Limonin, a Component of Immature Citrus Fruits, Activates Anagen Signaling in Dermal Papilla Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Immature Citrus Fruit Extract
2.2. Cell Culture
2.3. Proliferation Assay
2.4. Western Blot Analysis
2.5. Immunofluorescence Staining
2.6. Statistical Analysis
3. Results
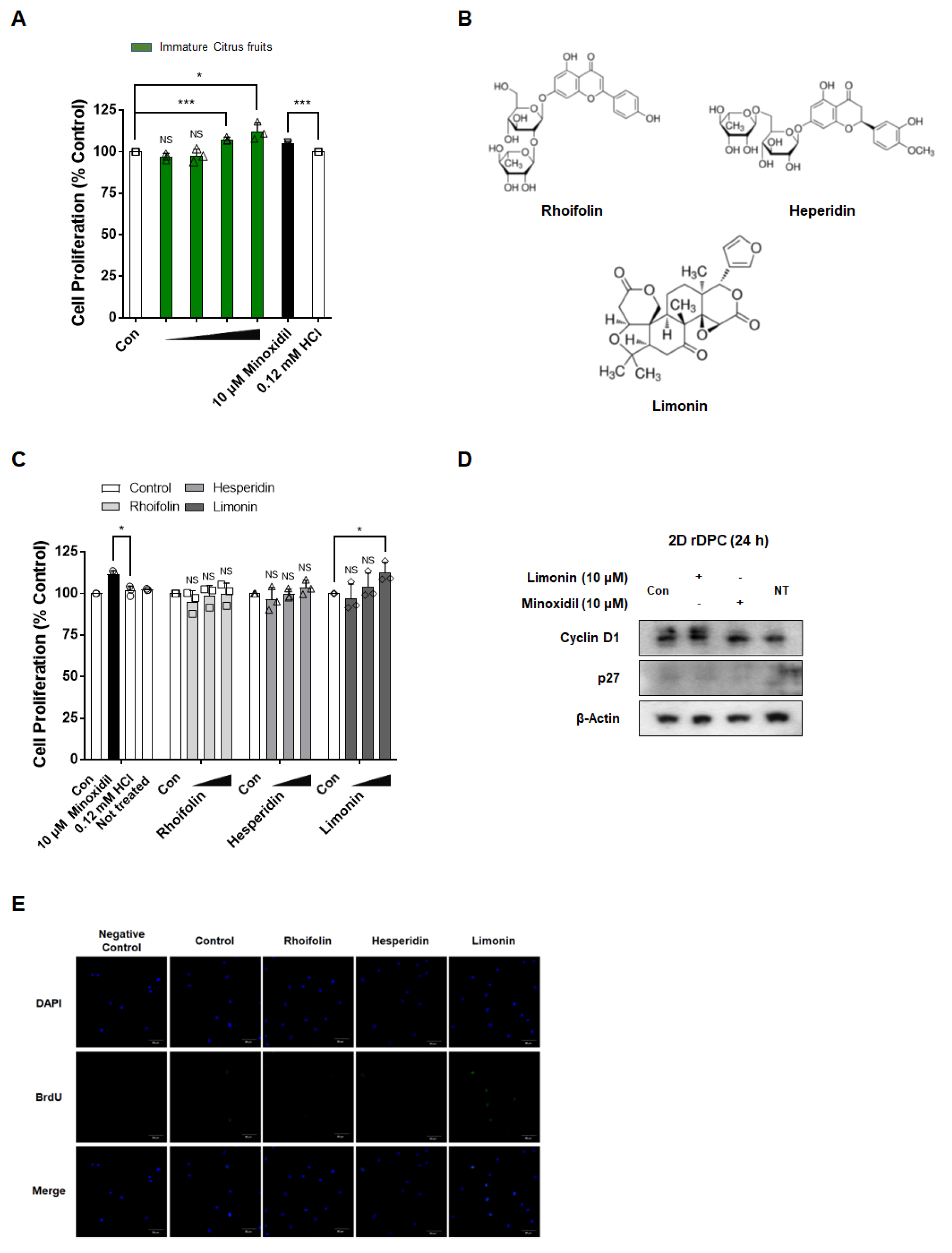
3.1. Immature Citrus Fruit Peel-Derived Limonin Increases the Proliferation of 2D Dermal Papilla Cells
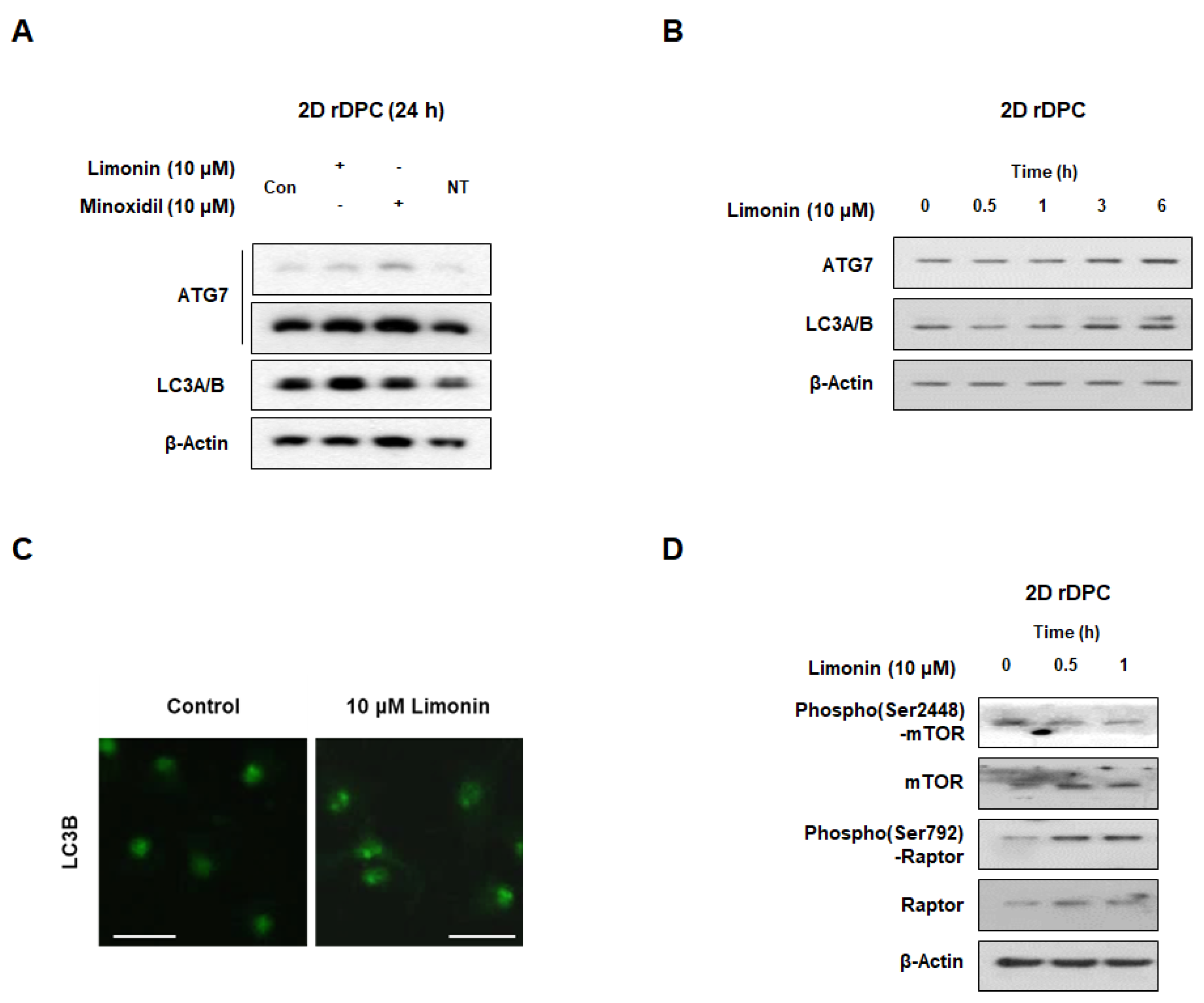
3.2. Limonin Activates Autophagy in 2D Dermal Papilla Cells
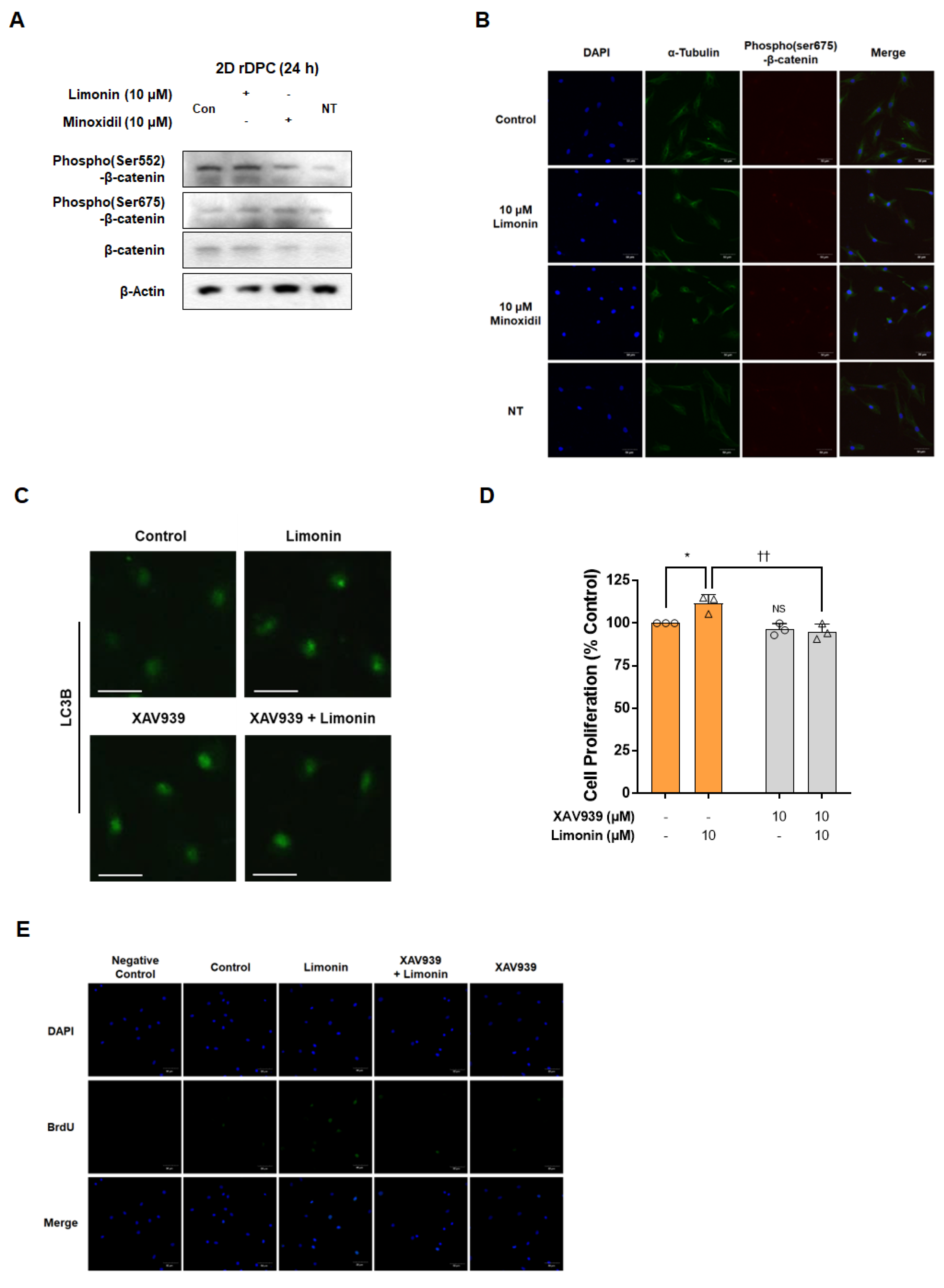
3.3. Limonin Stimulates Autophagy by Activation of Wnt/β-Catenin Signaling in 2D Dermal Papilla Cells
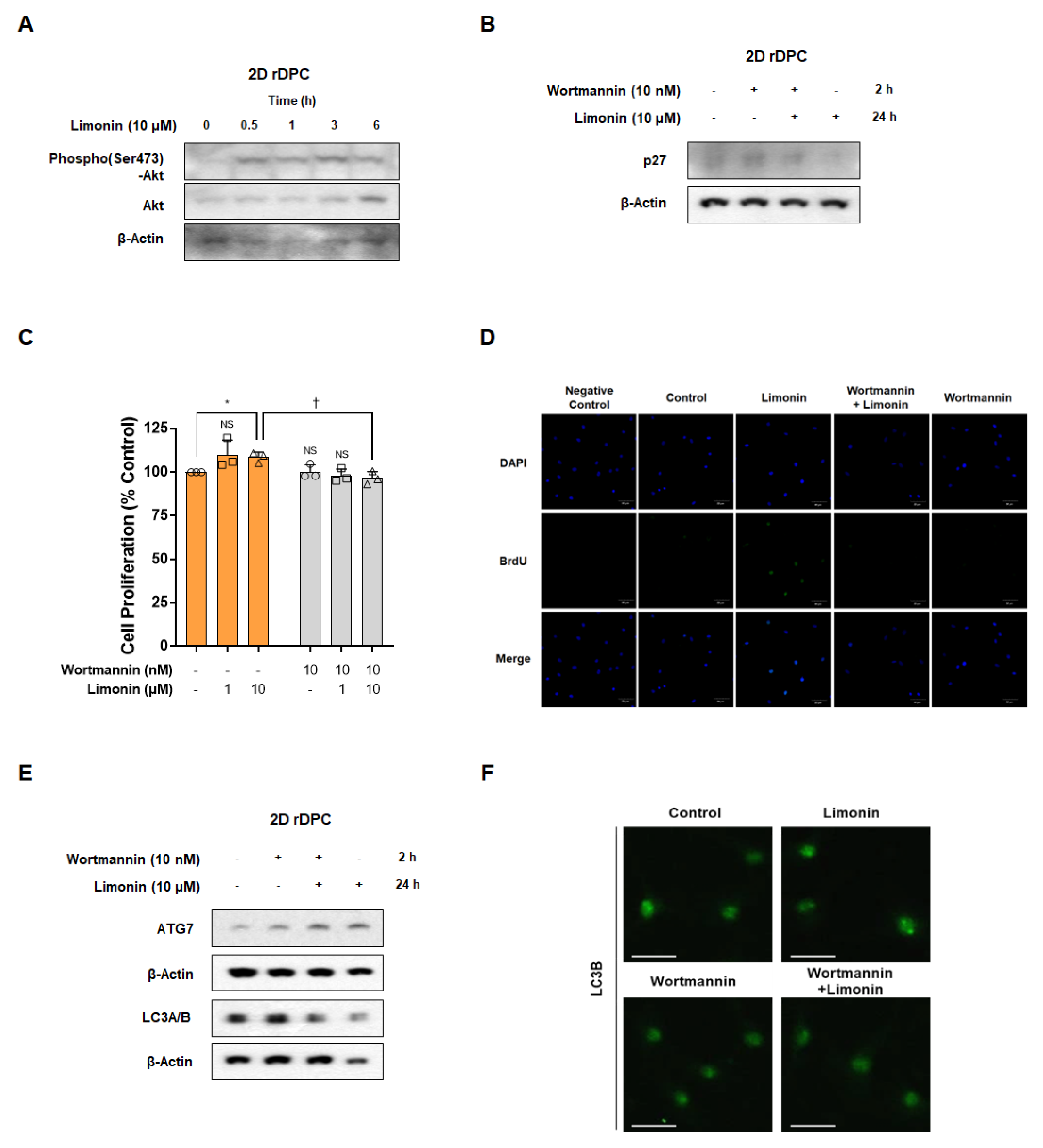
3.4. Inhibition of the PI3K/AKT Pathway Attenuated Limonin-Mediated Proliferation by Activation of the Autophagy Pathway in 2D Dermal Papilla Cells
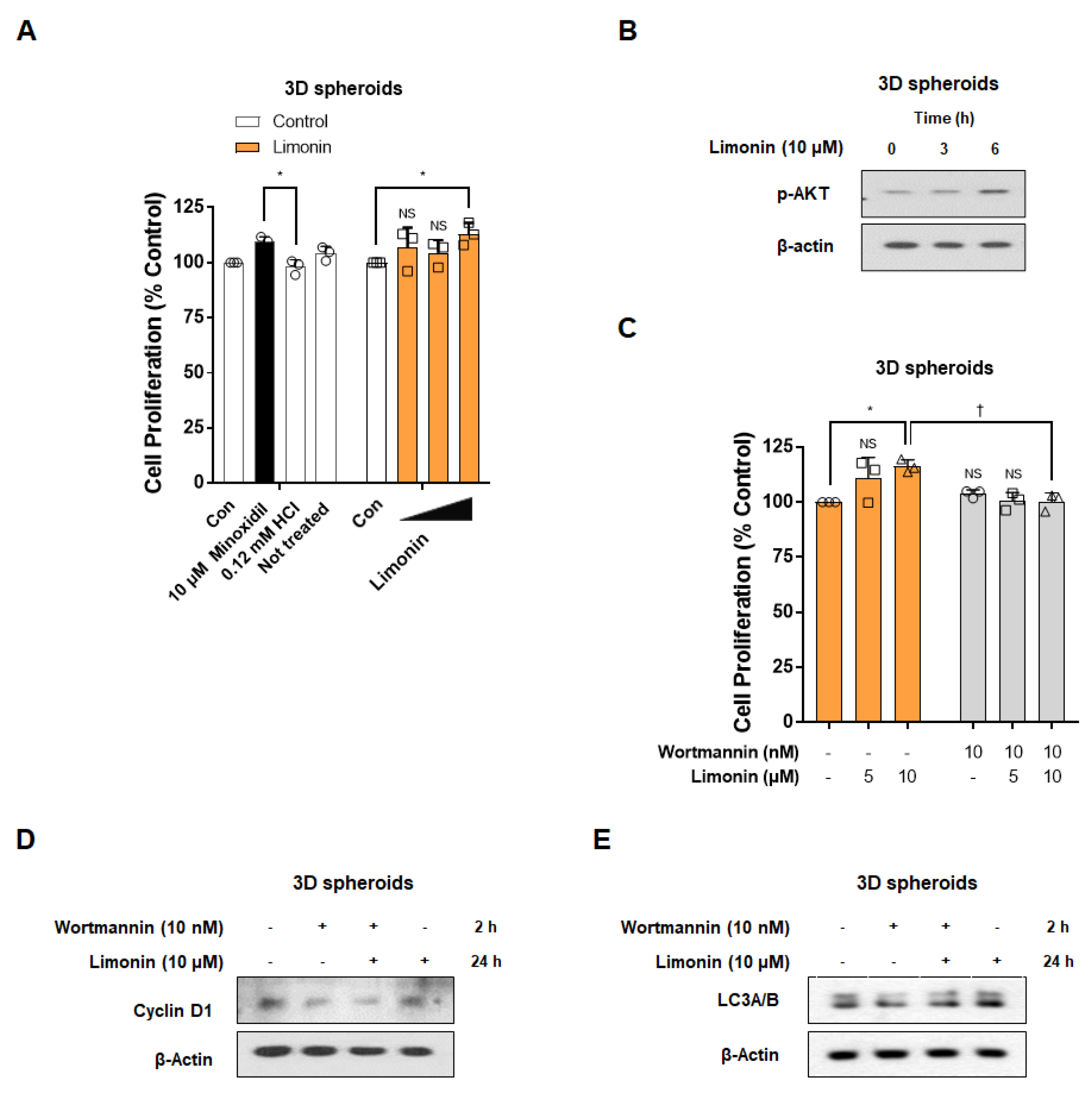
3.5. Limonin Promotes the Proliferation of 3D Spheroids through Activation of PI3K/AKT Pathway-Mediated Autophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Signori, V. Review of the current understanding of the effect of ultraviolet and visible radiation on hair structure and options for photoprotection. J. Cosmet. Sci. 2004, 55, 95–113. [Google Scholar] [CrossRef]
- Kim, J.Y.; Dao, H. Physiology, Integument; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. New Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmuller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iino, M.; Ehama, R.; Nakazawa, Y.; Iwabuchi, T.; Ogo, M.; Tajima, M.; Arase, S. Adenosine stimulates fibroblast growth factor-7 gene expression via adenosine A2b receptor signaling in dermal papilla cells. J. Investig. Dermatol. 2007, 127, 1318–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef]
