Association between Diet Quality and Index of Non-Alcoholic Steatohepatitis in a Large Population of People with Type 2 Diabetes: Data from the TOSCA.IT Study
Abstract
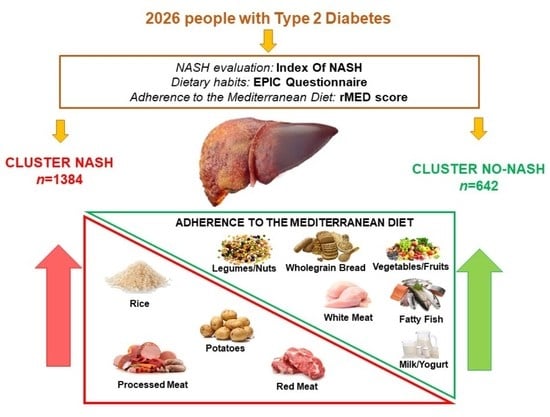
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Assessment of Anthropometric and Laboratory Parameters
2.3. Assessment of Dietary Intake, Food Consumption and Adherence to the Mediterranean Diet
2.4. Assessment of Non-Alcoholic Steatohepatitis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Colombo, M.; Cortez-Pinto, H.; Huang, T.T.; Miller, V.; Ninburg, M.; Schattenberg, J.M.; Seim, L.; Wong, V.W.S.; Zelber-Sagi, S. NAFLD—Sounding the alarm on a silent epidemic. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Targher, G. From nonalcoholic fatty liver disease to metabolic dysfunction-associated fatty liver disease: More than a single-letter change in an acronym. Hepatoma Res. 2021, 7, 47. [Google Scholar] [CrossRef]
- Cusi, K. Time to Include Nonalcoholic Steatohepatitis in the Management of Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Lomonaco, R.; Bril, F.; Portillo-Sanchez, P.; Ortiz-Lopez, C.; Orsak, B.; Biernacki, D.; Lo, M.; Suman, A.; Weber, M.H.; Cusi, K. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Bril, F.; Cusi, K. Management of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Call to Action. Diabetes Care 2017, 40, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Bertolini, L.; Rodella, S.; Tessari, R.; Zenari, L.; Lippi, G.; Arcaro, G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007, 30, 2119–2121. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Marra, F.; Marchesini, G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia 2008, 51, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Otgonsuren, M.; Estep, M.J.; Hossain, N.; Younossi, E.; Frost, S.; Henry, L.; Hunt, S.; Fang, Y.; Goodman, Z.; Younossi, Z.M. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J. Gastroenterol. Hepatol. 2014, 29, 2006–2013. [Google Scholar] [CrossRef]
- Iqbal, U.; Perumpail, B.J.; Akhtar, D.; Kim, D.; Ahmed, A. The Epidemiology, Risk Profiling and Diagnostic Challenges of Nonalcoholic Fatty Liver Disease. Medicines 2019, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of Nutritional Changes on Nonalcoholic Fatty Liver Disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef] [Green Version]
- Della Pepa, G.; Vetrani, C.; Lombardi, G.; Bozzetto, L.; Annuzzi, G.; Rivellese, A.A. Isocaloric Dietary Changes and Non-Alcoholic Fatty Liver Disease in High Cardiometabolic Risk Individuals. Nutrients 2017, 26, 1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valtueña, S.; Pellegrini, N.; Ardigò, D.; Del Rio, D.; Numeroso, F.; Scazzina, F.; Monti, L.; Zavaroni, I.; Brighenti, F. Dietary glycemic index and liver steatosis. Am. J. Clin. Nutr. 2006, 84, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Yang, A.; Mao, L.; Quan, Y.; Cui, J.; Sun, Y. Association Between Dietary Fiber Intake and Non-alcoholic Fatty Liver Disease in Adults. Front. Nutr. 2020, 7, 593735. [Google Scholar] [CrossRef] [PubMed]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Salomone, F.; Webb, M.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Dig. Liver Dis. 2019, 51, 1698–1705. [Google Scholar] [CrossRef] [Green Version]
