Analysis of Faecal Samples
2.6. SCFAs Quantification
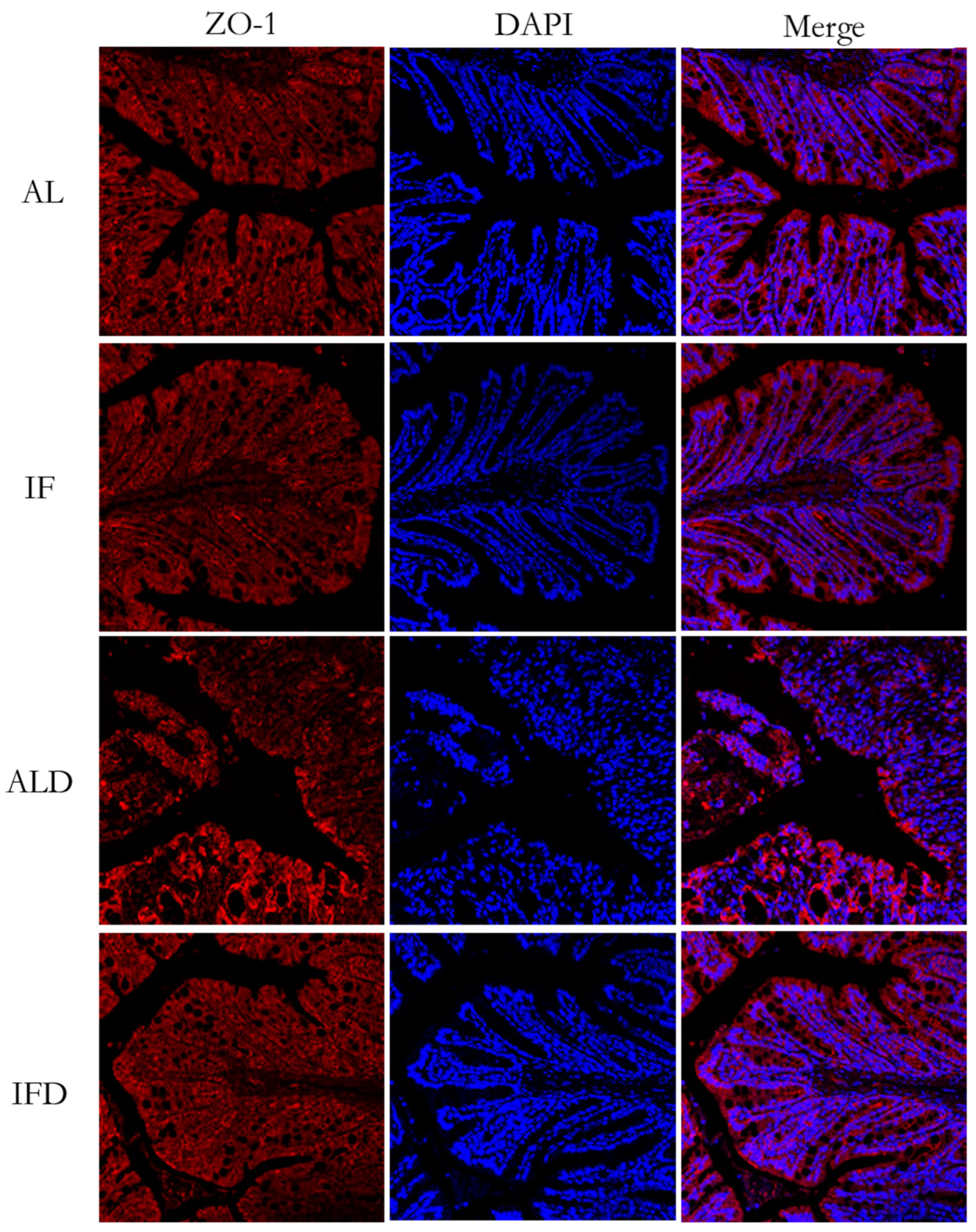
2.7. Histopathological Evaluation and Immunofluorescence Analyses
2.8. Serum Cytokine Analysis
2.9. Statistical Analysis
3. Results: Short-Term IF and Long-Term IF Experiments
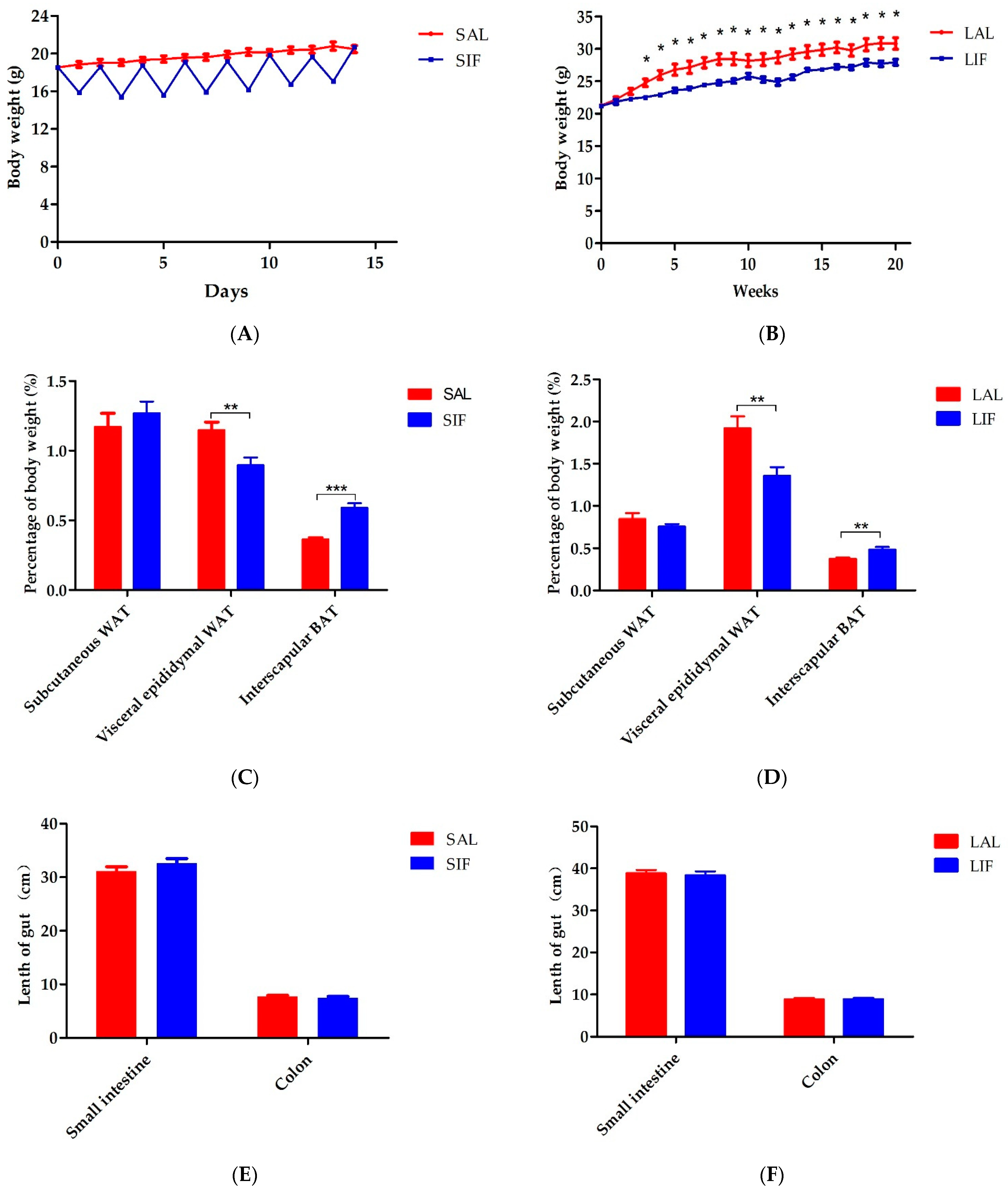
3.1. IF Impacts Body Weight and Adipose Tissue Contents
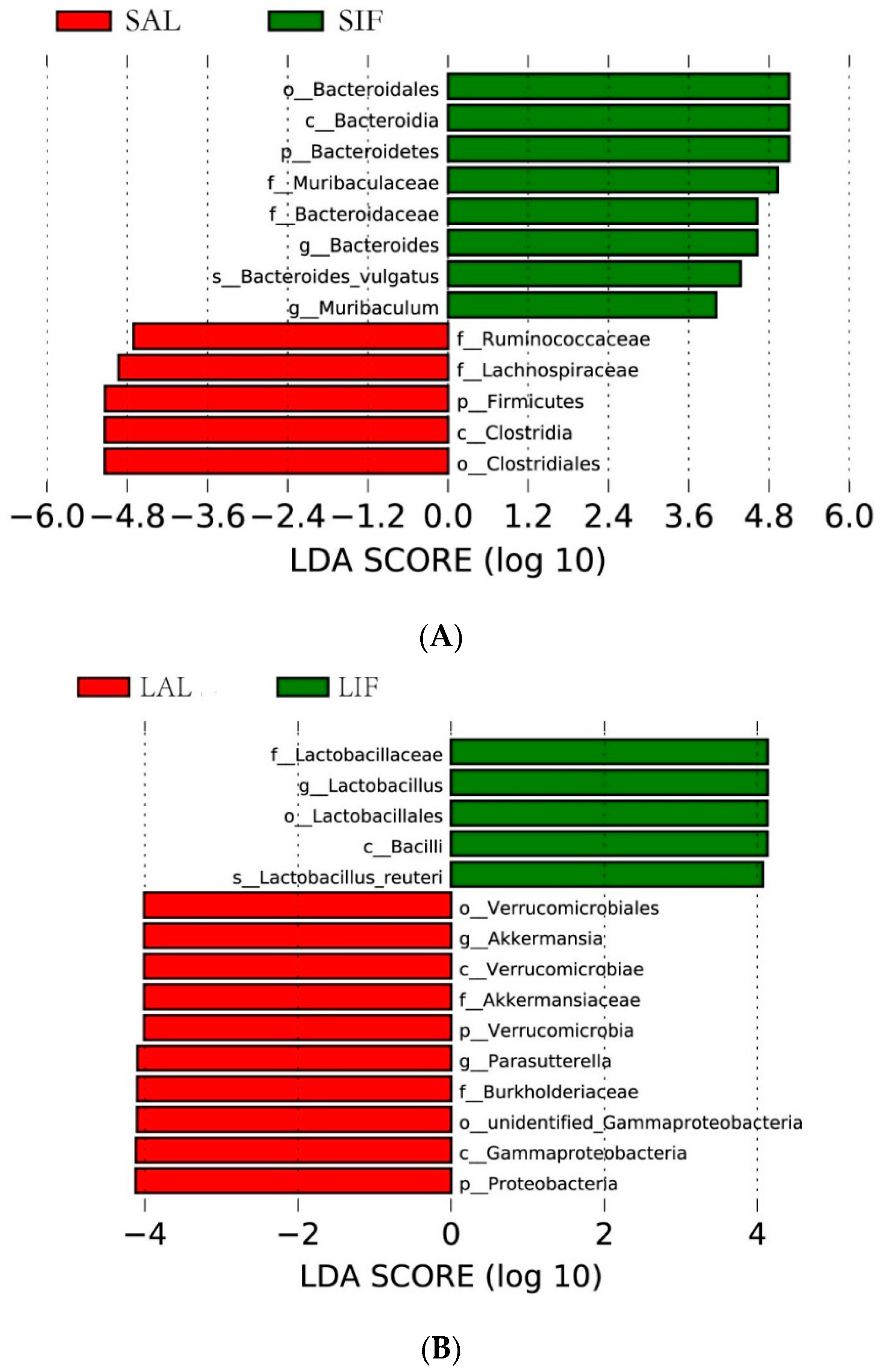
3.2. Influence of IF on the Gut Microbiota
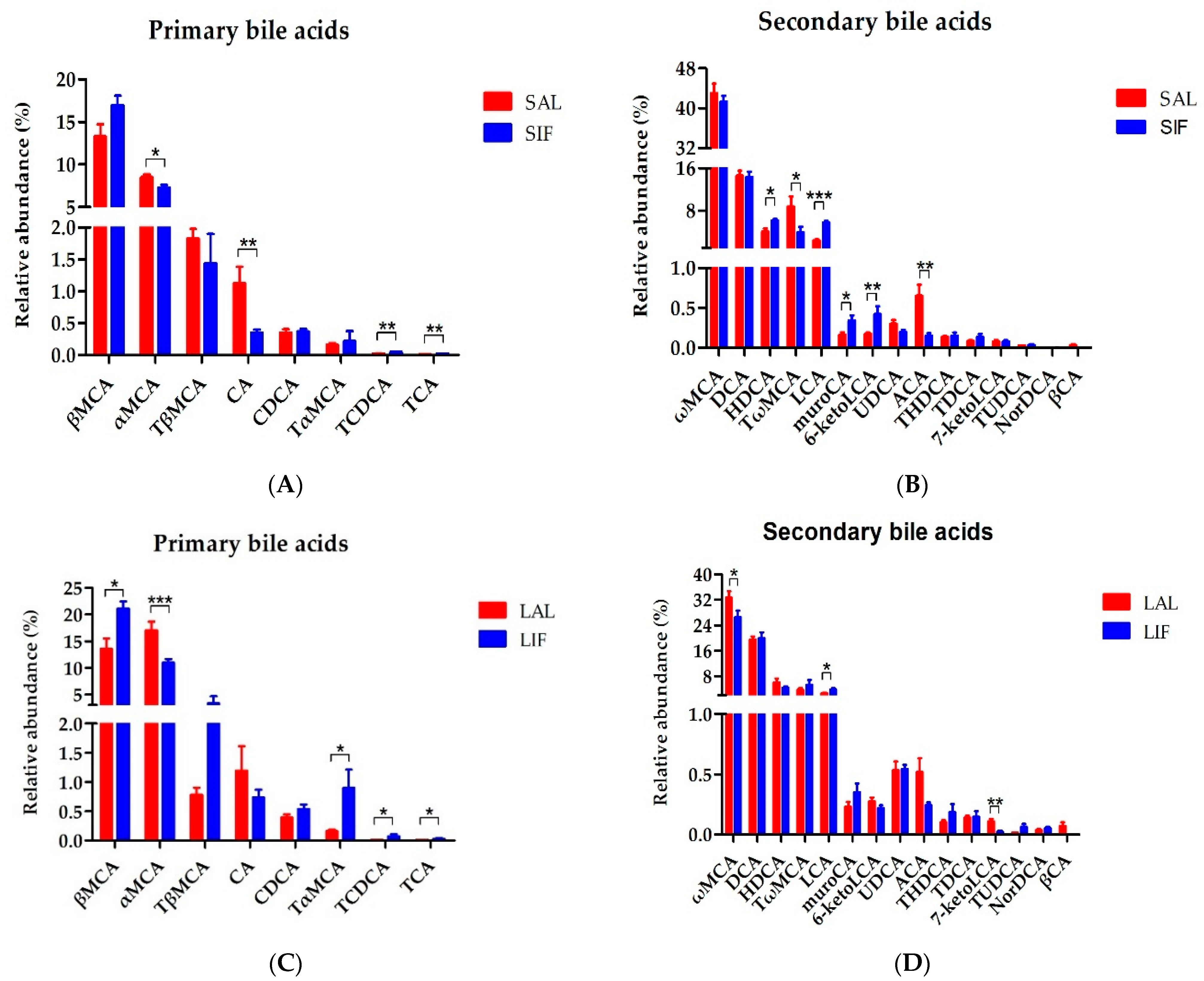
3.3. Bile Acids Profiles of Faeces after IF
3.4. Metabolomic Profiling Analysis after IF
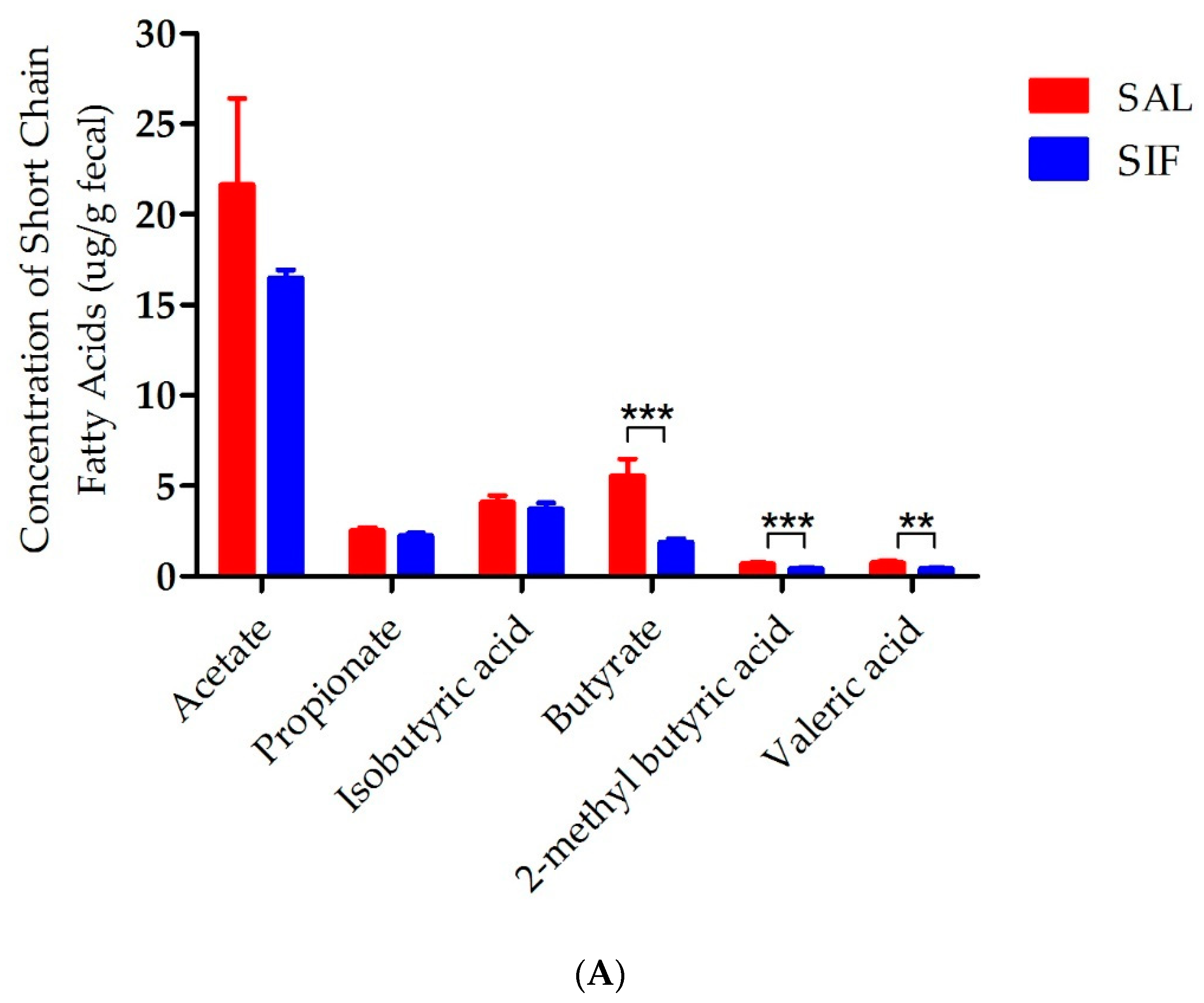
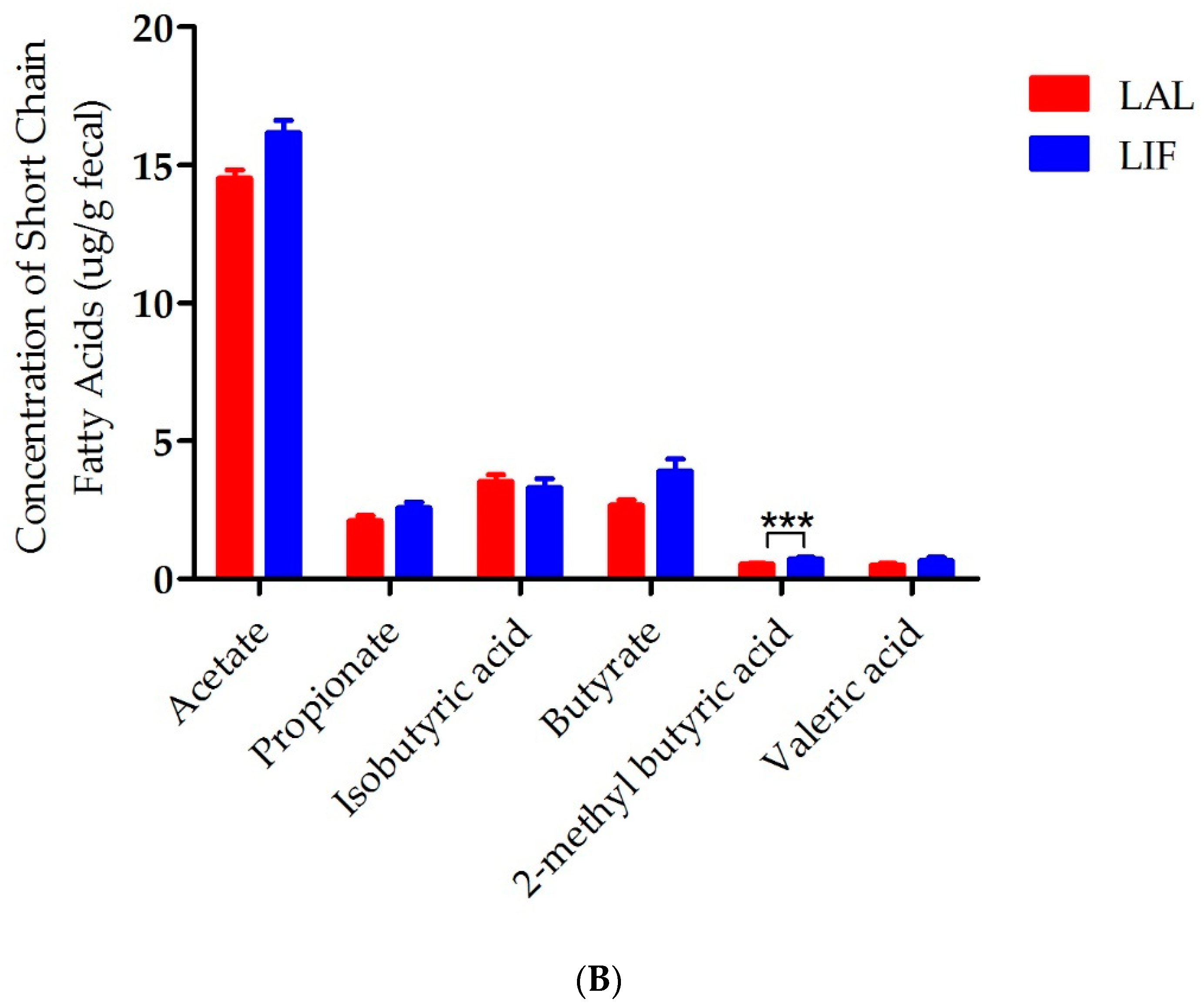
3.5. Influence of IF on SCFAs in the Gut
4. IF Alleviates DSS-Induced Colitis
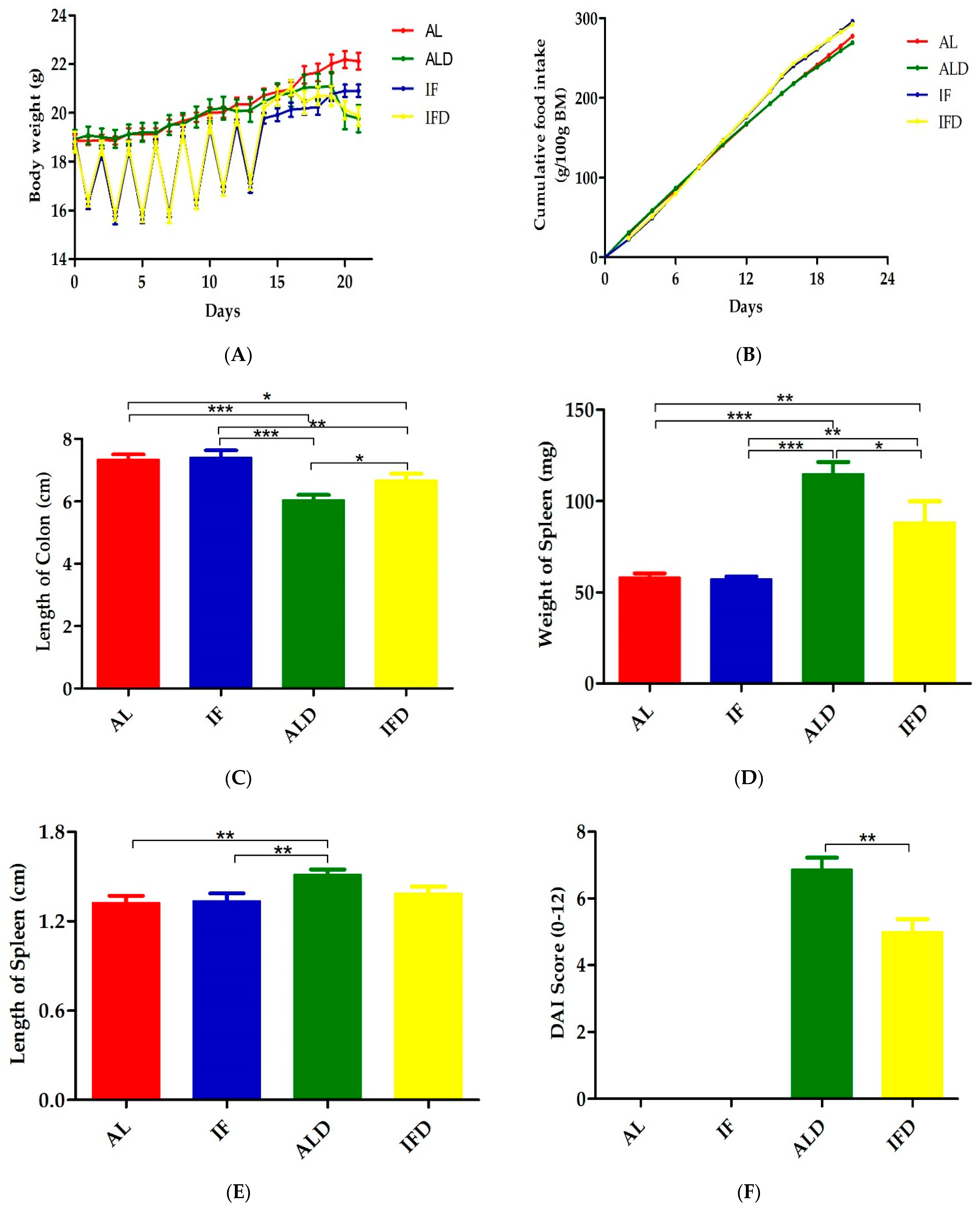
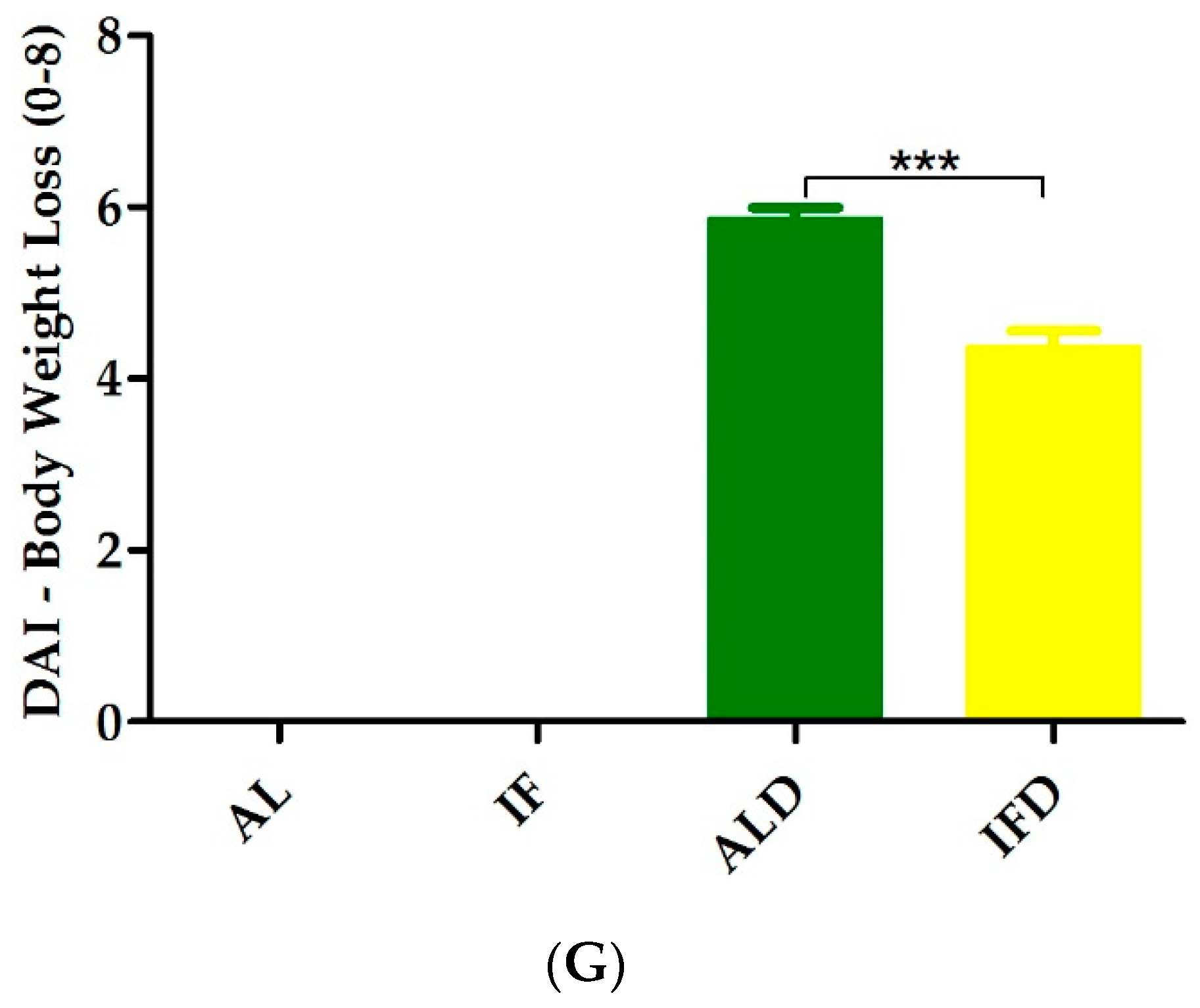
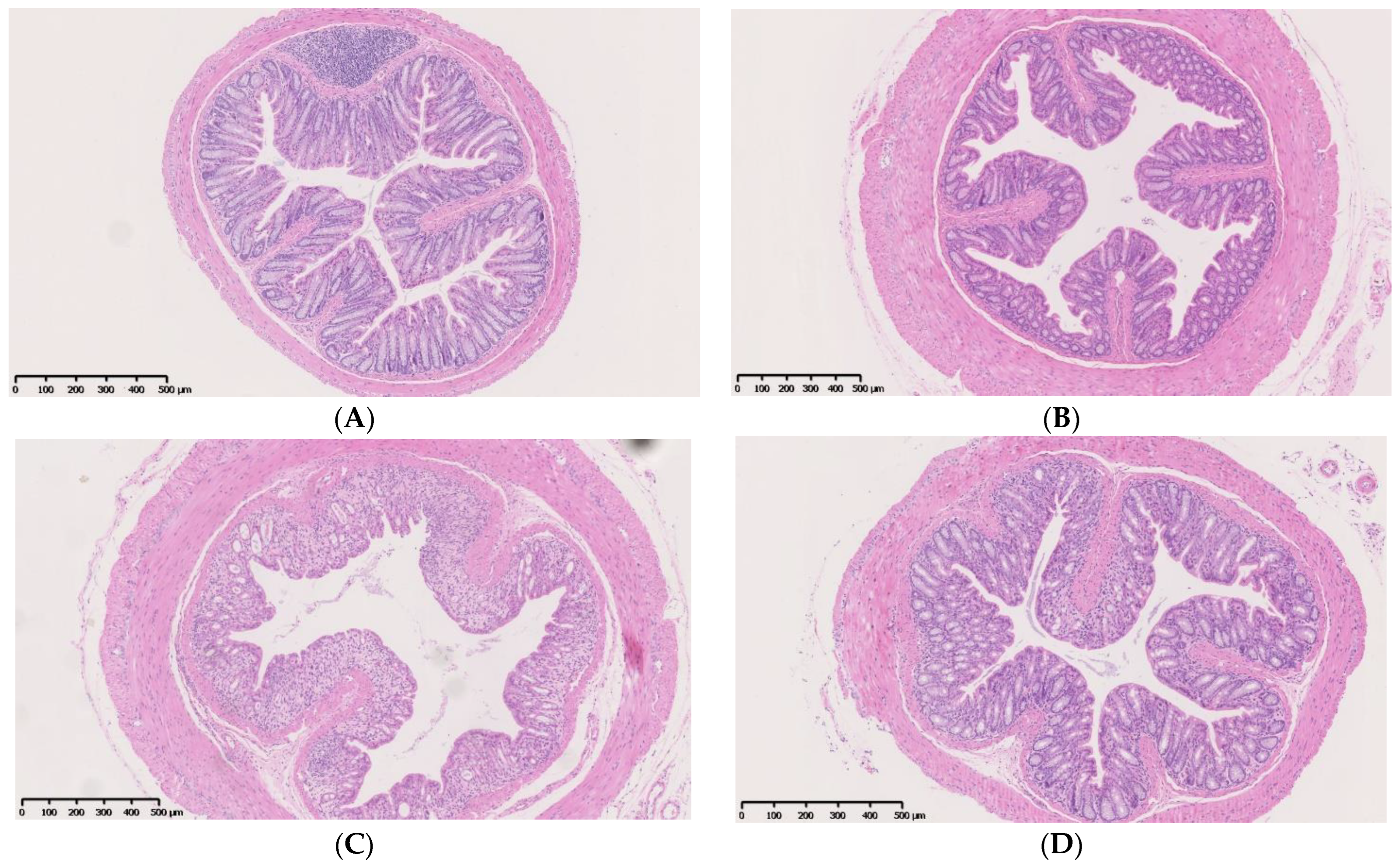
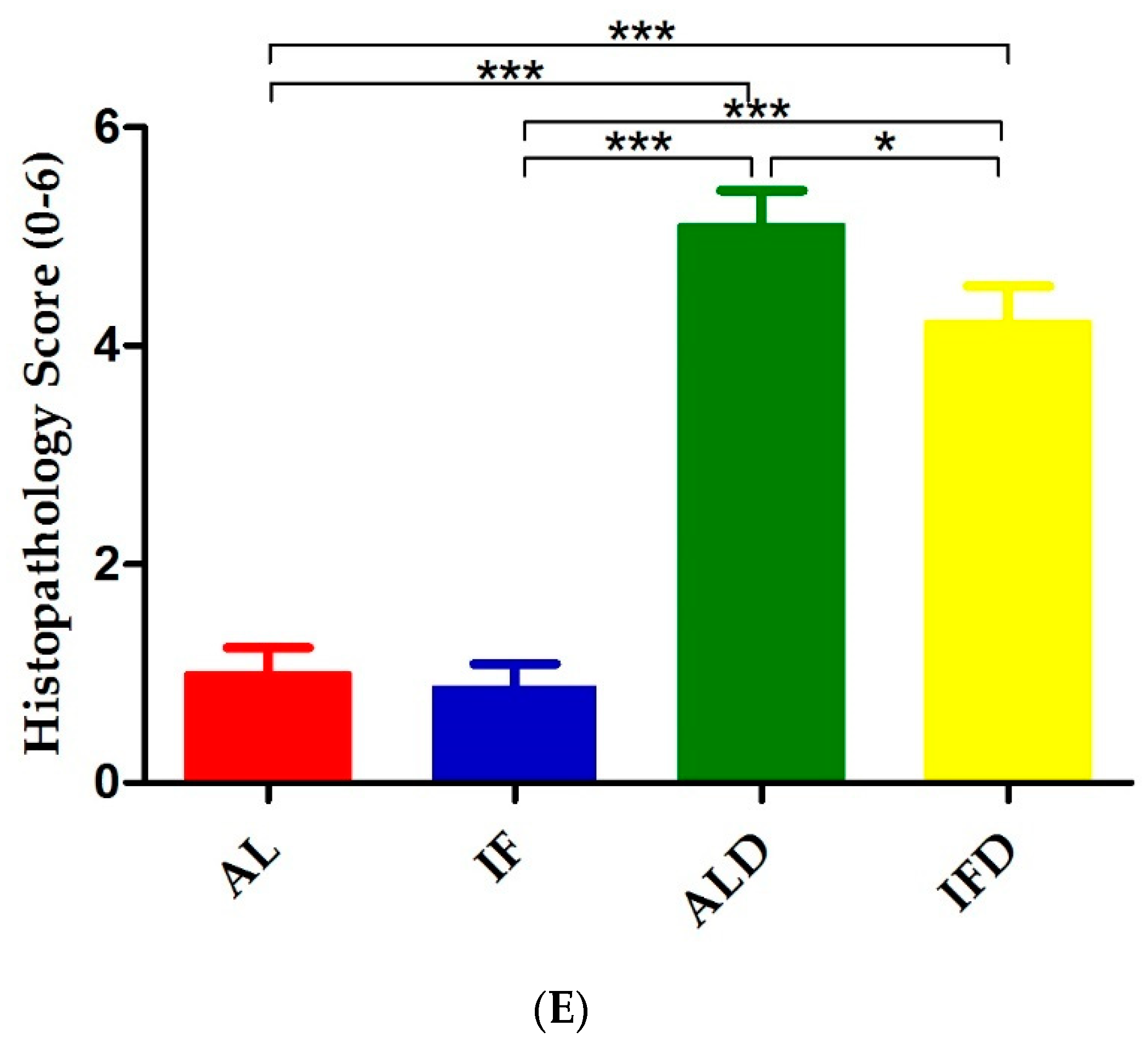
4.1. IF Alleviates Colitis Symptoms and Colon Injury after DSS Administration
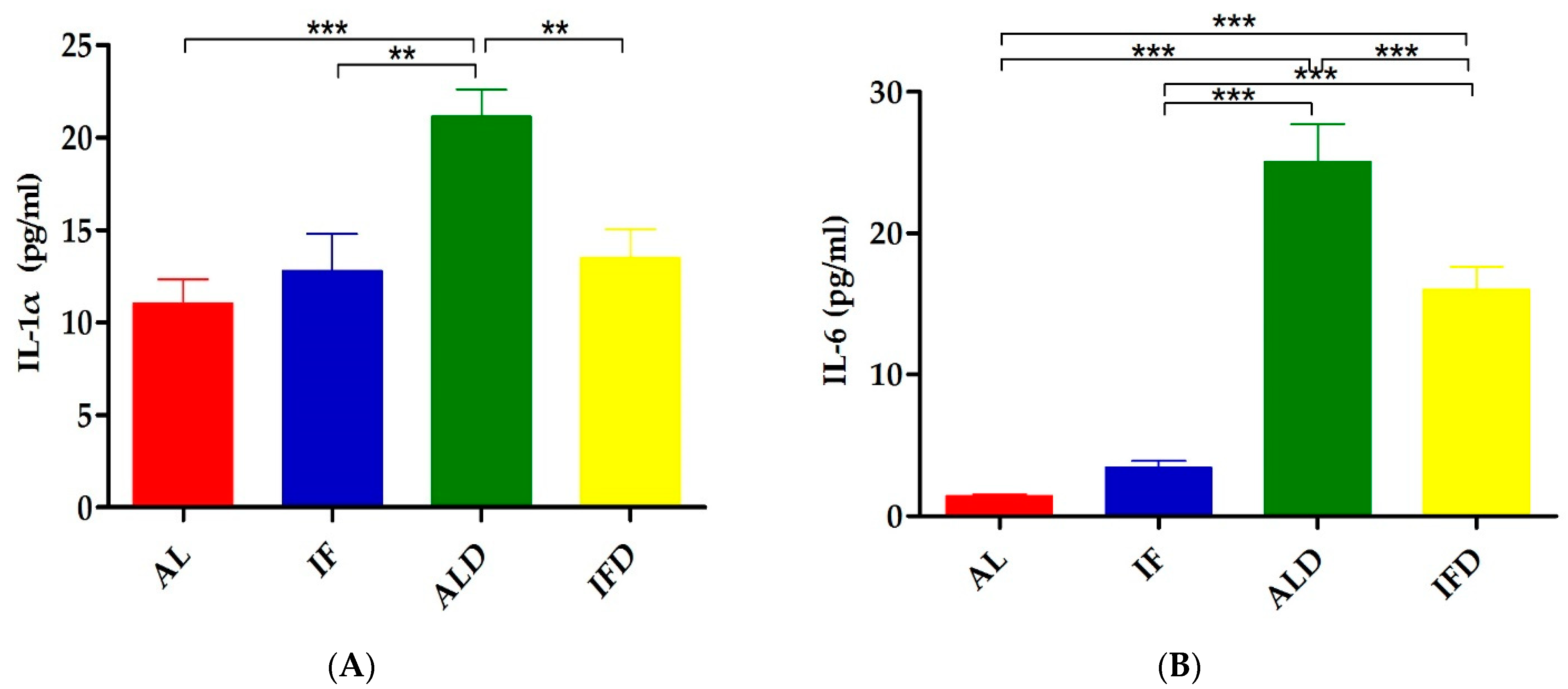
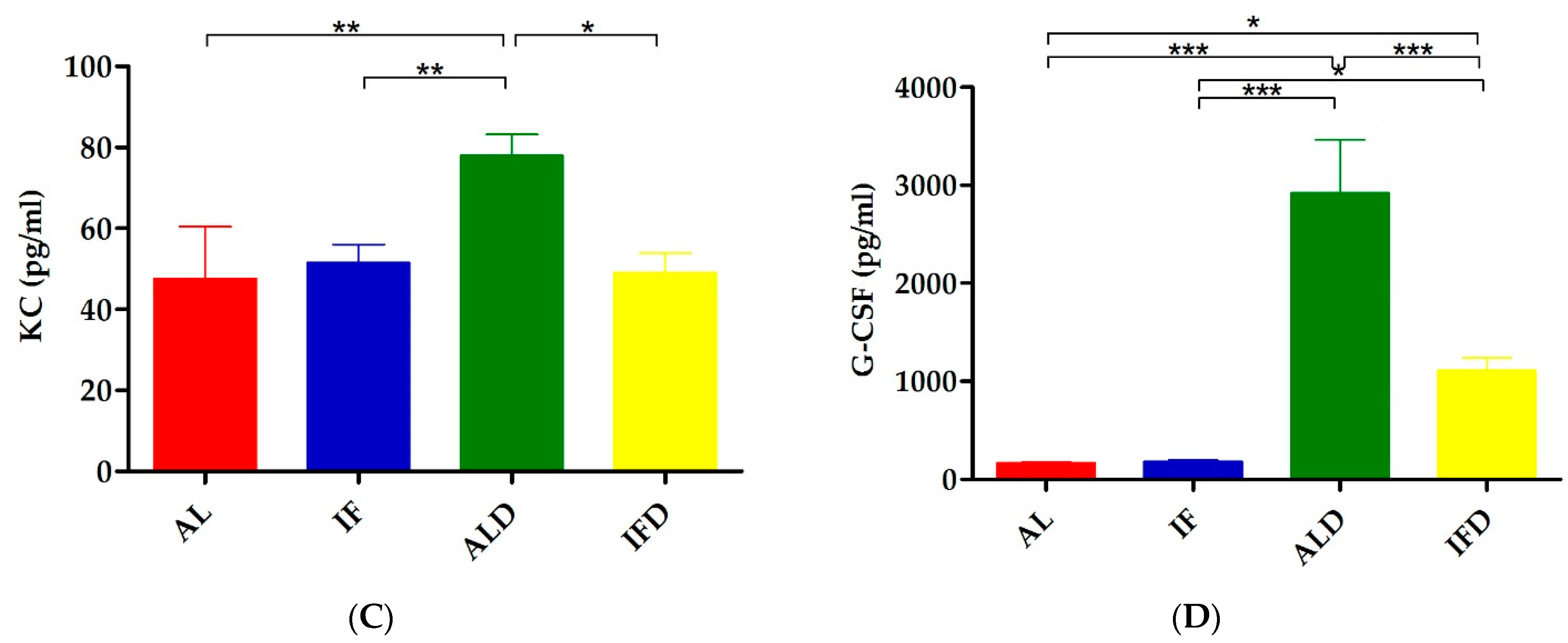
4.2. IF Relieves DSS-Induced Systemic Inflammation
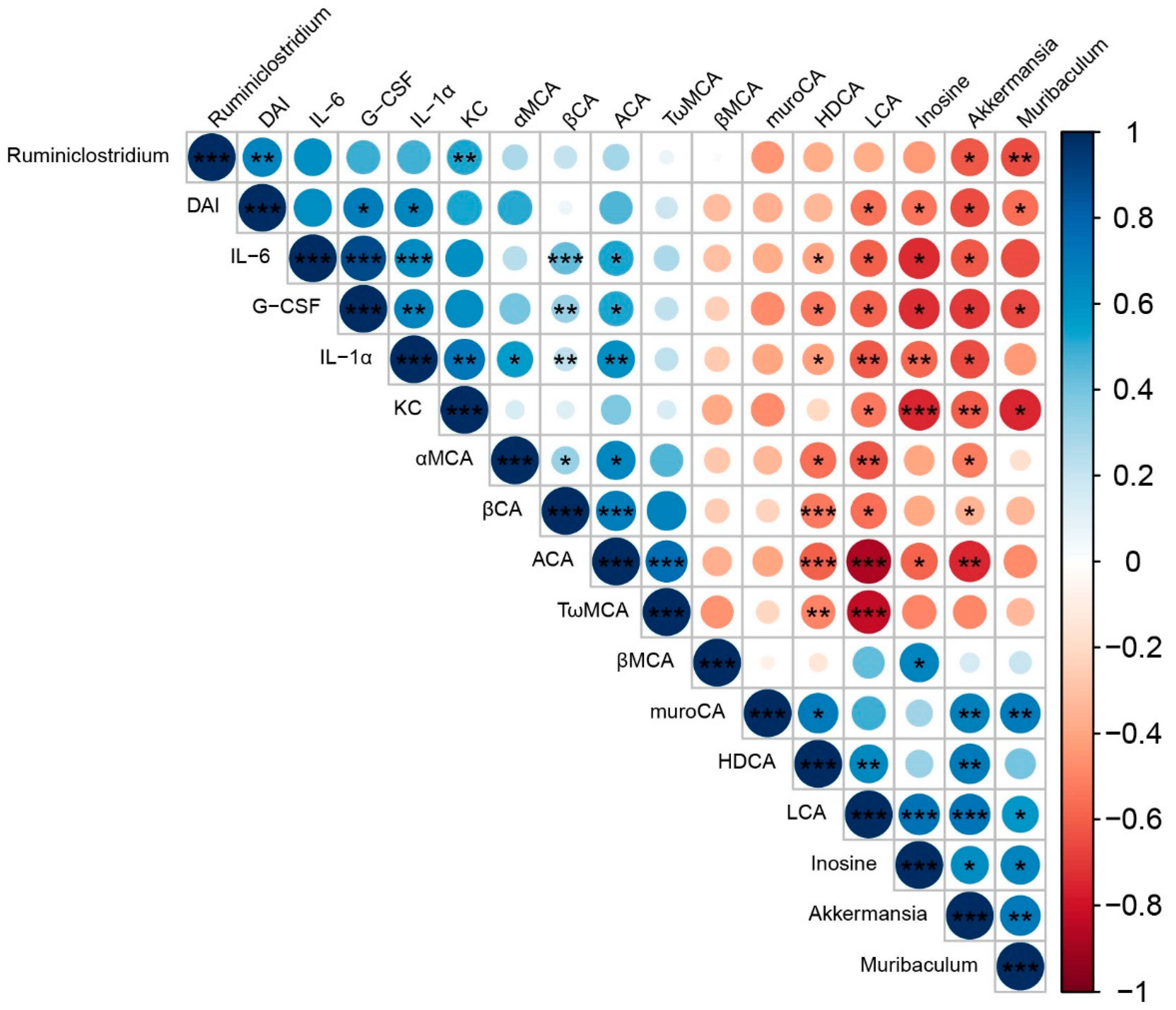
4.3. Elucidating the Mechanism Responsible for IF
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kroemer, G.; López-Otín, C.; Madeo, F.; de Cabo, R. Carbotoxicity-Noxious Effects of Carbohydrates. Cell 2018, 175, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Patikorn, C.; Roubal, K.; Veettil, S.K.; Chandran, V.; Pham, T.; Lee, Y.Y.; Giovannucci, E.L.; Varady, K.A.; Chaiyakunapruk, N. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw. Open 2021, 4, e2139558. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.; Leeuwenburgh, C. Fasting or caloric restriction for healthy aging. Exp. Gerontol. 