The Influence of Nutrition and Physical Activity on Exercise Performance after Mild COVID-19 Infection in Endurance Athletes-CESAR Study
Abstract
1. Introduction
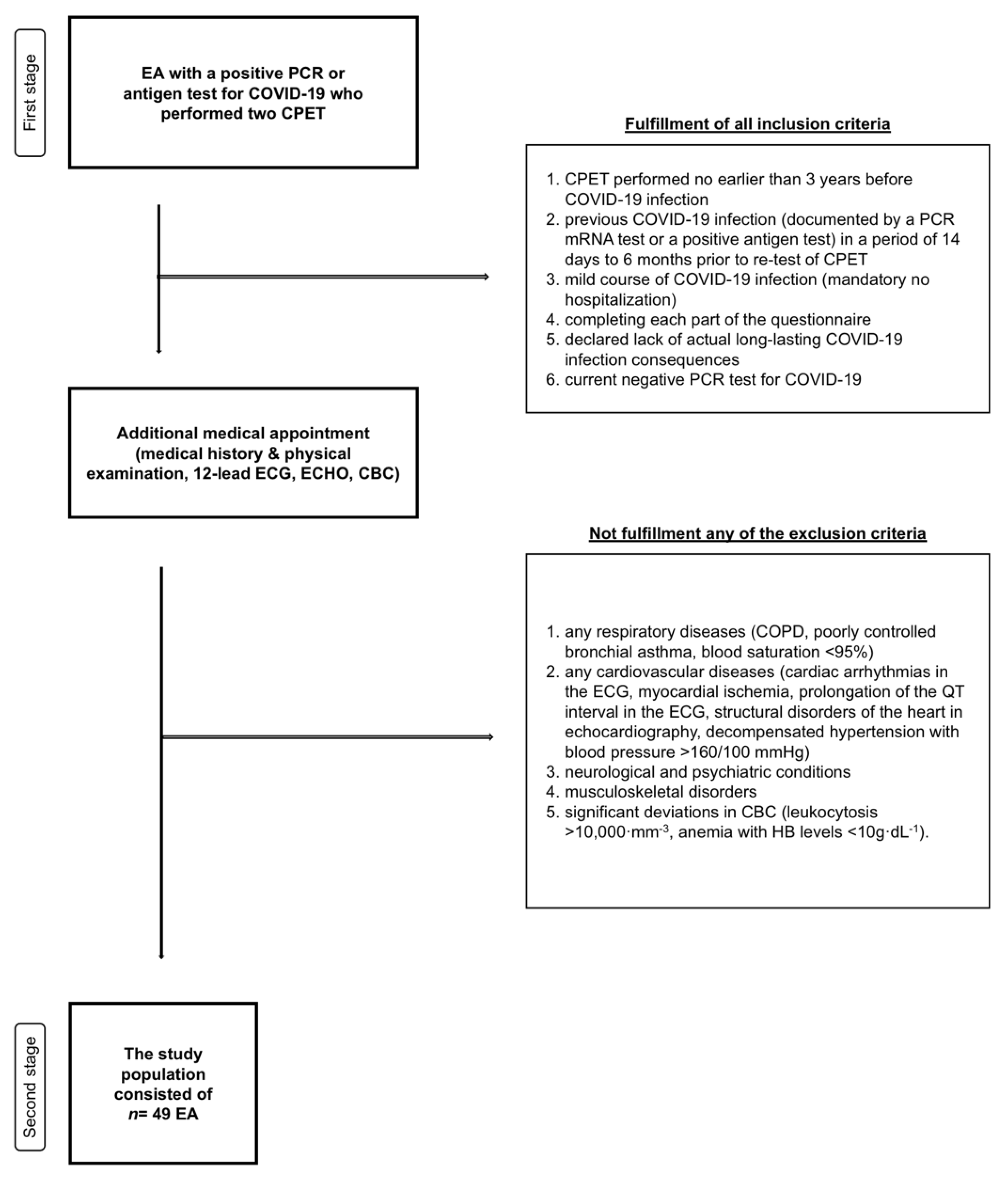
2. Materials and Methods
2.1. Study Design
2.2. Survey Tool
2.3. CPET and Somatic Measurements
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Basic Population Data
3.2. CPET Characteristics
3.3. Nutrition and Eating Habits
3.4. Physical Activity
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsang, H.F.; Chan, L.W.C.; Cho, W.C.S.; Yu, A.C.S.; Yim, A.K.Y.; Chan, A.K.C.; Ng, L.P.W.; Wong, Y.K.E.; Pei, X.M.; Li, M.J.W.; et al. An update on COVID-19 pandemic: The epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev. Anti Infect. Ther. 2021, 19, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, K.; Jodczyk, A.M.; Dlugolecki, P.; Emerla, S.; Stanska, W.; Kasiak, P.S.; Gasior, J.S.; Parol, D.; Mamcarz, A.; Sliz, D. Factors Associated with Willingness to Receive a COVID-19 Vaccine in Adult Polish Population-A Cross-Sectional Survey. Vaccines 2022, 10, 1715. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Bhola, S.; Thakur, P.; Patel, S.K.S.; Kulshrestha, S.; Ratho, R.K.; Kumar, P. Waves and variants of SARS-CoV-2: Understanding the causes and effect of the COVID-19 catastrophe. Infection 2022, 50, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Balanzá-Martínez, V.; Kapczinski, F.; de Azevedo Cardoso, T.; Atienza-Carbonell, B.; Rosa, A.R.; Mota, J.C.; De Boni, R.B. The assessment of lifestyle changes during the COVID-19 pandemic using a multidimensional scale. Rev. Psiquiatr. Salud. Ment. 2021, 14, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Romero-Blanco, C.; Rodríguez-Almagro, J.; Onieva-Zafra, M.D.; Parra-Fernández, M.L.; Prado-Laguna, M.D.C.; Hernández-Martínez, A. Physical Activity and Sedentary Lifestyle in University Students: Changes during Confinement Due to the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 6567. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Santos, L. The impact of nutrition and lifestyle modification on health. Eur. J. Intern. Med. 2022, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Rishi, P.; Thakur, K.; Vij, S.; Rishi, L.; Singh, A.; Kaur, I.P.; Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Diet, Gut Microbiota and COVID-19. Indian J. Microbiol. 2020, 60, 420–429. [Google Scholar] [CrossRef]
- Fikenzer, S.; Fikenzer, K.; Laufs, U.; Falz, R.; Pietrek, H.; Hepp, P. Impact of COVID-19 lockdown on endurance capacity of elite handball players. J. Sports Med. Phys. Fit. 2021, 61, 977–982. [Google Scholar] [CrossRef]
- Krzywański, J.; Mikulski, T.; Krysztofiak, H.; Pokrywka, A.; Sobierajski, T.; Młyńczak, M.; Piechuta, A.; Kuchar, E. Vaccine versus infection—COVID-19-related loss of training time in elite athletes. J. Sci. Med. Sport 2022, 25, 950–959. [Google Scholar] [CrossRef]