- Salehi-Sahlabadi, A.; Teymoori, F.; Jabbari, M.; Momeni, A.; Mokari-Yamchi, A.; Sohouli, M.; Hekmatdoost, A. Dietary polyphenols and the odds of non-alcoholic fatty liver disease: A case-control study. Clin. Nutr. ESPEN 2021, 41, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.A.; Hodson, L. Managing NAFLD in Type 2 Diabetes: The Effect of Lifestyle Interventions, a Narrative Review. Adv. Ther. 2020, 37, 1381–1406. [Google Scholar] [CrossRef] [Green Version]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Ge, L.; Wang, Q.; He, J.; Qin, T.; Cao, L.; Cao, C.; Liu, D.; Liu, X.; Yang, K. Nutritional Recommendations for Type 2 Diabetes: An International Review of 15 Guidelines. Can. J. Diabetes 2022, 2, S1499–S2671. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Rivellese, A.A.; Bonora, E.; Babini, A.C.; Sartore, G.; Corsi, L.; Buzzetti, R.; Citro, G.; Baldassarre, M.P.A.; et al. Pasta Consumption and Connected Dietary Habits: Associations with Glucose Control, Adiposity Measures, and Cardiovascular Risk Factors in People with Type 2 Diabetes-TOSCA.IT Study. Nutrients 2019, 30, 101. [Google Scholar]
- Vaccaro, O.; Masulli, M.; Bonora, E.; Del Prato, S.; Giorda, C.B.; Maggioni, A.P.; Mocarelli, P.; Nicolucci, A.; Rivellese, A.A.; Squatrito, S.; et al. Addition of either pioglitazone or a sulfonylurea in type 2 diabetic patients inadequately controlled with metformin alone: Impact on cardiovascular events. A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, O.; Masulli, M.; Nicolucci, A.; Bonora, E.; Del Prato, S.; Maggioni, A.P.; Rivellese, A.A.; Squatrito, S.; Giorda, C.B.; Sesti, G.; et al. Effects on the incidence of cardiovascular events of the addition of pioglitazone versus sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): A randomized, multicenter trial. Lancet Diabetes Endocrinol. 2017, 5, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori J. 2003, 89, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26, S152–S160. [Google Scholar] [CrossRef] [Green Version]
- Vitale, M.; Masulli, M.; Cocozza, S.; Anichini, R.; Babini, A.C.; Boemi, M.; Bonora, E.; Buzzetti, R.; Carpinteri, R.; Caselli, C.; et al. TOSCA.IT Study Group. Sex differences in food choices, adherence to dietary recommendations and plasma lipid profile in type 2 diabetes—The TOSCA.IT study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 879–885. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Rivellese, A.A.; Bonora, E.; Cappellini, F.; Nicolucci, A.; Squatrito, S.; Antenucci, D.; Barrea, A.; Bianchi, C.; et al. TOSCA.IT Study Group. Dietary intake and major food sources of polyphenols in people with type 2 diabetes: The TOSCA.IT Study. Eur. J. Nutr. 2018, 57, 679–688. [Google Scholar] [CrossRef]
- Salvini, S.; Parpinel, M.; Gnagnarella, P.; Maisonneuve, P.; Turrini, A. Banca Dati di Composizione Degli Alimenti per Studi Epidemiologici in Italia; Istituto Europeo di Oncologia: Milan, Italy, 1998. [Google Scholar]
- Carnovale, E.; Marletta, L. Tabella di Composizione Degli Alimenti; Crea-Nut (ex INRAN): Rome, Italy, 2000. [Google Scholar]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1. U.S. Department of Agriculture, Agricultural Research Service. 2014; Nutrient Data Laboratory Home Page. Available online: http://www.ars.usda.gov/nutrientdata/flav (accessed on 16 September 2022).
