Short-Term Adaptations in Skeletal Muscle Mitochondrial Oxidative Capacity and Metabolic Pathways to Breaking up Sedentary Behaviors in Overweight or Obese Adults
Abstract
:1. Introduction
2. Methods
2.1. Experimental Protocol
2.2. Participants
2.3. Screening Visit
2.4. Study Interventions
2.5. Standardized Diet
2.6. Skeletal Muscle Biopsy
2.7. Preparation of Permeabilized Muscle Fiber Bundles
2.8. Skeletal Muscle Mitochondrial Respiration Protocols
2.9. RNA Sequencing
2.10. Pathway Analysis
2.11. Data and Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Mitochondrial Capacity for Fat Oxidation Is Enhanced by a Single-Continuous Bout of PA, but Not by Multiple Short Bouts of PA
3.3. Correlations between Skeletal Muscle Mitochondrial Oxidative Capacity and Whole-Body Metabolic Outcomes
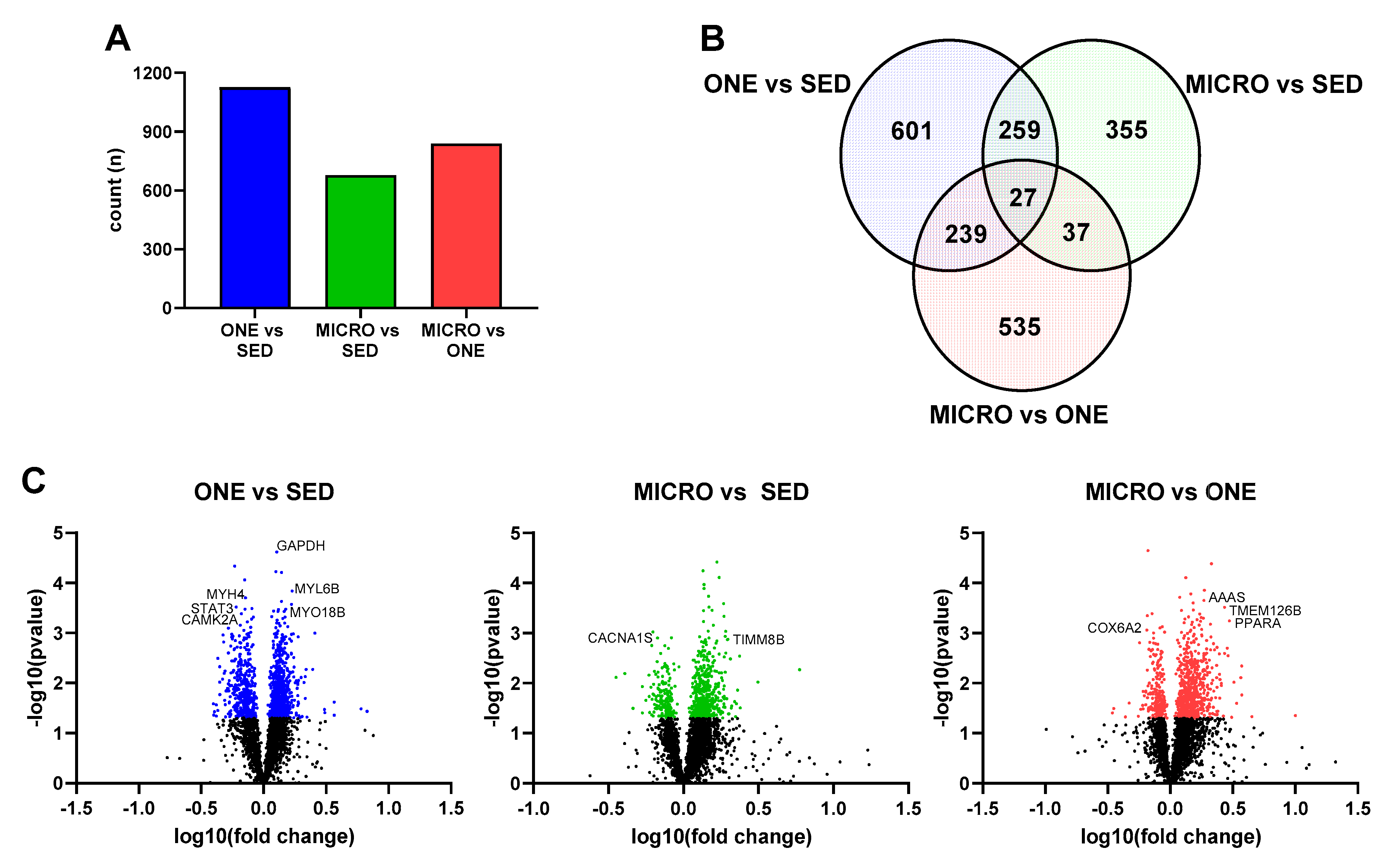
3.4. Differential Gene Expression Leads to Similar Pathway Enrichment for MICRO and ONE with More Biological Pathways Enriched in ONE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Altenburg, T.M.; et al. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Healy, G.N.; Dunstan, D.W.; Salmon, J.; Cerin, E.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Breaks in sedentary time: Beneficial associations with metabolic risk. Diabetes Care 2008, 31, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.H.; Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur. Heart J. 2011, 32, 590–597. [Google Scholar] [CrossRef]
- Blankenship, J.M.; Granados, K.; Braun, B. Effects of subtracting sitting versus adding exercise on glycemic control and variability in sedentary office workers. Appl. Physiol. Nutr. Metab. 2014, 39, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.C.; Blankenship, J.M.; Larsen, R.; Sacre, J.W.; Sethi, P.; Straznicky, N.E.; Cohen, N.; Cerin, E.; Lambert, G.W.; Owen, N.; et al. Interrupting prolonged sitting in type 2 diabetes: Nocturnal persistence of improved glycaemic control. Diabetologia 2016, 60, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, P.C.; Larsen, R.N.; Sethi, P.; Sacre, J.W.; Straznicky, N.E.; Cohen, N.D.; Cerin, E.; Lambert, G.W.; Owen, N.; Kingwell, B.A.; et al. Benefits for Type 2 Diabetes of Interrupting Prolonged Sitting with Brief Bouts of Light Walking or Simple Resistance Activities. Diabetes Care 2016, 39, 964–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Cerin, E.; Hamilton, M.T.; Shaw, J.E.; Bertovic, D.A.; Zimmet, P.Z.; Salmon, J.; et al. Breaking Up Prolonged Sitting Reduces Postprandial Glucose and Insulin Responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Holmstrup, M.; Fairchild, T.; Keslacy, S.; Weinstock, R.; Kanaley, J. Multiple short bouts of exercise over 12-h period reduce glucose excursions more than an energy-matched single bout of exercise. Metabolism 2014, 63, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homer, A.R.; Fenemor, S.; Perry, T.L.; Rehrer, N.J.; Cameron, C.; Skeaff, C.M.; Peddie, M.C. Regular activity breaks combined with physical activity improve postprandial plasma triglyceride, nonesterified fatty acid, and insulin responses in healthy, normal weight adults: A randomized crossover trial. J. Clin. Lipidol. 2017, 11, 1268–1279.e1. [Google Scholar] [CrossRef]