- Inui, S.; Fukuzato, Y.; Nakajima, T.; Yoshikawa, K.; Itami, S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: A clue to understand paradoxical effects of androgen on human hair growth. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1967–1969. [Google Scholar] [CrossRef]
- Kraft, C.; Martens, S. Mechanisms and regulation of autophagosome formation. Curr. Opin. Cell Biol. 2012, 24, 496–501. [Google Scholar] [CrossRef]
- Parodi, C.; Hardman, J.A.; Allavena, G.; Marotta, R.; Catelani, T.; Bertolini, M.; Paus, R.; Grimaldi, B. Autophagy is essential for maintaining the growth of a human (mini-)organ: Evidence from scalp hair follicle organ culture. PLoS Biol. 2018, 16, e2002864. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Kang, J.I.; Hyun, J.W.; Koh, Y.S.; Kang, J.H.; Hyun, C.G.; Yoon, K.S.; Lee, K.S.; Lee, C.M.; Kim, T.Y.; et al. Myristoleic Acid Promotes Anagen Signaling by Autophagy through Activating Wnt/beta-Catenin and ERK Pathways in Dermal Papilla Cells. Biomol. Ther. 2021, 29, 211–219. [Google Scholar] [CrossRef]
- Jaller, J.A.; MacQuhae, F.; Nichols, A.J. Chapter 26–Clinical Trials and Hair Loss. In Alopecia; Miteva, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–284. [Google Scholar]
- Burton, J.L.; Marshall, A. Hypertrichosis due to minoxidil. Br. J. Dermatol. 1979, 101, 593–595. [Google Scholar] [CrossRef]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.D. Androgens and alopecia. Mol. Cell. Endocrinol. 2002, 198, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Hibberts, N.; Howell, A.; Randall, V. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J. Endocrinol. 