2013, 48, 1003–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, G.P.; Murphy, T.; Stangl, D.; Ahmet, S.; Morisse, B.; Nix, A.; Aimone, L.J.; Aimone, J.B.; Kuro, O.M.; Gage, F.H.; et al. Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of longevity gene Klotho. Mol. Psychiatry 2021, 26, 6365–6379. [Google Scholar] [CrossRef] [PubMed]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476.e466. [Google Scholar] [CrossRef]
- Lessan, N.; Ali, T. Energy Metabolism and Intermittent Fasting: The Ramadan Perspective. Nutrients 2019, 11, 1192. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e674. [Google Scholar] [CrossRef] [Green Version]
- Hatchwell, L.; Harney, D.J.; Cielesh, M.; Young, K.; Koay, Y.C.; O’Sullivan, J.F.; Larance, M. Multi-omics Analysis of the Intermittent Fasting Response in Mice Identifies an Unexpected Role for HNF4α. Cell Rep. 2020, 30, 3566–3582.e3564. [Google Scholar] [CrossRef] [Green Version]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Rose, W.A., 2nd; Sakamoto, K.; Leifer, C.A. TLR9 is important for protection against intestinal damage and for intestinal repair. Sci. Rep. 2012, 2, 574. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, P.A.; Crowley, J.R.; Sambandam, N.; Muegge, B.D.; Costello, E.K.; Hamady, M.; Knight, R.; Gordon, J.I. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl. Acad. Sci. USA 2009, 106, 11276–11281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negm, M.; Bahaa, A.; Farrag, A.; Lithy, R.M.; Badary, H.A.; Essam, M.; Kamel, S.; Sakr, M.; Abd El Aaty, W.; Shamkh, M.; et al. Effect of Ramadan intermittent fasting on inflammatory markers, disease severity, depression, and quality of life in patients with inflammatory bowel diseases: A prospective cohort study. BMC Gastroenterol. 