Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9–Dependent Adipose Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. HFD-Fed Mouse Model
2.4. Experimental Design
2.5. Quantitative PCR (qPCR)
2.6. Immunoblotting
2.7. ELISA
2.8. Statistical Analysis
3. Results
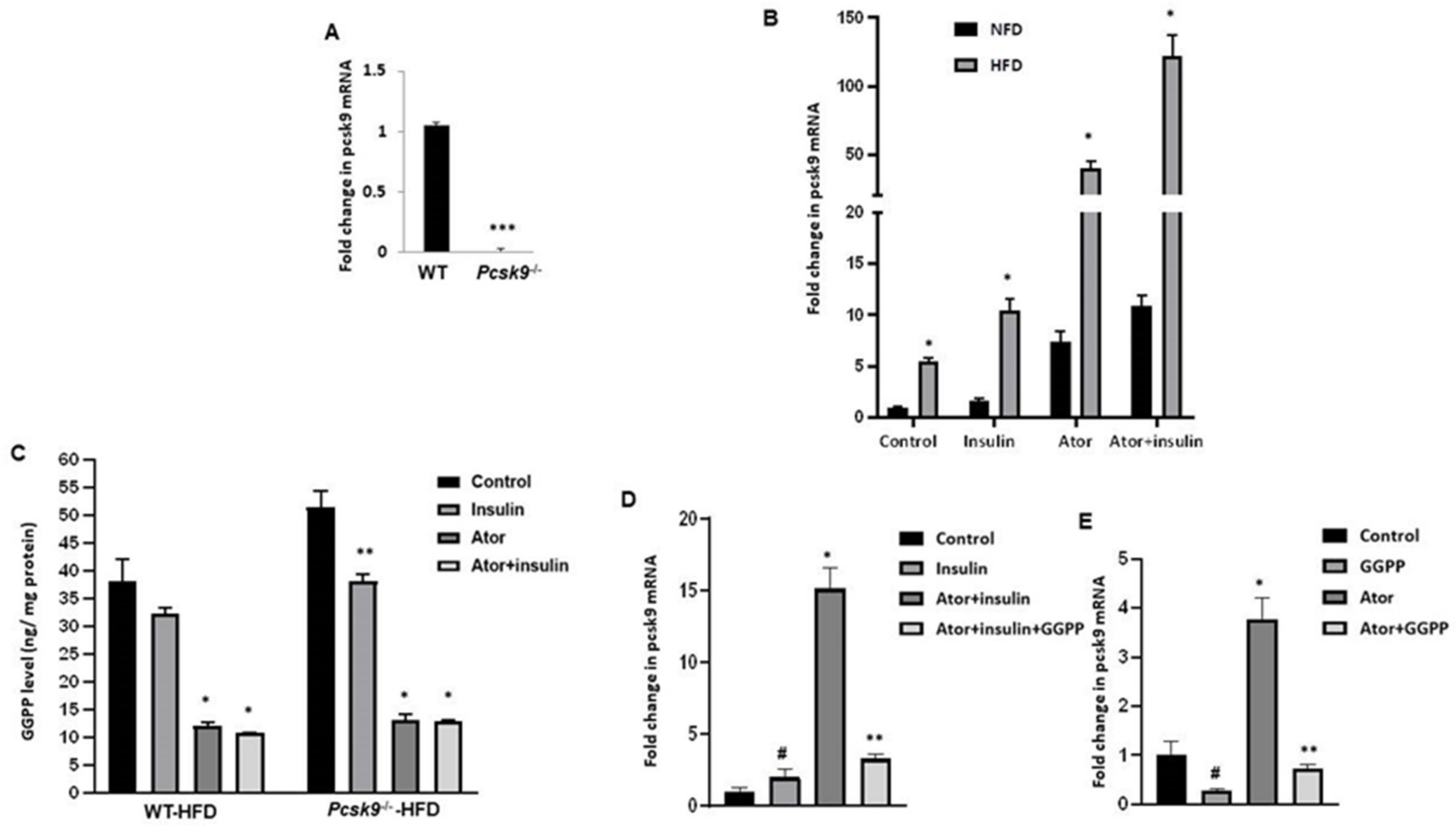
3.1. Atorvastatin Upregulates PCSK9 mRNA in Adipose Tissue
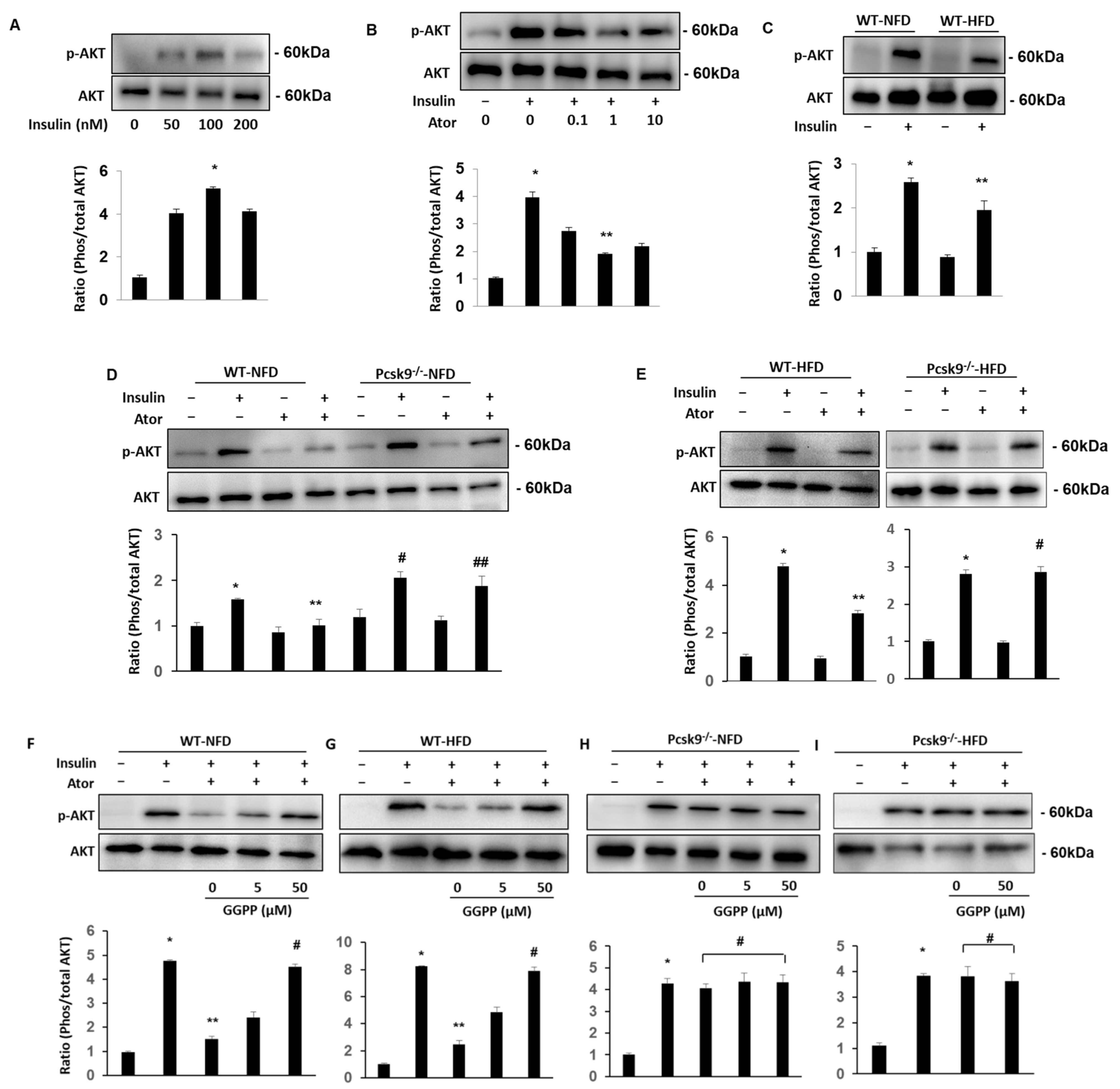
3.2. PCSK9 Deficiency Protects Statin-Inhibited Insulin Signaling in Adipose Tissue
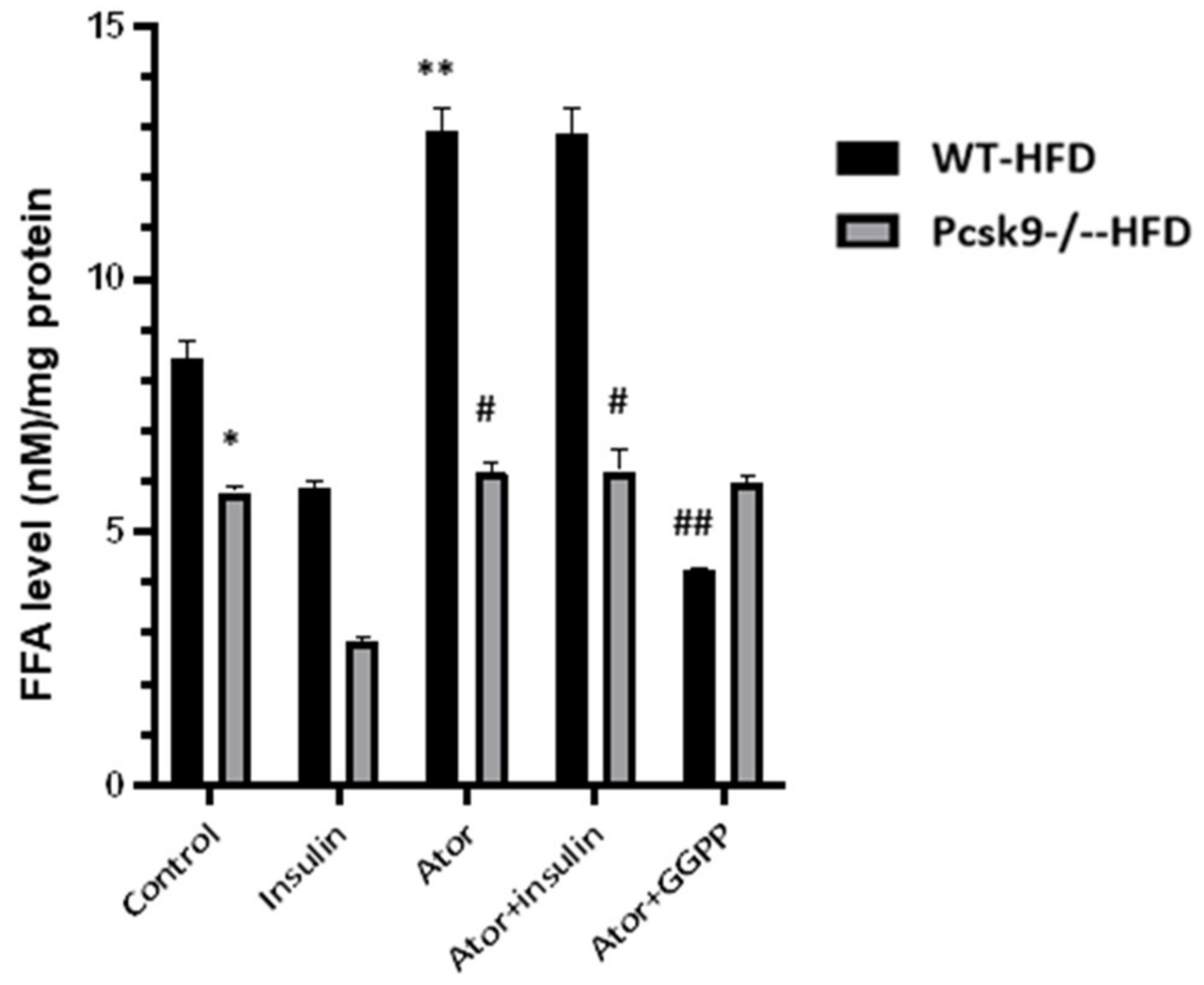
3.3. PCSK9 Deficiency Decreases Statin-Stimulated FFAs in Adipose Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug. Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.K.; Mcmurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Preiss, D.; Seshasai, S.R.; Welsh, P.; Murphy, S.A.; Ho, J.E.; Waters, D.D.; DeMicco, D.A.; Barter, P.; Cannon, C.P.; Sabatine, M.S.; et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011, 305, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Buffon, A.; Andreotti, F.; Kozinski, M.; Welton, N.; Fabiszak, T.; Caputo, S.; Grzesk, G.; Kubica, A.; Swiatkiewicz, I.; et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am. J. Cardiol. 2013, 111, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Mansi, I.A.; Chansard, M.; Lingvay, I.; Zhang, S.; Halm, E.A.; Alvarez, C.A. Association of Statin Therapy Initiation with Diabetes Progression: A Retrospective Matched-Cohort Study. JAMA Intern. Med. 2021, 181, 1562–1574. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Preiss, D.; Kuchenbaecker, K.B.; Holmes, M.V.; Engmann, J.E.L.; Shah, T.; Sofat, R.; Stender, S.; Johnson, P.C.D.; Scott, R.A.; et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet 2015, 385, 351–361. [Google Scholar] [CrossRef]