- Larsen, R.N.; Kingwell, B.A.; Robinson, C.; Hammond, L.; Cerin, E.; Shaw, J.E.; Healy, G.N.; Hamilton, M.T.; Owen, N.; Dunstan, D.W. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin. Sci. 2015, 129, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peddie, M.C.; Bone, J.L.; Rehrer, N.J.; Skeaff, C.M.; Gray, A.; Perry, T.L. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: A randomized crossover trial. Am. J. Clin. Nutr. 2013, 98, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dijk, J.W.; Venema, M.; van Mechelen, W.; Stehouwer, C.D.; Hartgens, F.; van Loon, L.J. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care 2013, 36, 3448–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, N.P.; Rynders, C.A.; Goldstrohm, D.A.; Pan, Z.; Lange, A.H.; Mendez, C.; Melanson, E.L.; Bessesen, D.H.; Bergouignan, A. Effect of frequent interruptions of sedentary time on nutrient metabolism in sedentary overweight male and female adults. J. Appl. Physiol. 1985, 2019. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Matthews, C.E.; Dashti, S.G.; Doherty, A.R.; Bergouignan, A.; van Roekel, E.H.; Dunstan, D.W.; Wareham, N.J.; Yates, T.E.; Wijndaele, K.; et al. Sedentary Behavior and Chronic Disease: Mechanisms and Future Directions. J. Phys. Act. Health 2020, 17, 52–61. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latouche, C.; Jowett, J.B.M.; Carey, A.L.; Bertovic, D.A.; Owen, N.; Dunstan, D.W.; Kingwell, B.A. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J. Appl. Physiol. 2013, 114, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Bergouignan, A.; Latouche, C.; Heywood, S.; Grace, M.S.; Reddy-LuthmoodooM; Natoli, A.K.; Owen, N.; Dunstan, D.W.; Kingwell, B.A. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: Ancillary analysis from randomized clinical trials. Sci. Rep. 2016, 6, 32044. [Google Scholar] [CrossRef]
- Thyfault, J.P. Setting the stage: Possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.; Carrasquilla, G.D.; Ingerslev, L.R.; Hochreuter, M.Y.; Hansson, S.; Pillon, N.J.; Donkin, I.; Versteyhe, S.; Zierath, J.R.; Kilpeläinen, T.O.; et al. Epigenetic rewiring of skeletal muscle enhancers after exercise training supports a role in whole-body function and human health. Mol. Metab. 2021, 53, 101290. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Katsiaras, A.; Kelley, D.E. Enhanced Fat Oxidation Through Physical Activity Is Associated with Improvements in Insulin Sensitivity in Obesity. Diabetes 2003, 52, 2191–2197. [Google Scholar] [CrossRef] [Green Version]
- De Jong, N.P.; Debache, I.; Pan, Z.; Garnotel, M.; Lyden, K.; Sueur, C.; Simon, C.; Bessesen, D.H.; Bergouignan, A. Breaking up Sedentary Time in Overweight/Obese Adults on Work Days and Non-Work Days: Results from a Feasibility Study. Int. J. Environ. Res. Public Health 2018, 15, 2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [PubMed]