1998, 156, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 1725–1736. [Google Scholar] [CrossRef] [Green Version]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Brit. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates beta-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef]
- Michelet, J.F.; Commo, S.; Billoni, N.; Mahe, Y.F.; Bernard, B.A. Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. J. Investig. Dermatol. 1997, 108, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.M.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.A.; Kim, G.S.; Lee, J.E.; Park, S.; Yi, S.; Lee, S.J.; Kim, J.H.; Jin, J.S.; Abd El-Aty, A.M.; Shim, J.H.; et al. Flavonoid profiles of immature and mature fruit tissues of Citrus grandis Osbeck (Dangyuja) and overall contribution to the antioxidant effect. Biomed. Chromatogr. 2015, 29, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.J.; Han, S.C.; Yi, E.J.; Kang, H.K.; Yoo, E.S. The Inhibitory Effect of Premature Citrus unshiu Extract on Atopic Dermatitis In Vitro and In Vivo. Toxicol. Res. 2011, 27, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Tamaru, E.; Watanabe, M.; Nomura, Y. Dietary immature Citrus unshiu alleviates UVB- induced photoaging by suppressing degradation of basement membrane in hairless mice. Heliyon 2020, 6, e04218. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Yang, H.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Limonoids from Dictamnus dasycarpus protect against glutamate-induced toxicity in primary cultured rat cortical cells. J. Mol. Neurosci. 2010, 42, 9–16. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Kumar, V.; Rathore, K.S.; Patil, B.S. Citrus Limonin and Its Glucoside Inhibit Colon Adenocarcinoma Cell Proliferation through Apoptosis. J. Agr. Food Chem. 2011, 59, 2314–2323. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Hamdan, D.; Farrag, N.; El-Shazly, A.; Wink, M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur. J. Pharmacol. 2010, 626, 139–145. [Google Scholar] [CrossRef]
- Ohnemus, U.; Unalan, M.; Handjiski, B.; Paus, R. Topical estrogen accelerates hair regrowth in mice after chemotherapy-induced alopecia by favoring the dystrophic catagen response pathway to damage. J. Investig. Dermatol. 2004, 122, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Olah, A.; Gherardini, J.; Bertolini, M.; Cheret, J.; Ponce, L.; Kloepper, J.; Biro, T.; Soeberdt, M.; Abels, C.; Paus, R. The Thyroid Hormone Analogue KB2115 (Eprotirome) Prolongs Human Hair Growth (Anagen) Ex Vivo. J. Investig. Dermatol. 2016, 136, 1711–1714. [Google Scholar] [CrossRef] [Green Version]
- Jahoda, C.A.; Horne, K.A.; Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984, 311, 560–562. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, J.; Fu, D.; Liu, Z.; Wang, H.; Wang, J.; Qu, Q.; Li, K.; Fan, Z.; Hu, Z.; et al. Transcriptome Analysis Reveals an Inhibitory Effect of Dihydrotestosterone-Treated 2D- and 3D-Cultured Dermal Papilla Cells on Hair Follicle Growth. Front. Cell Dev. Biol. 2021, 9, 724310. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Kobayashi, T.; Sasaki, T.; Shimizu, A.; Amagai, M. Restoration of the intrinsic properties of human dermal papilla in vitro. J. Cell Sci. 2012, 125, 4114–4125. [Google Scholar] [CrossRef] [Green Version]