- Henriksbo, B.D.; Tamrakar, A.K.; Xu, J.; Duggan, B.M.; Cavallari, J.F.; Phulka, J.; Stampfli, M.R.; Ashkar, A.A.; Schertzer, J.D. Statins Promote Interleukin-1β-Dependent Adipocyte Insulin Resistance Through Lower Prenylation, Not Cholesterol. Diabetes 2019, 68, 1441–1448. [Google Scholar] [CrossRef]
- Henriksbo, B.D.; Lau, T.C.; Cavallari, J.F.; Denou, E.; Chi, W.; Lally, J.S.; Crane, J.D.; Duggan, B.M.; Foley, K.P.; Fullerton, M.D.; et al. Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance. Diabetes 2014, 63, 3742–3747. [Google Scholar] [CrossRef]
- Leander, K.; Mälarstig, A.; van’t Hooft, F.M.; Hyde, C.; Hellénius, M.L.; Troutt, J.S.; Konrad, R.J.; Öhrvik, J.; Hamsten, A.; de Faire, U. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation 2016, 133, 1230–1239. [Google Scholar] [CrossRef]
- Dixon, D.L.; Trankle, C.; Buckley, L.; Parod, E.; Carbone, S.; Van Tassell, B.W.; Abbate, A. A review of PCSK9 inhibition and its effects beyond LDL receptors. J. Clin. Lipidol. 2016, 10, 1073–1080. [Google Scholar] [CrossRef]
- Schmidt, A.F.; I Swerdlow, D.I.; Holmes, M.V.; Patel, R.S.; Fairhurst-Hunter, Z.; Lyall, D.M.; Hartwig, F.P.; Horta, B.L.; Hyppönen, E.; Power, C.; et al. PCSK9 genetic variants and risk of type 2 diabetes: A mendelian randomisation study. Lancet Diabetes Endocrinol. 2017, 5, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, M.; Troutt, J.; van Greevenbroek, M.; Ferreira, I.; Feskens, E.; van der Kallen, C.; Schaper, N.; Schalkwijk, C.; Konrad, R.; Stehouwer, C. Plasma proprotein convertase subtilisin kexin type 9 is not altered in subjects with impaired glucose metabolism and type 2 diabetes mellitus, but its relationship with non-HDL cholesterol and apolipoprotein B may be modified by type 2 diabetes mellitus: The CODAM study. Atherosclerosis 2011, 217, 263–267. [Google Scholar] [PubMed]