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database. 2013. Available online: https://doi.org/10.1093/database/bat070 (accessed on 26 September 2022). [CrossRef]
- Buckland, G.; González, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Calabrese, I.; Rivellese, A.A.; Bonora, E.; Signorini, S.; Perriello, G.; Squatrito, S.; Buzzetti, R.; Sartore, G.; et al. TOSCA.IT Study Group. Impact of a Mediterranean Dietary Pattern and Its Components on Cardiovascular Risk Factors, Glucose Control, and Body Weight in People with Type 2 Diabetes: A Real-Life Study. Nutrients 2018, 10, 1067. [Google Scholar] [CrossRef] [Green Version]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Fan, J.G.; Cao, H.X. Role of diet and nutritional management in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2013, 28, 81–87. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ratziu, V.; Oren, R. Nutrition and physical activity in NAFLD: An overview of the epidemiological evidence. World J. Gastroenterol. 2011, 17, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Li, Y.; Guo, X.; Zhong, L.; Tang, S. Food groups and the likelihood of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Br. J. Nutr. 2020, 124, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.; Ma, J.; Patel, K.; Berger, S.; Lau, J.; Lichtenstein, A.H. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 833–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayram, H.M.; Majoo, F.M.; Ozturkcan, A. Polyphenols in the prevention and treatment of non-alcoholic fatty liver disease: An update of preclinical and clinical studies. Clin. Nutr. ESPEN. 2021, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.A.M.; Masroor, A.; Khorochkov, A.; Prieto, J.; Singh, K.B.; Nnadozie, M.C.; Abdal, M.; Shrestha, N.; Mohammed, L. The Role of Vitamins in Non-Alcoholic Fatty Liver Disease: A Systematic Review. Cureus 2021, 13, e16855. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, Fiber, and NAFLD: Is There Any Connection? Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef]
- Bozzetto, L.; Annuzzi, G.; Ragucci, M.; Di Donato, O.; Della Pepa, G.; Della Corte, G.; Griffo, E.; Anniballi, G.; Giacco, A.; Mancini, M.; et al. Insulin resistance, postprandial GLP-1 and adaptive immunity are the main predictors of NAFLD in a homogeneous population at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 623–629. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef]
- Hashemian, M.; Poustchi, H.; Merat, S.; Abnet, C.; Malekzadeh, R.; Etemadi, A. Red Meat Consumption and Risk of Nonalcoholic Fatty Liver Disease in a Population with Low Red Meat Consumption. Curr. Dev. Nutr. 2020, 29, 1413. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Ghasemi-Nasab, M.; Riasatian, M. Mediterranean diet for patients with non-alcoholic fatty liver disease, a systematic review and meta-analysis of observational and clinical investigations. J. Diabetes Metab. Disord. 2020, 19, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [PubMed]