- Abbastabar, M.; Kheyrollah, M.; Azizian, K.; Bagherlou, N.; Tehrani, S.S.; Maniati, M.; Karimian, A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: A double-edged sword protein. DNA Repair 2018, 69, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, E.J.; Kim, M.K.; Jeon, Y.J.; Kang, S.M.; Koh, Y.S.; Yoo, E.S.; Kang, H.K. The promoting effect of Ishige sinicola on hair growth. Mar. Drugs 2013, 11, 1783–1799. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Kang, J.I.; Park, D.B.; Lee, Y.K.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S.; Kim, J.A.; Kim, Y.H.; Kang, H.K. Promotion effect of acankoreoside J, a lupane-triterpene in Acanthopanax koreanum, on hair growth. Arch. Pharm. Res. 2012, 35, 1495–1503. [Google Scholar] [CrossRef]
- Fujita, N.; Sato, S.; Katayama, K.; Tsuruo, T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 2002, 277, 28706–28713. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Li, W.; Man, X.Y.; Li, C.M.; Chen, J.Q.; Zhou, J.; Cai, S.Q.; Lu, Z.F.; Zheng, M. VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.M.; Wang, X.X.; Mo, M.H.; Zeng, S.B.; Xu, R.H.; Wang, X.S.; Wu, Y.J. PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration. Stem Cell Res. Ther. 2020, 11, 144. [Google Scholar] [CrossRef]
- Tanizawa, H.; Ohkawa, Y.; Takino, Y.; Miyase, T.; Ueno, A.; Kageyama, T.; Hara, S. Studies on natural antioxidants in citrus species. I. Determination of antioxidative activities of citrus fruits. Chem. Pharm. Bull. 1992, 40, 1940–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amelio, I.; Lena, A.M.; Bonanno, E.; Melino, G.; Candi, E. miR-24 affects hair follicle morphogenesis targeting Tcf-3. Cell Death Dis. 2013, 4, e922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prall, O.W.; Sarcevic, B.; Musgrove, E.A.; Watts, C.K.; Sutherland, R.L. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J. Biol. Chem. 1997, 272, 10882–10894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.Y.; Boo, H.J.; Kang, J.I.; Kim, M.K.; Yoo, E.S.; Hyun, J.W.; Koh, Y.S.; Kim, G.Y.; Maeng, Y.H.; Hyun, C.L.; et al. (1S,2S,3E,7E,11E)-3,7,11,15-Cembratetraen-17,2-olide, a cembrenolide diterpene from soft coral Lobophytum sp., inhibits growth and induces apoptosis in human colon cancer cells through reactive oxygen species generation. Biol. Pharm. Bull. 2012, 35, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.I.; Choi, Y.K.; Koh, Y.S.; Hyun, J.W.; Kang, J.H.; Lee, K.S.; Lee, C.M.; Yoo, E.S.; Kang, H.K. Vanillic Acid Stimulates Anagen Signaling via the PI3K/Akt/ β-Catenin Pathway in Dermal Papilla Cells. Biomol. Ther. 2020, 28, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Sharov, A.A.; Sharova, T.Y.; Mardaryev, A.N.; Tommasi di Vignano, A.; Atoyan, R.; Weiner, L.; Yang, S.; Brissette, J.L.; Dotto, G.P.; Botchkarev, V.A. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc. Natl. Acad. Sci. USA 2006, 103, 18166–18171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, K.; Wang, G.; Yang, L.; Qu, Q.; Fan, Z.; Sun, Y.; Huang, J.; Miao, Y.; Hu, Z. Impairment of autophagy may be associated with follicular miniaturization in androgenetic alopecia by inducing premature catagen. J. Dermatol. 2021, 48, 289–300. [Google Scholar] [CrossRef]
- Vujic, N.; Bradic, I.; Goeritzer, M.; Kuentzel, K.B.; Rainer, S.; Kratky, D.; Radovic, B. ATG7 is dispensable for LC3-PE conjugation in thioglycolate-elicited mouse peritoneal macrophages. Autophagy 2021, 17, 3402–3407. [Google Scholar] [CrossRef]