- Da Dalt, L.; Ruscica, M.; Bonacina, F.; Balzarotti, G.; Dhyani, A.; Di Cairano, E.; Baragetti, A.; Arnaboldi, L.; De Metrio, S.; Pellegatta, F.; et al. PCSK9 deficiency reduces insulin secretion and promotes glucose intolerance: The role of the low-density lipoprotein receptor. Eur. Heart J. 2019, 40, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Banach, M.; Corsini, A.; Sirtori, C.R.; Ferri, N.; Ruscica, M. Changes in circulating pro-protein convertase subtilisin/kexin type 9 levels-experimental and clinical approaches with lipid-lowering agents. Eur. J. Prev. Cardiol. 2019, 26, 930–949. [Google Scholar] [CrossRef]
- Dubuc, G.; Chamberland, A.; Wassef, H.; Davignon, J.; Seidah, N.G.; Bernier, L.; Prat, A. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1454–1459. [Google Scholar] [CrossRef]
- Dong, B.; Wu, M.; Li, H.; Kraemer, F.; Adeli, K.; Seidah, N.; Park, S.W.; Liu, J. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: Mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 2010, 51, 1486–1495. [Google Scholar] [CrossRef]
- Bordicchia, M.; Spannella, F.; Ferretti, G.; Bacchetti, T.; Vignini, A.; Di Pentima, C.; Mazzanti, L.; Sarzani, R. PCSK9 is Expressed in Human Visceral Adipose Tissue and Regulated by Insulin and Cardiac Natriuretic Peptides. Int. J. Mol. Sci. 2019, 20, 245. [Google Scholar] [CrossRef]
- Roubtsova, A.; Munkonda, M.N.; Awan, Z.; Marcinkiewicz, J.; Chamberland, A.; Lazure, C.; Cianflone, K.; Seidah, N.G.; Prat, A. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 785–791. [Google Scholar] [CrossRef]