- Gervasi, S.F.; Pengue, L.; Damato, L.; Monti, R.; Pradella, S.; Pirronti, T.; Bartoloni, A.; Epifani, F.; Saggese, A.; Cuccaro, F.; et al. Is extensive cardiopulmonary screening useful in athletes with previous asymptomatic or mild SARS-CoV-2 infection? Br. J. Sports Med. 2021, 55, 54–61. [Google Scholar] [CrossRef]
- Roberts, C.; Gill, N.; Sims, S. The Influence of COVID-19 Lockdown Restrictions on Perceived Nutrition Habits in Rugby Union Players. Front. Nutr. 2020, 7, 589737. [Google Scholar] [CrossRef] [PubMed]
- Firoozjah, M.H.; Shahrbanian, S.; Homayouni, A.; Hower, H. Comparison of eating disorders symptoms and body image between individual and team sport adolescent athletes during the COVID-19 pandemic. J. Eat. Disord. 2022, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Kasiak, P.S.; Adamczyk, N.; Jodczyk, A.M.; Kaproń, A.; Lisowska, A.; Mamcarz, A.; Śliż, D. COVID-19 Pandemic Consequences among Individuals with Eating Disorders on a Clinical Sample in Poland-A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 8484. [Google Scholar] [CrossRef]
- Abdel Moneim, A.; Radwan, M.A.; Yousef, A.I. COVID-19 and cardiovascular disease: Manifestations, pathophysiology, vaccination, and long-term implication. Curr. Med. Res. Opin. 2022, 38, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef]
- Naeije, R.; Caravita, S. CPET for Long COVID-19. JACC Heart Fail. 2022, 10, 214–215. [Google Scholar] [CrossRef]
- Wiecha, S.; Price, S.; Cieśliński, I.; Kasiak, P.S.; Tota, Ł.; Ambroży, T.; Śliż, D. Transferability of Cardiopulmonary Parameters between Treadmill and Cycle Ergometer Testing in Male Triathletes-Prediction Formulae. Int. J. Environ. Res. Public Health 2022, 19, 1830. [Google Scholar] [CrossRef]
- Moulson, N.; Gustus, S.K.; Scirica, C.; Petek, B.J.; Vanatta, C.; Churchill, T.W.; Guseh, J.S.; Baggish, A.; Wasfy, M.M. Diagnostic evaluation and cardiopulmonary exercise test findings in young athletes with persistent symptoms following COVID-19. Br. J. Sports Med. 2022, 56, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of Cardiopulmonary Stress Testing for Patients With Unexplained Dyspnea Post-Coronavirus Disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Kysel, P.; Haluzíková, D.; Doležalová, R.P.; Laňková, I.; Lacinová, Z.; Kasperová, B.J.; Trnovská, J.; Hrádková, V.; Mráz, M.; Vilikus, Z.; et al. The Influence of Cyclical Ketogenic Reduction Diet vs. Nutritionally Balanced Reduction Diet on Body Composition, Strength, and Endurance Performance in Healthy Young Males: A Randomized Controlled Trial. Nutrients 2020, 12, 2832. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Wiecha, S.; Cieśliński, I.; Śliż, D.; Kasiak, P.S.; Lach, J.; Gruba, G.; Kowalski, T.; Mamcarz, A. Differences between Treadmill and Cycle Ergometer Cardiopulmonary Exercise Testing Results in Triathletes and Their Association with Body Composition and Body Mass Index. Int. J. Environ. Res. Public Health 2022, 19, 3557. [Google Scholar] [CrossRef] [PubMed]