2022, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [Google Scholar] [CrossRef] [PubMed]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, Y.; Luo, X.M.; Ma, Y.; Liu, J.; Wang, H. Intermittent Fasting Reshapes the Gut Microbiota and Metabolome and Reduces Weight Gain More Effectively Than Melatonin in Mice. Front. Nutr. 2021, 8, 784681. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.L.; Wang, S.N.; Miao, C.Y. Influence of Microbiota on Intestinal Immune System in Ulcerative Colitis and Its Intervention. Front. Immunol. 2017, 8, 1674. [Google Scholar] [CrossRef] [Green Version]
- Qian, K.; Chen, S.; Wang, J.; Sheng, K.; Wang, Y.; Zhang, M. A β-N-acetylhexosaminidase Amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. 2022, 13, 2216–2227. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Lee, Y.S.; Lee, S.H.; Kim, M.N.; Eunju, O.; Kim, K.S.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Fan, L.; Qi, Y.; Xu, C.; Hu, Y.; Chen, S.; Liu, W.; Liu, W.; Si, J. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. Microbiol. Spectr. 2021, 9, e0073021. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz Azizi Raftar, S.; Ashrafian, F.; Yadegar, A.; Lari, A.; Moradi, H.R.; Shahriary, A.; Azimirad, M.; Alavifard, H.; Mohsenifar, Z.; Davari, M.; et al. The Protective Effects of Live and Pasteurized Akkermansia muciniphila and Its Extracellular Vesicles against HFD/CCl4-Induced Liver Injury. Microbiol. Spectr. 2021, 9, e0048421. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals 2020, 10, 1154. [Google Scholar] [CrossRef]
- Guo, J.; Ma, B.; Wang, Z.; Chen, Y.; Tian, W.; Dong, Y. Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota. Nutrients 2022, 14, 2069. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, J.; Feng, B.; Lin, F.; Zhou, J.; Liu, J.; Shi, X.; Lu, X.; Pan, Q.; Yu, J.; et al. Immunosuppressive effect of mesenchymal stem cells on lung and gut CD8(+) T cells in lipopolysaccharide-induced acute lung injury in mice. Cell Prolif. 2021, 54, e13028. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Buey, R.M.; Revuelta, J.L. Increased production of inosine and guanosine by means of metabolic engineering of the purine pathway in Ashbya gossypii. Microb. Cell Factories 2015, 14, 58. [Google Scholar] [CrossRef]