| Cluster NASH (n. 642) | Cluster NO-NASH (n. 1384) | p-Value | |
|---|---|---|---|
| Age (years) | 62 ± 7 | 63 ± 6 | 0.006 |
| Smoking (%) | 34.4 | 32.6 | 0.209 |
| Diabetes duration (years) | 8 ± 5 | 9 ± 6 | 0.004 |
| BMI (kg/m2) | 32 ± 4 | 29 ± 4 | <0.0001 |
| Waist circumference (cm) | 108 ± 11 | 102 ± 11 | <0.0001 |
| Hip circumference (cm) | 109 ± 11 | 105 ± 10 | <0.0001 |
| Waist/Hip ratio | 0.99 ± 0.98 | 0.96 ± 0.91 | 0.003 |
| Systolic blood pressure (mm/Hg) | 136 ± 16 | 134 ± 15 | 0.004 |
| Diastolic blood pressure (mm/Hg) | 81 ± 9 | 80 ± 9 | 0.002 |
| HbA1c (%) | 7.8 ± 0.5 | 7.6 ± 0.5 | <0.0001 |
| Plasma Glucose (mg/dL) | 185 ± 39 | 159 ± 32 | <0.0001 |
| Plasma Insulin (μU/mL) | 23.5 ± 12.2 | 9.4 ± 3.6 | <0.0001 |
| HOMA-IR | 10.7 ± 7.0 | 3.6 ± 1.4 | <0.0001 |
| Plasma HDL-cholesterol (mg/dL) | 43 ± 12 | 48 ± 12 | <0.0001 |
| Plasma LDL-cholesterol (mg/dL) | 104 ± 30 | 101 ± 36 | 0.045 |
| Plasma Triglycerides (mg/dL) | 184 ± 114 | 137 ± 66 | <0.0001 |
| C-reactive protein (mg/dL) | 0.5 ± 2.3 | 0.4 ± 1.7 | 0.246 |
| eGFR (ml/min/1.73 m2) | 91.4 ± 2.6 | 92.7 ± 2.5 | 0.311 |
| AST (U/L) | 24 ± 12 | 18 ± 9 | <0.0001 |
| ALT (U/L) | 25 ± 15 | 17 ± 10 | <0.0001 |
| GGT (U/L) | 47 ± 54 | 31 ± 28 | <0.0001 |
| Use of antihypertensive drugs (%) | 95.3 | 91.2 | 0.652 |
| Use of Lipid lowering drugs (%) | 63.6 | 61.1 | 0.441 |
| Cluster NASH (n. 642) | Cluster NO-NASH (n. 1384) | p-Value | |
|---|---|---|---|
| Energy (Kcal/day) | 1755 ± 618 | 1843 ± 692 | 0.006 |
| Total Proteins (% TEI) | 18.4 ± 2.6 | 18.2 ± 2.6 | 0.272 |
| Proteins from animal food sources (% TEI) | 12.7 ± 3.2 | 12.5 ± 3.2 | 0.276 |
| Proteins from vegetable food sources (% TEI) | 5.7 ± 1.1 | 5.7 ± 1.1 | 0.574 |
| Total Lipids (% TE) | 37.1 ± 6.2 | 36.6 ± 6.1 | 0.089 |
| Saturated fatty acids (% TEI) | 12.2 ± 2.5 | 12.0 ± 2.5 | 0.179 |
| Monounsaturated fatty acids (% TEI) | 18.3 ± 3.8 | 18.0 ± 3.8 | 0.105 |
| Polyunsaturated fatty acids (% TEI) | 4.5 ± 1.1 | 4.5 ± 1.1 | 0.253 |
| n-3 (% TEI) | 0.55 ± 0.11 | 0.55 ± 0.12 | 0.930 |
| n-6 (% TEI) | 3.60 ± 1.03 | 3.56 ± 1.03 | 0.394 |
| Total cholesterol (mg/1000 kcal/day) | 185 ± 53 | 181 ± 54 | 0.130 |
| Total Carbohydrates (% TEI) | 44.4 ± 7.7 | 45.1 ± 7.4 | 0.081 |
| Added sugars (% TEI) | 2.37 ± 3.01 | 2.24 ± 3.24 | 0.376 |
| Fibre (g/1000 kcal/day) | 10.5 ± 2.6 | 11.0 ± 2.7 | <0.0001 |
| Glycemic Index | 51.6 ± 3.5 | 51.9 ± 3.4 | 0.172 |
| Glycemic Load (%) | 105.1 ± 46.6 | 111.4 ± 50.1 | 0.019 |
| Alcohol (g/day) | 9.9 ± 15.2 | 11.0 ± 15.3 | 0.146 |
| Vitamin-C (mg/day) | 105 ± 49 | 115 ± 58 | <0.0001 |
