Combined Phyllostachys pubescens and Scutellaria baicalensis Prevent High-Fat Diet-Induced Obesity via Upregulating Thermogenesis and Energy Expenditure by UCP1 in Male C57BL/6J Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction
2.2. Obesity Induction and BS21 Administration in Animals
2.3. Collection of Blood, Liver, Muscle, and Brown or White Adipose Tissues
2.4. Serum Biochemical Analyses and ELISA
2.5. Liver TG Level Measurement
2.6. Histological Analyses of Liver and Adipose Tissues
2.7. RNA Isolation and Real-Time PCR Analysis
2.8. Cold Tolerance Test
2.9. Immunofluorescence Staining of UCP1
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Effects of BS21 on Body Weight, Body Weight Gain, Food Intake, and FER
3.2. Effects of BS21 on Serum Biochemical Parameters
3.3. Serum Leptin, Adiponectin, and Insulin Growth Factor (IGF) 1 Levels
3.4. Adipose Tissue Weight
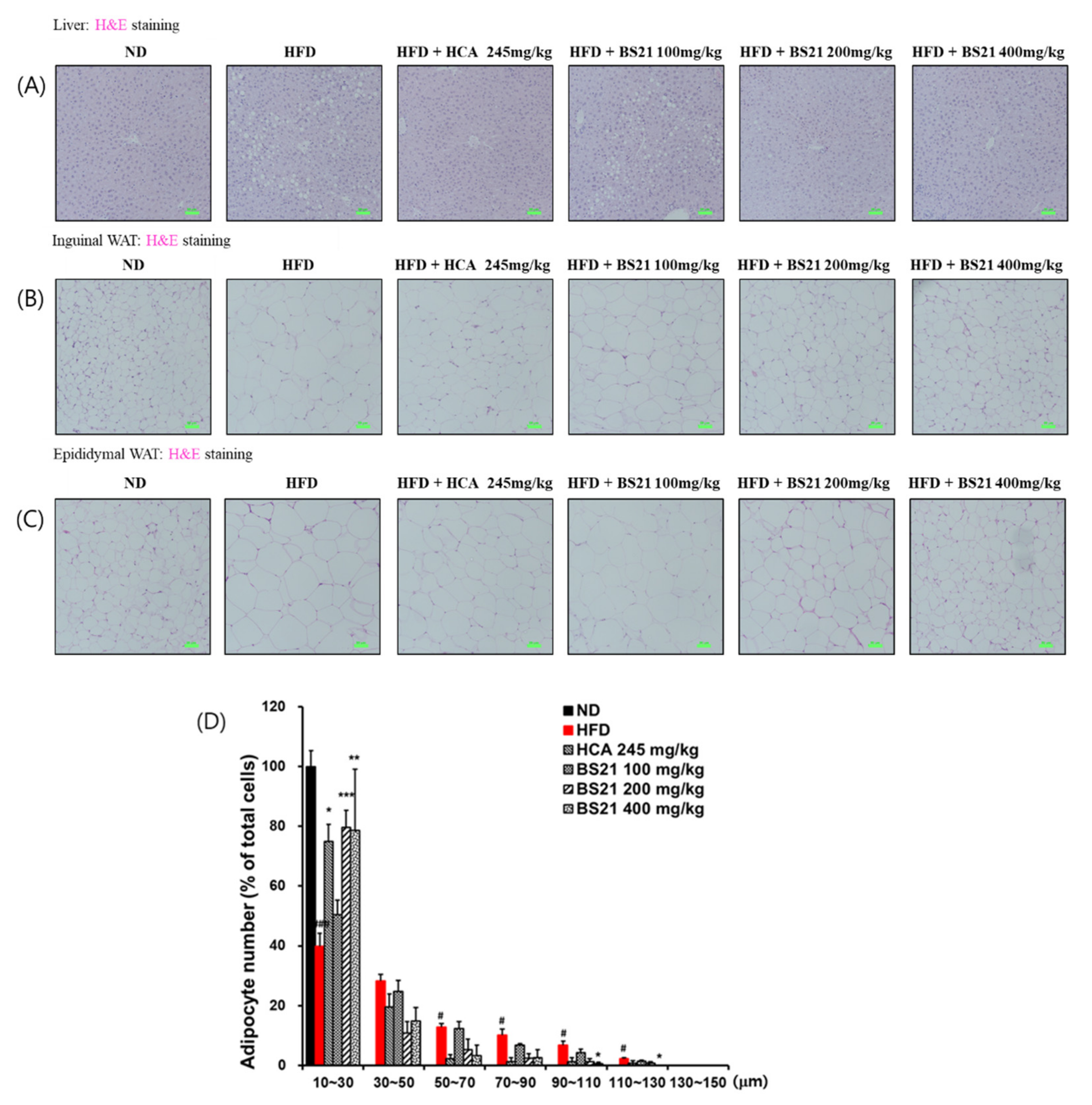
3.5. Histologic Examination
3.6. Effects of BS21 on Lipogenesis or Lipolysis-Related Gene Expression in EWAT
3.7. Energy Expenditure and Thermogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spieglman, B.M.; Filer, J.S. Obesity and the regulation of energy balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Dalbeth, N.; So, A. Hyperuricaemia and gout: State of the art and future perspectives. Ann. Rheum. Dis. 2010, 69, 1738–1743. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, L.P.; Anunciado-Koza, R. UCP1: Its involvement and utility in obesity. Int. J. Obes. 2008, 32, S32–S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.K.; Pahlavani, M.; Ramalingam, L.; Scoggin, S.; Moustaid-Moussa, N. Uncoupling protein 1-independent effects of eicosapentaenoic acid in brown adipose tissue of diet-induced obese female mice. J. Nutr. Biochem. 2021, 98, 108819. [Google Scholar] [CrossRef]
- Saltiel, A.R. New therapeutic approaches for the treatment of obesity. Sci. Transl. Med. 2016, 8, 323rv2. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Apovian, C.M. Current Pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. Herbal preparations for obesity: Are they useful? Prim. Care 2003, 30, 441–463. [Google Scholar] [CrossRef]
- Cho, E.A.; Kim, S.Y.; Na, I.H.; Kim, D.C.; In, M.J.; Chae, H.J. antioxidant and anticoagulant activities of water and ethanol extracts of Phyllostachys pubescence leaf produced in Geoje. J. Appl. Biol. Chem. 2010, 53, 170–173. [Google Scholar] [CrossRef] [Green Version]
- In, M.J.; Park, M.K.; Kim, S.Y.; Chae, H.J.; Chae, M.W.; Sone, J.; Ji, H.S.; Han, K.S.; Kim, D.C. Composition analysis and antioxidative activity of maengjong-juk (Phyllostachys pubescence) leaves tea. J. Appl. Biol. Chem. 2010, 53, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.L.; Park, Y.S. Effects of Scutellaria baicalensis water extract on lipid metabolism and antioxidant defense system in rats fed high fat diet. J. Korean Soc. Food Sci. Nutr. 2010, 39, 219–226. [Google Scholar] [CrossRef]