- Niemann, B.; Haufs-Brusberg, S.; Puetz, L.; Feickert, M.; Jaeckstein, M.Y.; Hoffmann, A.; Zurkovic, J.; Heine, M.; Trautmann, E.M.; Müller, C.E.; et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature 2022, 609, 361–368. [Google Scholar] [CrossRef]
- Luu, M.; Visekruna, A. Microbial metabolites: Novel therapeutic tools for boosting cancer therapies. Trends Cell Biol. 2021, 31, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Li, Y.; Zhao, M.; Kuang, J.; Liang, D.; Wang, J.; Wei, M.; Rajani, C.; Ma, X.; et al. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 2022, 13, 2060. [Google Scholar] [CrossRef]
- Rau, M.; Stieger, B.; Monte, M.J.; Schmitt, J.; Jahn, D.; Frey-Wagner, I.; Raselli, T.; Marin, J.J.; Müllhaupt, B.; Rogler, G.; et al. Alterations in Enterohepatic Fgf15 Signaling and Changes in Bile Acid Composition Depend on Localization of Murine Intestinal Inflammation. Inflamm. Bowel Dis. 2016, 22, 2382–2389. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley Leaf Insoluble Dietary Fiber Alleviated Dextran Sulfate Sodium-Induced Mice Colitis by Modulating Gut Microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Ren, X.; Zhang, Y.; Ke, Z.; Zhou, J.; Wang, Y.; Zhang, Y.; Liu, Y. Cholecystectomy-induced secondary bile acids accumulation ameliorates colitis through inhibiting monocyte/macrophage recruitment. Gut Microbes 2022, 14, 2107387. [Google Scholar] [CrossRef]