- Chai, M.; Jiang, M.; Vergnes, L.; Fu, X.; de Barros, S.C.; Doan, N.B.; Huang, W.; Chu, J.; Jiao, J.; Herschman, H.; et al. Stimulation of Hair Growth by Small Molecules that Activate Autophagy. Cell Rep. 2019, 27, 3413–3421.e3413. [Google Scholar] [CrossRef] [Green Version]
- Frudd, K.; Burgoyne, T.; Burgoyne, J.R. Oxidation of Atg3 and Atg7 mediates inhibition of autophagy. Nat. Commun. 2018, 9, 95. [Google Scholar] [CrossRef]
- Hall, M.N. mTOR-what does it do? Transpl. Proc. 2008, 40, S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Ber, Y.; Shiloh, R.; Gilad, Y.; Degani, N.; Bialik, S.; Kimchi, A. DAPK2 is a novel regulator of mTORC1 activity and autophagy. Cell Death Differ. 2015, 22, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling and cancer. Genes Dev. 2000, 14, 1837–1851. [Google Scholar] [CrossRef]
- Hino, S.; Tanji, C.; Nakayama, K.I.; Kikuchi, A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 2005, 25, 9063–9072. [Google Scholar] [CrossRef] [Green Version]
- Monick, M.M.; Carter, A.B.; Robeff, P.K.; Flaherty, D.M.; Peterson, M.W.; Hunninghake, G.W. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J. Immunol. 2001, 166, 4713–4720. [Google Scholar] [CrossRef] [Green Version]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Kwack, M.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dickkopf 1 promotes regression of hair follicles. J. Investig. Dermatol. 2012, 132, 1554–1560. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.S.; Pyo, H.K.; Oh, Y.J.; Han, J.H.; Lee, S.R.; Chung, J.H.; Eun, H.C.; Kim, K.H. Promotive effect of minoxidil combined with all-trans retinoic acid (tretinoin) on human hair growth in vitro. J. Korean Med. Sci. 2007, 22, 283–289. [Google Scholar] [CrossRef] [PubMed]
| Antibodies | Species | Dilution | Supplier |
|---|---|---|---|
| Cyclin D1 | Mouse | 1:1000 | BD Biosciences |