| β-carotene (mg/day) | 2286 ± 1307 | 2649 ± 1830 | <0.0001 |
| Vitamin E (mg/day) | 6.42 ± 2.27 | 7.00 ± 2.90 | <0.0001 |
| Vitamin D (mg/day) | 2.47 ± 1.29 | 2.53 ± 1.53 | 0.398 |
| TEAC | 5.47 ± 2.25 | 6.00 ± 2.42 | <0.0001 |
| TRAP | 8.15 ± 3.64 | 8.91 ± 3.76 | <0.0001 |
| FRAP | 17.04 ± 7.11 | 18.46 ± 7.45 | <0.0001 |
| Total polyphenols (mg/1000 kcal/day) | 377.4 ± 163.1 | 386.1 ± 165.4 | 0.026 |
| Cluster NASH (n. 642) | Cluster NO-NASH (n. 1384) | p-Value | |
|---|---|---|---|
| Cereals | 97.9 ± 35.1 | 94.8 ± 36.6 | 0.075 |
| Pasta | 28.2 ± 17.7 | 26.8 ± 16.3 | 0.016 |
| Rice | 3.55 ± 4.36 | 3.09 ± 4.04 | 0.021 |
| Bread | 35.6 ± 31.1 | 36.1 ± 32.6 | 0.738 |
| Wholegrain Bread | 6.7 ± 15.8 | 8.7 ± 17.6 | 0.019 |
| Legumes and Nuts | 13.6 ± 10.6 | 14.7 ± 11.8 | 0.049 |
| Vegetables | 95.7 ± 49.6 | 97.4 ± 49.7 | 0.047 |
| Potatoes | 11.7 ± 12.5 | 10.1 ± 14.0 | 0.013 |
| Fruits | 151.1 ± 86.1 | 164.3 ± 92.9 | 0.002 |
| Meat | 73.7 ± 30.7 | 68.4 ± 29.1 | <0.0001 |
| Red meat | 56.3 ± 26.8 | 52.7 ± 25.8 | 0.004 |
| White meat | 1.5 ± 0.9 | 1.9 ± 0.5 | 0.001 |
| Processed meat | 15.6 ± 11.2 | 14.0 ± 10.1 | 0.003 |
| Fish | 23.7 ± 18.8 | 23.0 ± 17.1 | 0.390 |
| Fatty fish | 14.6 ± 12.1 | 16.3 ± 11.8 | 0.005 |
| Lean fish | 8.5 ± 6.0 | 8.2 ± 6.5 | 0.147 |
| Dairy products | 100.4 ± 54.9 | 125.3 ± 60.1 | <0.0001 |
| Cheese | 20.1 ± 13.0 | 20.0 ± 13.4 | 0.965 |
| Milk and Yogurt | 80.6 ± 76.0 | 102.9 ± 88.0 | <0.0001 |
| Eggs | 10.6 ± 7.2 | 10.4 ± 7.5 | 0.543 |
| Vegetable oils | 14.0 ± 6.0 | 13.9 ± 6.0 | 0.897 |
| Oils from animal origin | 1.56 ± 1.54 | 1.43 ± 1.42 | 0.067 |
| rMED score | 8.1 ± 2.6 | 9.7 ± 2.5 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, M.; Della Pepa, G.; Costabile, G.; Bozzetto, L.; Cipriano, P.; Signorini, S.; Leoni, V.; Riccardi, G.; Vaccaro, O.; Masulli, M. Association between Diet Quality and Index of Non-Alcoholic Steatohepatitis in a Large Population of People with Type 2 Diabetes: Data from the TOSCA.IT Study. Nutrients 2022, 14, 5339. https://doi.org/10.3390/nu14245339
Vitale M, Della Pepa G, Costabile G, Bozzetto L, Cipriano P, Signorini S, Leoni V, Riccardi G, Vaccaro O, Masulli M. Association between Diet Quality and Index of Non-Alcoholic Steatohepatitis in a Large Population of People with Type 2 Diabetes: Data from the TOSCA.IT Study. Nutrients. 2022; 14(24):5339. https://doi.org/10.3390/nu14245339
Chicago/Turabian StyleVitale, Marilena, Giuseppe Della Pepa, Giuseppina Costabile, Lutgarda Bozzetto, Paola Cipriano, Stefano Signorini, Valerio Leoni, Gabriele Riccardi, Olga Vaccaro, and Maria Masulli. 2022. "Association between Diet Quality and Index of Non-Alcoholic Steatohepatitis in a Large Population of People with Type 2 Diabetes: Data from the TOSCA.IT Study" Nutrients 14, no. 24: 5339. https://doi.org/10.3390/nu14245339