- Tian, Y.; Gui, W.; Koo, I.; Smith, P.B.; Allman, E.L.; Nichols, R.G.; Rimal, B.; Cai, J.; Liu, Q.; Patterson, A.D. The microbiome modulating activity of bile acids. Gut Microbes 2020, 11, 979–996. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Huang, K.; Mukherjee, S.; DesMarais, V.; Albanese, J.M.; Rafti, E.; Draghi Ii, A.; Maher, L.A.; Khanna, K.M.; Mani, S.; Matson, A.P. Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatr. Res. 2018, 83, 1031–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e958. [Google Scholar] [CrossRef]
- Zou, F.; Qiu, Y.; Huang, Y.; Zou, H.; Cheng, X.; Niu, Q.; Luo, A.; Sun, J. Effects of short-chain fatty acids in inhibiting HDAC and activating p38 MAPK are critical for promoting B10 cell generation and function. Cell Death Dis. 2021, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef] [Green Version]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719.e2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, T.; Otsubo, T.; Hagiwara, T.; Inazuka, F.; Kobayashi, E.; Fukuda, S.; Inoue, T.; Higuchi, K.; Kawamura, Y.I.; Dohi, T. Intermittent fasting prompted recovery from dextran sulfate sodium-induced colitis in mice. J. Clin. Biochem. Nutr. 2017, 61, 100–107. [Google Scholar] [CrossRef]
| Index | 2-Week Intermittent Fasting | 20-Week Intermittent Fasting | ||
|---|---|---|---|---|
| SAL | SIF | LAL | LIF | |
| OTUs | 601 ± 191 | 520 ± 76 | 230 ± 25 | 253 ± 20 |
| Ace | 756 ± 241 | 671 ± 145 | 246 ± 27 | 269 ± 22 |
| Chao1 | 741 ± 224 | 655 ± 146 | 246 ± 28 | 268 ± 22 |
| Shannon index | 5.56 ± 0.44 | 5.2 ± 0.28 | 4.01 ± 0.57 | 4.26 ± 0.43 |
| Simpson index | 0.94 ± 0.02 | 0.93 ± 0.01 | 0.86 ± 0.06 | 0.87 ± 0.05 |
| Metabolites | SAL vs. SIF | LAL vs. LIF | |||||
|---|---|---|---|---|---|---|---|
| RT | VIP Value | FC | p Value | VIP Value | FC | p Value | |
| 1,2-ethanediamine | 28.91 | 1.1 | 1.8 | <0.01 | 1.403 | 2.18 | 0.01 |
| 2,6-diaminopimelic acid | 20.77 | 2.19 | 4.96 | <0.01 | 2.498 | 7.77 | <0.01 |
| 2-hydroxy-3-methylbutyric acid | 8.5 | 1.39 | 2.08 | <0.01 | 1.753 | 3.77 | <0.01 |