- Shu, X.; Wu, J.; Zhang, T.; Ma, X.; Du, Z.; Xu, J.; You, J.; Wang, L.; Chen, N.; Luo, M.; et al. Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes Ferroptosis-Related Senescence in Adipose Tissue. Nutrients 2022, 14, 4365. [Google Scholar] [CrossRef]
- Lalli, C.A.; Pauli, J.R.; Prada, P.O.; Cintra, D.E.; Ropelle, E.R.; Velloso, L.A.; Saad, M.J.A. Statin modulates insulin signaling and insulin resistance in liver and muscle of rats fed a high-fat diet. Metabolism 2008, 57, 57–65. [Google Scholar] [CrossRef]
- Yeh, Y.S.; Goto, T.; Takahashi, N.; Egawa, K.; Takahashi, H.; Jheng, H.F.; Kim, Y.I.; Kawada, T. Geranylgeranyl pyrophosphate performs as an endogenous regulator of adipocyte function via suppressing the LXR pathway. Biochem. Biophys. Res. Commun. 2016, 478, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-S.; Jheng, H.-F.; Iwase, M.; Kim, M.; Mohri, S.; Kwon, J.; Kawarasaki, S.; Li, Y.; Takahashi, H.; Ara, T.; et al. The Mevalonate Pathway Is Indispensable for Adipocyte Survival. iScience 2018, 9, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, M.-F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.-W.; Shen, D.; Zhang, J.-Z.; Zhou, N.; He, J.; et al. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Anderwald, C.; Krssak, M.; Bayerle-Eder, M.; Esterbauer, H.; Pfeiler, G.; Brehm, A.; Nowotny, P.; Hofer, A.; Waldhäusl, W.; et al. Effects of high-dose simvastatin therapy on glucose metabolism and ectopic lipid deposition in nonobese type 2 diabetic patients. Diabetes Care 2009, 32, 209–214. [Google Scholar] [CrossRef]
- Sahebkar, A.; Simental-Mendía, L.E.; Guerrero-Romero, F.; Golledge, J.; Watts, G.F. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: A systematic review and meta-analysis of clinical trials. Diabetes Obes. Metab. 2015, 17, 1042–1055. [Google Scholar] [CrossRef]