- Poudel, B.; Nepali, S.; Xin, M.; Ki, H.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. Flavonoids from Triticum aestivum inhibit adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol. Med. Rep. 2015, 12, 3139–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.H.; Lee, S.H.; Kim, B.Y.; Park, A.Y.; Kim, J.Y. Extracts of Scutellaria baicalensis reduced body weight and blood triglyceride in db/db mice. Phytother. Res. 2013, 27, 244–250. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.H.; Cha, J. Antiobesity effects of the combined plant extracts varying the combination ratio of Phyllostachys pubescens leaf extract and Scutellaria baicalensis root extract. Evid.-Based Complement. Altern. Med. 2016, 2016, 9735276. [Google Scholar]
- Sung, Y.Y.; Son, E.; Im, G.; Kim, D.S. Herbal combination of Phyllostachys pubescens and Scutellaria baicalensis inhibits adipogenesis and promotes browning via AMPK activation in 3T3-L1 adipocytes. Plants 2020, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yang, W.K.; Kim, H.Y.; Min, B.; Caturla, N.; Jones, J.; Park, Y.C.; Lee, Y.C.; Kim, S.H. Metabolaid combination of Lemon Verbena and Hibiscus flower extract prevents high-fat diet-induced obesity through AMP-Activated protein kinase activation. Nutrients 2018, 10, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, S.K.; Ricci, M.R.; Russell, C.D.; Laferrère, B. Regulation of leptin production in humans. J. Nutr. 2000, 130, 3127S–3131S. [Google Scholar] [CrossRef]
- Landrier, J.F.; Kasiri, E.; Karkeni, E.; Mihály, J.; Béke, G.; Weiss, K.; Lucas, R.; Aydemir, G.; Salles, J.; Walrand, S.; et al. Reduced adiponectin expression after high-fat diet is associated with selective up-regulation of ALDH1A1 and further retinoic acid receptor signaling in adipose tissue. FASEB J. 2017, 31, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Laws, A. Free fatty acids, insulin resistance and lipoprotein metabolism. Curr. Opin. Lipidol. 1996, 7, 172–177. [Google Scholar] [CrossRef]
- Fève, B. Adipogenesis: Cellular and molecular aspects, Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [Green Version]
- Hejazi, K.; Hosseini, S.R.A.; Fathi, M.; Ziaaldini, M.M. The regulation of the concentrations of peroxisome proliferator-activated receptor gamma coactivator 1-alpha and Sirtuin 1 protein in the soleus muscle by aerobic exercise training in obese wistar rats. J. Kermanshah Univ. Med. Sci. 2020, 24, e101849. [Google Scholar] [CrossRef]
- Payab, M.; Abedi, M.; Foroughi Heravani, N.; Hadavandkhani, M.; Arabi, M.; Tayanloo-Beik, A.; Sheikh Hosseini, M.; Gerami, H.; Khatami, F.; Larijani, B.; et al. Brown adipose tissue transplantation as a novel alternative to obesity treatment: A systematic review. Int. J. Obes. 2021, 45, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Chang, S.H.; Yang, D.K.; Song, N.J.; Yun, U.J.; Park, K.W. Sesamol increases Ucp1 expression in white adipose tissues and stimulates energy expenditure in high-fat diet-fed obese mice. Nutrients 2020, 12, 1459. [Google Scholar] [CrossRef] [PubMed]