| 2-hydroxycaproic acid | 9.87 | 1.29 | 1.94 | <0.01 | 1.632 | 2.93 | <0.01 |
| 2-indolecarboxylic acid | 21.34 | 1.75 | 3.44 | <0.01 | 1.051 | 1.65 | <0.01 |
| 2-keto-l-gluconate | 21.25 | 1.16 | 1.72 | <0.01 | 1.346 | 2.19 | <0.01 |
| 3,5-dihydroxyphenylglycine | 32.04 | 2.27 | 6.77 | <0.01 | 1.777 | 3.28 | <0.01 |
| 3-epicholic acid | 37.42 | 2.03 | 0.16 | 0.01 | 1.426 | 0.34 | <0.01 |
| 5-methyluridine | 30.53 | 2.36 | 7.09 | <0.01 | 1.833 | 3.9 | <0.01 |
| 6-deoxyglucose | 19.95 | 1.64 | 2.65 | <0.01 | 1.384 | 2.38 | <0.01 |
| 6-hydroxy-2-aminohexanoic acid | 16.43 | 1.54 | 2.55 | <0.01 | 1.048 | 1.75 | 0.04 |
| Aspartate | 13.59 | 1.44 | 0.43 | <0.01 | 1.346 | 0.13 | 0.04 |
| Butane-2,3-diol | 5.83 | 1.54 | 2.48 | <0.01 | 1.13 | 1.75 | <0.01 |
| Butanedioic acid | 11.34 | 1.82 | 3.14 | <0.01 | 1.086 | 1.59 | 0.02 |
| Cellobiose | 21.4 | 1.12 | 0.49 | <0.01 | 1.008 | 0.55 | <0.01 |
| Cholesterol | 28.53 | 1.37 | 0.37 | <0.01 | 1.486 | 0.34 | <0.01 |
| Citramalic acid | 14.6 | 1.81 | 3.16 | <0.01 | 1.077 | 1.62 | 0.02 |
| Cyclohexylamine | 8.54 | 1.22 | 0.35 | 0.03 | 1.524 | 0.25 | 0.02 |
| D-arabinose | 18.82 | 1.52 | 2.43 | <0.01 | 1.201 | 1.98 | <0.01 |
| Deoxycholic acid | 37.57 | 1.91 | 0.17 | <0.01 | 1.733 | 0.19 | 0.01 |
| D-tagatose | 23.09 | 2.14 | 3.4 | <0.01 | 1.337 | 2.16 | <0.01 |
| D-xylose | 20.89 | 1.2 | 2.66 | 0.04 | 1.927 | 7.62 | 0.02 |
| Galactaric acid | 24.84 | 1.23 | 1.97 | <0.01 | 1.172 | 1.87 | 0.02 |
| Galactinol | 22.86 | 1.74 | 4.52 | 0.03 | 2.445 | 9.9 | <0.01 |
| Galacto-hexodialdose | 22.29 | 1.53 | 2.28 | <0.01 | 1.431 | 3.27 | <0.01 |
| Galactose | 22.84 | 1.65 | 3.63 | 0.02 | 2.047 | 9.5 | 0.02 |
| Gluconic acid lactone | 17.36 | 1.36 | 0.39 | 0.02 | 1.39 | 0.35 | 0.01 |
| Glycolic acid | 7.01 | 1.41 | 2.01 | <0.01 | 1.293 | 2.11 | <0.01 |