- Gruba, G.; Kasiak, P.S.; Gębarowska, J.; Adamczyk, N.; Sikora, Z.; Jodczyk, A.M.; Mamcarz, A.; Śliż, D. PaLS Study of Sleep Deprivation and Mental Health Consequences of the COVID-19 Pandemic among University Students: A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2021, 18, 9581. [Google Scholar] [CrossRef]
- Jodczyk, A.M.; Gruba, G.; Sikora, Z.; Kasiak, P.S.; Gębarowska, J.; Adamczyk, N.; Mamcarz, A.; Śliż, D. PaLS Study: How Has the COVID-19 Pandemic Influenced Physical Activity and Nutrition? Observations a Year after the Outbreak of the Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 9632. [Google Scholar] [CrossRef]
- Jodczyk, A.M.; Kasiak, P.S.; Adamczyk, N.; Gebarowska, J.; Sikora, Z.; Gruba, G.; Mamcarz, A.; Sliz, D. PaLS Study: Tobacco, Alcohol and Drugs Usage among Polish University Students in the Context of Stress Caused by the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 1261. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl Physiol (1985) 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Singh, I.; Joseph, P.; Heerdt, P.M.; Cullinan, M.; Lutchmansingh, D.D.; Gulati, M.; Possick, J.D.; Systrom, D.M.; Waxman, A.B. Persistent Exertional Intolerance After COVID-19: Insights From Invasive Cardiopulmonary Exercise Testing. Chest 2022, 161, 54–63. [Google Scholar] [CrossRef]
- Mercier, J.; Grosbois, J.M.; Préfaut, C. Interpreting exercise tests. Rev. Pneumol Clin. 1997, 53, 289–296. [Google Scholar] [PubMed]
- Milovancev, A.; Avakumovic, J.; Lakicevic, N.; Stajer, V.; Korovljev, D.; Todorovic, N.; Bianco, A.; Maksimovic, N.; Ostojic, S.; Drid, P. Cardiorespiratory Fitness in Volleyball Athletes Following a COVID-19 Infection: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 4059. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, G.A.; Torres-Castro, R.; Intelangelo, L.; Vila Ortiz, B.; Lista-Paz, A. Does COVID-19 Affect the Exercise Capacity of Non-hospitalized Patients? Cureus 2021, 13, e18135. [Google Scholar] [CrossRef] [PubMed]
- Goergen, J.; Bavishi, A.; Eimer, M.; Zielinski, A.R. COVID-19: The Risk to Athletes. Curr. Treat. Options Cardiovasc. Med. 2021, 23, 68. [Google Scholar] [CrossRef]
- Cassar, M.P.; Tunnicliffe, E.M.; Petousi, N.; Lewandowski, A.J.; Xie, C.; Mahmod, M.; Samat, A.H.A.; Evans, R.A.; Brightling, C.E.; Ho, L.P.; et al. Symptom Persistence Despite Improvement in Cardiopulmonary Health—Insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine 2021, 41, 101159. [Google Scholar] [CrossRef]