- Dozio, E.; Ruscica, M.; Vianello, E.; Macchi, C.; Sitzia, C.; Schmitz, G.; Tacchini, L.; Romanelli, M.M.C. PCSK9 Expression in Epicardial Adipose Tissue: Molecular Association with Local Tissue Inflammation. Mediat. Inflamm. 2020, 2020, 1348913. [Google Scholar] [CrossRef]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef]
- Parisi, V.; Petraglia, L.; D’Esposito, V.; Cabaro, S.; Rengo, G.; Caruso, A.; Grimaldi, M.G.; Baldascino, F.; De Bellis, A.; Vitale, D.; et al. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int. J. Cardiol. 2019, 274, 326–330. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, X.; Wu, J.; Zhang, T.; Ma, X.; Du, Z.; Xu, J.; You, J.; Wang, L.; Chen, N.; Luo, M.; et al. Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9–Dependent Adipose Insulin Resistance. Nutrients 2022, 14, 5314. https://doi.org/10.3390/nu14245314
Shu X, Wu J, Zhang T, Ma X, Du Z, Xu J, You J, Wang L, Chen N, Luo M, et al. Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9–Dependent Adipose Insulin Resistance. Nutrients. 2022; 14(24):5314. https://doi.org/10.3390/nu14245314
Chicago/Turabian StyleShu, Xin, Jiaqi Wu, Tao Zhang, Xiaoyu Ma, Zuoqin Du, Jin Xu, Jingcan You, Liqun Wang, Ni Chen, Mao Luo, and et al. 2022. "Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9–Dependent Adipose Insulin Resistance" Nutrients 14, no. 24: 5314. https://doi.org/10.3390/nu14245314
APA StyleShu, X., Wu, J., Zhang, T., Ma, X., Du, Z., Xu, J., You, J., Wang, L., Chen, N., Luo, M., & Wu, J. (2022). Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9–Dependent Adipose Insulin Resistance. Nutrients, 14(24), 5314. https://doi.org/10.3390/nu14245314