| Inosine | 32.3 | 2.57 | 11.3 | <0.01 | 2.131 | 5.85 | <0.01 |
| Inulotriose | 31.78 | 2.24 | 6.31 | <0.01 | 2.133 | 7.41 | <0.01 |
| Isochlorogenic acid | 25.34 | 1.37 | 2.14 | 0.01 | 1.292 | 0.45 | <0.01 |
| Kynurenic acid | 31.85 | 2.61 | 11.9 | <0.01 | 1.927 | 3.33 | <0.01 |
| L-asparagine | 18.92 | 1.49 | 0.32 | 0.02 | 1.369 | 0.14 | 0.03 |
| Leucinic acid | 9.76 | 1.36 | 1.98 | <0.01 | 1.517 | 2.78 | <0.01 |
| Melibiose | 28.06 | 1.62 | 2.86 | <0.01 | 2.082 | 6.05 | <0.01 |
| Methyl beta-d-glucopyranoside | 23.24 | 1.64 | 2.73 | <0.01 | 1.351 | 2.34 | 0.02 |
| N-carbamoylaspartate | 21.1 | 1.84 | 3.21 | <0.01 | 1.622 | 2.71 | <0.01 |
| Phenyllactic acid | 17.01 | 1.24 | 1.81 | <0.01 | 1.523 | 2.86 | <0.01 |
| p-hydroxylphenyllactic acid | 14.66 | 1.74 | 2.81 | <0.01 | 1.548 | 2.67 | <0.01 |
| Tranexamic acid | 14.23 | 1.24 | 0.5 | <0.01 | 1.171 | 0.54 | <0.01 |
| Xylofuranose | 17.7 | 1.46 | 2.72 | <0.01 | 1.901 | 5.13 | <0.01 |
| Inflammatory Cytokines (pg/mL) | AL Group | IF Group | ALD Group | IFD Group |
|---|---|---|---|---|
| IL-1α | 11.06 ± 3.63 | 12.78 ± 5.74 | 21.13 ± 4.18 *** | 13.5 ± 4.37 ## |
| IL-1β | 2.32 ± 1.45 | 5.27 ± 3.56 | 12.82 ± 5.66 *** | 9.57 ± 2.23 |
| IL-2 | 5.23 ± 4.96 | 4.22 ± 1.28 | 8.74 ± 2.96 * | 7.54 ± 2.32 |
| IL-3 | 3.60 ± 1.11 | 4.70 ± 1.23 | 8.14 ± 2.73 *** | 7.20 ± 2.51 |
| IL-6 | 1.41 ± 0.36 | 3.40 ± 1.47 | 25.06 ± 7.52 *** | 16.04 ± 4.48 ### |
| IL-9 | 6.35 ± 1.47 | 10.78 ± 3.37 | 15.80 ± 9.10 ** | 13.25 ± 4.22 |
| IL-12P40 | 821.25 ± 99.80 | 1028.30 ± 263.93 | 1361.20 ± 372.46 ** | 1354.28 ± 397.44 |
| IL-12P70 | 119.24 ± 21.53 | 154.53 ± 52.00 | 221.96 ± 97.32 ** | 207.09 ± 81.13 |
| IL-17 | 125.89 ± 30.09 | 154.09 ± 34.62 | 233.67 ± 72.18 *** | 254.26 ± 49.73 |
| IFN-γ | 12.11 ± 2.17 | 16.35 ± 4.57 | 24.72 ± 10.51 ** | 22.34 ± 6.40 |
| TNF-α | 45.89 ± 12.23 | 60.53 ± 21.1 | 108.86 ± 44.35 *** | 105.80 ± 34.11 |