- Barbagelata, L.; Masson, W.; Iglesias, D.; Lillo, E.; Migone, J.F.; Orazi, M.L.; Maritano Furcada, J. Cardiopulmonary Exercise Testing in Patients with Post-COVID-19 Syndrome. Med. Clin. 2022, 159, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, O.V.; Rorat, M.; Barg, W. Oxygen metabolism markers as predictors of mortality in severe COVID-19. Int. J. Infect. Dis. 2021, 103, 452–456. [Google Scholar] [CrossRef]
- Ahmed, I. COVID-19—Does exercise prescription and maximal oxygen uptake (VO(2) max) have a role in risk-stratifying patients? Clin. Med. 2020, 20, 282–284. [Google Scholar] [CrossRef]
- Pal, R.; Singh, S.N.; Chatterjee, A.; Saha, M. Age-related changes in cardiovascular system, autonomic functions, and levels of BDNF of healthy active males: Role of yogic practice. Age 2014, 36, 9683. [Google Scholar] [CrossRef]
- Komici, K.; Bianco, A.; Perrotta, F.; Dello Iacono, A.; Bencivenga, L.; D’Agnano, V.; Rocca, A.; Bianco, A.; Rengo, G.; Guerra, G. Clinical Characteristics, Exercise Capacity and Pulmonary Function in Post-COVID-19 Competitive Athletes. J. Clin. Med. 2021, 10, 3053. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J. Nutritional aspects of endurance exercise in humans. Proc. Nutr. Soc. 1994, 53, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Greenhaff, P.L.; Leiper, J.B.; Ball, D.; Lambert, C.P.; Gleeson, M. Diet composition and the performance of high-intensity exercise. J. Sports Sci. 1997, 15, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M. Nutritional aspects in ultra-endurance exercise. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Csulak, E.; Petrov, Á.; Kováts, T.; Tokodi, M.; Lakatos, B.; Kovács, A.; Staub, L.; Suhai, F.I.; Szabó, E.L.; Dohy, Z.; et al. The Impact of COVID-19 on the Preparation for the Tokyo Olympics: A Comprehensive Performance Assessment of Top Swimmers. Int. J. Environ. Res. Public Health 2021, 18, 9770. [Google Scholar] [CrossRef]
- Lodi, E.; Scavone, A.; Carollo, A.; Guicciardi, C.; Reggianini, L.; Savino, G.; Modena, M.G. Return to sport after the COVID-19 pandemic. How to behave? G. Ital. Cardiol. 2020, 21, 514–522. [Google Scholar] [CrossRef]
- Dove, J.; Gage, A.; Kriz, P.; Tabaddor, R.R.; Owens, B.D. COVID-19 and Review of Current Recommendations for Return to Athletic Play. Rhode Isl. Med. J. 2020, 103, 15–20. [Google Scholar]
- Haddad, M.; Abbes, Z.; Mujika, I.; Chamari, K. Impact of COVID-19 on Swimming Training: Practical Recommendations during Home Confinement/Isolation. Int. J. Environ. Res. Public Health 2021, 18, 4767. [Google Scholar] [CrossRef]
- Heuberger, J.; Gal, P.; Stuurman, F.E.; de Muinck Keizer, W.A.S.; Mejia Miranda, Y.; Cohen, A.F. Repeatability and predictive value of lactate threshold concepts in endurance sports. PLoS ONE 2018, 13, e0206846. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010-2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. A defined, plant-based diet utilized in an outpatient cardiovascular clinic effectively treats hypercholesterolemia and hypertension and reduces medications. Clin. Cardiol 2018, 41, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Monnard, C.R.; Dulloo, A.G. Polyunsaturated fatty acids as modulators of fat mass and lean mass in human body composition regulation and cardiometabolic health. Obes. Rev. 2021, 22 (Suppl. 2), e13197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shirai, N.; Suzuki, H. Relationship between the effect of dietary fat on swimming endurance and energy metabolism in aged mice. Ann. Nutr. Metab. 2011, 58, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Rust, P.; Ekmekcioglu, C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. Adv. Exp. Med. Biol. 2017, 956, 61–84. [Google Scholar] [CrossRef]
- Veniamakis, E.; Kaplanis, G.; Voulgaris, P.; Nikolaidis, P.T. Effects of Sodium Intake on Health and Performance in Endurance and Ultra-Endurance Sports. Int. J. Environ. Res. Public Health 2022, 19, 3651. [Google Scholar] [CrossRef]
- Barrea, L.; Grant, W.B.; Frias-Toral, E.; Vetrani, C.; Verde, L.; de Alteriis, G.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Dietary Recommendations for Post-COVID-19 Syndrome. Nutrients 2022, 14, 1305. [Google Scholar] [CrossRef]
| Variable | Males (n = 43) | Females (n = 6) | |
|---|---|---|---|
| Age (years) | 40.7 ± 7.0 | 38.1 ± 6.4 | |
| Height (cm) | 178.5 ± 6.8 | 178.4 ± 6.9 | |
| CPET modality | Treadmill | 25 (51.0%) | 4 (8.2%) |
| Cycle ergometer | 16 (32.7%) | 2 (4.1%) | |
| Primary sport discipline | Running | 16 (32.7%) | 4 (8.2%) |
| Cycling | 13 (26.5%) | 1 (2.0%) | |
| Other | 14 (28.6%) | 1 (2.0%) | |
| Waived competition due to COVID-19 infection | Yes | 21 (42.9%) | 2 (4.1%) |
| No | 23 (46.9%) | 4 (8.2%) | |
| Pre-COVID-19 | Post-COVID-19 | p-value | |
| Weight (kg) | 76.6 ± 10.0 | 76.7 ± 10.9 | 0.951 |
| BMI (kg·m−2) | 24.0 ± 2.5 | 24.0 ± 2.7 | 0.931 |
| FFM (kg) | 63.4 ± 7.6 | 63.5 ± 8.0 | 0.774 |
| BF (%) | 17.1 ± 4.7 | 16.9 ± 5.1 | 0.604 |
| FATM (kg) | 13.3 ± 4.7 | 13.2 ± 5.2 | 0.848 |
| Variable | Pre-COVID-19 | Post-COVID-19 | p-Value |
|---|---|---|---|
| VO2AT (mL·kg·min−1) | 35.0 ± 6.5 | 32.4 ± 6.0 | <0.001 |
| VO2Ata (mL·min−1) | 2650.0 ± 470.9 | 2446.1 ± 400.3 | <0.001 |
| HRAT (beats·min−1) | 145.1 ± 10.9 | 141.1 ± 10.1 | 0.001 |
| VEAT (L·min−1) | 70.8 ± 18.7 | 68.1 ± 14.7 | 0.090 |
| SAT (km·h−1) | 11.4 ± 1.4 | 11.1 ± 1.3 | 0.044 |
| PAT (Watts) | 162.8 ± 25.9 | 154.8 ± 25.9 | 0.066 |
| fRAT (breaths·min−1) | 32.1 ± 9.0 | 32.1 ± 8.1 | 0.706 |
| LacAT (mmol·L−1) | 2.0 ± 0.9 | 2.1 ± 0.9 | 0.630 |
| VO2RCP (mL·kg·min−1) | 43.9 ± 7.4 | 40.5 ± 6.7 | <0.001 |
| VO2RCPa (mL·min−1) | 3324.3 ± 512.9 | 3063.7 ± 440.1 | <0.001 |
| HRRCP (beats·min−1) | 168.8 ± 9.2 | 165.1 ± 9.8 | <0.001 |
| VERCP (L·min−1) | 106.8 ± 21.7 | 98.9 ± 18.3 | <0.001 |
| SRCP (km·h−1) | 14.3 ± 1.9 | 13.8 ± 1.5 | <0.001 |
| PRCP (Watts) | 245.2 ± 42.0 | 232.2 ± 39.7 | 0.061 |