| p27 | Rabbit | 1:1000 | Santa Cruz |
| ATG7 | Rabbit | 1:1000 | Cell Signaling |
| LC3A/B | Rabbit | 1:1000 | Cell Signaling |
| LC3B | Rabbit | 1:1000 | Abcam |
| BrdU | Mouse | 1:200 | Thermo Fisher Scientific |
| Phospho(Ser2448)-mTOR | Rabbit | 1:1000 | Cell Signaling |
| mTOR | Rabbit | 1:1000 | Cell Signaling |
| Phospho(Ser792)-Raptor | Rabbit | 1:1000 | Cell Signaling |
| Raptor | Rabbit | 1:1000 | Cell Signaling |
| Phospho(Ser473)-Akt | Rabbit | 1:1000 | Cell Signaling |
| Akt | Rabbit | 1:1000 | Cell Signaling |
| Phospho(Ser552)-β-catenin | Rabbit | 1:1000 | Cell Signaling |
| Phospho(Ser675)-β-catenin | Rabbit | 1:1000 | Cell Signaling |
| β-catenin | Rabbit | 1:1000 | Cell Signaling |
| α-Tubulin | Mouse | 1:1000 | Santa Cruz |
| β-Actin | Mouse | 1:1000 | Santa Cruz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-I.; Choi, Y.K.; Han, S.-C.; Kim, H.G.; Hong, S.W.; Kim, J.; Kim, J.H.; Hyun, J.W.; Yoo, E.-S.; Kang, H.-K. Limonin, a Component of Immature Citrus Fruits, Activates Anagen Signaling in Dermal Papilla Cells. Nutrients 2022, 14, 5358. https://doi.org/10.3390/nu14245358
Kang J-I, Choi YK, Han S-C, Kim HG, Hong SW, Kim J, Kim JH, Hyun JW, Yoo E-S, Kang H-K. Limonin, a Component of Immature Citrus Fruits, Activates Anagen Signaling in Dermal Papilla Cells. Nutrients. 2022; 14(24):5358. https://doi.org/10.3390/nu14245358
Chicago/Turabian StyleKang, Jung-Il, Youn Kyoung Choi, Sang-Chul Han, Hyeon Gyu Kim, Seok Won Hong, Jungeun Kim, Jae Hoon Kim, Jin Won Hyun, Eun-Sook Yoo, and Hee-Kyoung Kang. 2022. "Limonin, a Component of Immature Citrus Fruits, Activates Anagen Signaling in Dermal Papilla Cells" Nutrients 14, no. 24: 5358. https://doi.org/10.3390/nu14245358