- Cereijo, R.; Giralt, M.; Villarroya, F. Thermogenic brown and beige/brite adipogenesis in humans. Ann. Med. 2015, 47, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, S. Baicalin attemuates diet-induced obesity partially through promoting thermogenesis in adipose tissue. Obes. Res. Clin. Pract. 2021, 15, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, M.S.; Chang, E.; Jung, S.; Ko, H.; Lee, E.; Lee, S.; Kim, C.T.; Kim, I.H.; Kim, Y. Tartary Buckwheat extract attenuated the obesity-induced inflammation and increased muscle PGC-1a/SIRT1 expression in high fat diet-induced obese rats. Nutrients 2019, 11, 654. [Google Scholar] [CrossRef] [Green Version]
- Graf, C.; Ferrari, N. Metabolic health-the role of adipo-myokines. Int. J. Mol. Sci. 2019, 20, 6159. [Google Scholar] [CrossRef] [Green Version]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2019, 228, e13367. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Mermier, C.M.; Conn, C.A. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J. Physiol. Biochem. 2015, 71, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Wang, F.; Donelan, W.; Zona, M.C.; Li, S.; Reeves, W.; Ding, Y.; Tang, D.; Yang, L. Effects of irisin on the differentiation and browning of human visceral white adipocytes. Am. J. Transl. Res. 2019, 11, 7410–7421. [Google Scholar] [PubMed]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Bjornholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F.P. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes 2016, 66, 598–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Jiang, X.; Ma, H.; Wang, Y.; Xue, P.; Liu, Y. SIRT1 and insulin resistance. J. Diabetes Complicat. 2016, 30, 178–183. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Tian, H.C.; Fan, Z.; Chen, Q.N.; Yang, Y.F.; Yu, J.; Wu, Y.X.; Xiao, Q. FGF19 protects skeletal muscle against obesity-induced muscle atrophy, metabolic derangement and abnormal irisin levels via the AMPK/SIRT-1/PGC-α pathway. J. Cell Mol. Med. 2021, 25, 3585–3600. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, Y.-Y.; Kim, S.-H.; Kim, D.-S. Combined Phyllostachys pubescens and Scutellaria baicalensis Prevent High-Fat Diet-Induced Obesity via Upregulating Thermogenesis and Energy Expenditure by UCP1 in Male C57BL/6J Mice. Nutrients 2022, 14, 446. https://doi.org/10.3390/nu14030446
Sung Y-Y, Kim S-H, Kim D-S. Combined Phyllostachys pubescens and Scutellaria baicalensis Prevent High-Fat Diet-Induced Obesity via Upregulating Thermogenesis and Energy Expenditure by UCP1 in Male C57BL/6J Mice. Nutrients. 2022; 14(3):446. https://doi.org/10.3390/nu14030446
Chicago/Turabian StyleSung, Yoon-Young, Seung-Hyung Kim, and Dong-Seon Kim. 2022. "Combined Phyllostachys pubescens and Scutellaria baicalensis Prevent High-Fat Diet-Induced Obesity via Upregulating Thermogenesis and Energy Expenditure by UCP1 in Male C57BL/6J Mice" Nutrients 14, no. 3: 446. https://doi.org/10.3390/nu14030446