| fRRCP (breaths·min−1) | 41.3 ± 8.7 | 40.1 ± 8.9 | 0.876 |
| LacRCP (mmol·L−1) | 4.9 ± 1.4 | 4.3 ± 1.1 | 0.013 |
| VO2max (mL·kg·min−1) | 47.8 ± 8.0 | 45.0 ± 7.1 | <0.001 |
| VO2maxa (mL·min−1) | 3623.5 ± 552.1 | 3406.0 ± 474.5 | <0.001 |
| HRmax (beats·min−1) | 180.8 ± 10.1 | 179.8 ± 10.0 | 0.273 |
| VEmax (L·min−1) | 143.0 ± 26.9 | 138.50 ± 23.9 | 0.068 |
| Smax (km·h−1) | 16.6 ± 1.6 | 16.4 ± 1.7 | 0.264 |
| Pmax (Watts) | 310.0 ± 37.2 | 312.2 ± 49.1 | 0.811 |
| fRmax (breaths·min−1) | 58.9 ± 14.4 | 57.3 ± 11.0 | 0.959 |
| Lacmax (mmol·L−1) | 9.7 ± 2.3 | 9.6 ± 2.4 | 0.880 |
| Question | Frequency | Lack of Answer | |||
|---|---|---|---|---|---|
| Once a Week or Less Often | 2–3 Times per Week | Majority of Days Within a Week | Every Day | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Consuming more meals per day than before the pandemic (including snacking) | 32 (65.3%) | 7 (14.3%) | 7 (14.3%) | 0 (0.0%) | 3 (6.1%) |
| Consuming less than 3 servings of wholegrain products daily (less than 90 g/day) | 20 (40.8%) | 18 (36.7%) | 6 (12.2%) | 2 (4.1%) | 3 (6.1%) |
| Consuming less than 400 g vegetables and fruits | 22 (44.9) | 11 (22.5%) | 11 (22.5%) | 2 (4.1%) | 3 (6.122%) |
| Consuming less than 2 glasses of unsweetened milk or other dairy products daily | 24 (49.0%) | 9 (18.4%) | 8 (16.3%) | 5 (10.2%) | 3 (6.1%) |
| Consuming products containing processed meat, (such as sausages, ham, frankfurters, etc.) | 20 (40.8%) 10.9 for Lacmax | 15 (30.6%) 16.6 for Lacmax | 8 (16.3%)20.1 for Lacmax | 2 (4.1%)4.5 for Lacmax | 4 (8.2%) |
| Replacing meat with protein-rich plant products such as nuts and legumes: beans, chickpeas, soy, lentils, fava beans, peas | 17 (34.7%) 19.1 for HRmax 10.9 for Lacmax | 14 (28.6%) 27.3 for HRmax 18.1 for Lacmax | 8 (16.3%)32.4 for HRmax19.2 for Lacmax | 7 (14.3%)16.3 for HRmax7.1 for Lacmax | 3 (6.1%) |
| Consuming products that aresources of animal fats or trans fatty acids present in products, such as pastries, candy bars, salty snacks, and fast-food products | 24 (49.00%) | 12 (24.5%) | 7 (14.3%) | 2 (4.1%) | 4 (8.2%) |
| Consuming products that are sources of unsaturated fatty acids, such as canola oil, olive oil, or fish | 9 (18.4%) 35.2 for FFM | 20 (40.8%) 19.6 for FFM | 11 (22.5%)21.1 for FFM | 6 (12.2%)23.4 for FFM | 3 (6.1%) |
| Drinking sweetened beverages or fruit juices instead of water | 40 (81.6%) | 5 (10.2%) | 2 (4.1%) | 0 (0.0%) | 2 (4.1%) |
| Adding salt to meals | 27 (55.1%) 26.7 for Smax/Pmax 21.0 for fRRCP | 9 (18.4%) 25.2 for Smax/Pmax 37.7 for fRRCP | 6 (12.2%)17.5 for Smax/Pmax20.5 for fRRCP | 4 (8.2%)7.1 for Smax/Pmax13.0 for fRRCP | 3 (6.1%) |
| Consuming meals while looking at the screen of a TV, computer, or other device | 21 (32.9%) | 12 (24.5%) | 9 (18.4%) | 4 (8.2%) | 3 (6.1%) |
| Paying attention to labels of chosen products during shopping, taking into account ingredients, amount of calories, etc. | 10 (20.4%) | 6 (12.2%) | 11 (22.4%) | 19 (38.8%) | 3 (6.1%) |