| IL-4 | 1.45 ± 1.51 | 3.73 ± 2.75 | 4.19 ± 2.68 * | 3.85 ± 2.51 |
| IL-10 | 23.87 ± 4.74 | 33.39 ± 7.49 | 54.97 ± 18.95 *** | 54.69 ± 16.33 |
| KC | 47.62 ± 36.27 | 51.54 ± 12.63 | 78.03 ± 14.70 ** | 49.06 ± 13.50 # |
| MCP-1 | 107.15 ± 28.00 | 180.90 ± 56.60 | 284.37 ± 63.82 *** | 261.27 ± 49.07 |
| MIP-1β | 23.30 ± 8.41 | 24.18 ± 8.21 | 47.94 ± 9.94 *** | 40.57 ± 11.28 |
| G-CSF | 166.68 ± 32.68 | 182.75 ± 30.30 | 2920.54 ± 1543.24 *** | 1111.86 ± 368.53 ### |
| GM-CSF | 20.96 ± 8.65 | 25.04 ± 9.78 | 44.82 ± 9.48 *** | 37.93 ± 10.15 |
| IL-5 | 4.55 ± 2.23 | 8.48 ± 4.43 | 8.57 ± 3.30 | 8.78 ± 5.72 |
| Eotaxin | 1531.17 ± 240.65 | 1645.73 ± 282.52 | 1108.29 ± 388.48 * | 1250.41 ± 356.2 |
| MIP-1α | 3.41 ± 0.78 | 3.13 ± 0.71 | 2.98 ± 0.72 | 2.62 ± 0.86 |
| RANTES | 153.38 ± 27.01 | 168.62 ± 23.41 | 133.68 ± 18.17 | 148.04 ± 24.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Man, D.; Shi, D.; Wu, W.; Wang, S.; Wang, K.; Li, Y.; Yang, L.; Bian, X.; Wang, Q.; et al. Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome. Nutrients 2022, 14, 5311. https://doi.org/10.3390/nu14245311
Wu J, Man D, Shi D, Wu W, Wang S, Wang K, Li Y, Yang L, Bian X, Wang Q, et al. Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome. Nutrients. 2022; 14(24):5311. https://doi.org/10.3390/nu14245311
Chicago/Turabian StyleWu, Jingjing, Da Man, Ding Shi, Wenrui Wu, Shuting Wang, Kaicen Wang, Yating Li, Liya Yang, Xiaoyuan Bian, Qiangqiang Wang, and et al. 2022. "Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome" Nutrients 14, no. 24: 5311. https://doi.org/10.3390/nu14245311
APA StyleWu, J., Man, D., Shi, D., Wu, W., Wang, S., Wang, K., Li, Y., Yang, L., Bian, X., Wang, Q., & Li, L. (2022). Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome. Nutrients, 14(24), 5311. https://doi.org/10.3390/nu14245311