| Self-assessed impact of COVID-19 pandemic and imposed restrictions on nutrition and dietary habits [in −5/0/+5 scale] | 0.09 ± 1.43 | 0 (0.0%) | |||
| CPET Variable | Survey Question | p-Value |
|---|---|---|
| Lacmax | How often did you eat processed meat products? | 0.030 |
| HRmax | How often did you replace meat with plant-based protein products? | 0.036 |
| Lacmax | How often did you replace meat with plant-based protein products? | 0.035 |
| FFM | How often unsaturated fats were the main source of fat in your diet? | 0.031 |
| Smax/Pmax | How often did you add salt to your meals? | 0.024 |
| fRRCP | How often did you add salt to your meals? | 0.033 |
| Type of Activity | Amount | Median (IQR) |
|---|---|---|
| Vigorous physical activity | Days/week | 3 (2.8–5) |
| Minutes/week | 60 (43.8–90) | |
| Moderate physical activity | Days/week | 3 (2–4.3) |
| Minutes/week | 50 (30–90) | |
| Walking | Days/week | 6.5 (4–7) |
| Minutes/week | 40 (20–60) | |
| Sitting time | Hours/day | 8 (5–9.3) |
| Weekly running distance (question only for runners) | 25 (0–50) | |
| Weekly cycling distance (question for cyclists) | 70 (3.75–200) | |
| Self-assessed fitness level in pre-COVID-19 [in 0–10 scale] | 8 (6–9) | |
| Self-assessed fitness level post-COVID-19 [in 0–10 scale] | 6 (4.8–8) | |
| Self-assessed impact of COVID-19 pandemic and imposed restrictions on fitness level [in −5/0/+5 scale] | −1 (−3–0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliż, D.; Wiecha, S.; Gąsior, J.S.; Kasiak, P.S.; Ulaszewska, K.; Postuła, M.; Małek, Ł.A.; Mamcarz, A. The Influence of Nutrition and Physical Activity on Exercise Performance after Mild COVID-19 Infection in Endurance Athletes-CESAR Study. Nutrients 2022, 14, 5381. https://doi.org/10.3390/nu14245381
Śliż D, Wiecha S, Gąsior JS, Kasiak PS, Ulaszewska K, Postuła M, Małek ŁA, Mamcarz A. The Influence of Nutrition and Physical Activity on Exercise Performance after Mild COVID-19 Infection in Endurance Athletes-CESAR Study. Nutrients. 2022; 14(24):5381. https://doi.org/10.3390/nu14245381
Chicago/Turabian StyleŚliż, Daniel, Szczepan Wiecha, Jakub S. Gąsior, Przemysław Seweryn Kasiak, Katarzyna Ulaszewska, Marek Postuła, Łukasz A. Małek, and Artur Mamcarz. 2022. "The Influence of Nutrition and Physical Activity on Exercise Performance after Mild COVID-19 Infection in Endurance Athletes-CESAR Study" Nutrients 14, no. 24: 5381. https://doi.org/10.3390/nu14245381
APA StyleŚliż, D., Wiecha, S., Gąsior, J. S., Kasiak, P. S., Ulaszewska, K., Postuła, M., Małek, Ł. A., & Mamcarz, A. (2022). The Influence of Nutrition and Physical Activity on Exercise Performance after Mild COVID-19 Infection in Endurance Athletes-CESAR Study. Nutrients, 14(24), 5381. https://doi.org/10.3390/nu14245381