- Gnaiger, E. Mitochondrial Pathways and Respiratory Control: An introduction to OXPHOS Analysis. Bioenerg. Commun. 2020, 2020, 2–22. [Google Scholar]
- Hohos, N.M.; Elliott, E.M.; Cho, K.J.; Lin, I.S.; Rudolph, M.C.; Skaznik-Wikiel, M.E. High-fat diet-induced dysregulation of ovarian gene expression is restored with chronic omega-3 fatty acid supplementation. Mol. Cell. Endocrinol. 2019, 499, 110615. [Google Scholar] [CrossRef] [PubMed]
- Presby, D.M.; Checkley, L.A.; Jackman, M.R.; Higgins, J.A.; Jones, K.L.; Giles, E.; Houck, J.A.; Webb, P.G.; Steig, A.J.; Johnson, G.C.; et al. Regular exercise potentiates energetically expensive hepatic de novo lipogenesis during early weight regain. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R684–R695. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.D.; Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 2010, 26, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Carli, J.F.M.; Trahan, G.D.; Jones, K.L.; Hirsch, N.; Rolloff, K.P.; Dunn, E.Z.; Friedman, J.E.; Barbour, L.A.; Hernandez, T.L.; MacLean, P.; et al. Single Cell RNA Sequencing of Human Milk-Derived Cells Reveals Sub-Populations of Mammary Epithelial Cells with Molecular Signatures of Progenitor and Mature States: A Novel, Non-invasive Framework for Investigating Human Lactation Physiology. J. Mammary Gland. Biol Neoplasia 2020, 25, 367–387. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Lee, S.; Dong, H.H. FoxO integration of insulin signaling with glucose and lipid metabolism. J. Endocrinol. 2017, 233, R67–R79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skovbro, M.; Boushel, R.; Hansen, C.N.; Helge, J.W.; Dela, F. High-fat feeding inhibits exercise-induced increase in mitochondrial respiratory flux in skeletal muscle. J. Appl. Physiol. 2011, 110, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.C.; Widegren, M.U.; Zierath, J.R. Exercise-induced mitogen-activated protein kinase signalling in skeletal muscle. Proc. Nutr. Soc. 2004, 63, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1–PGC-1α transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Musci, R.; Hamilton, K.; Linden, M. Exercise-Induced Mitohormesis for the Maintenance of Skeletal Muscle and Healthspan Extension. Sports 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieke, S.M.; Phillips, D.; McCoy, J.P.; Aponte, A.M.; Shen, R.-F.; Balaban, R.S.; Finkel, T. The Mammalian Target of Rapamycin (mTOR) Pathway Regulates Mitochondrial Oxygen Consumption and Oxidative Capacity. J. Biol. Chem. 2006, 281, 27643–27652. [Google Scholar] [CrossRef] [Green Version]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 1–10. [Google Scholar] [CrossRef]
- Ojuka, E.O. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc. Nutr. Soc. 2004, 63, 275–278. [Google Scholar] [CrossRef]



| Muscle Mitochondrial Respiration (Oroboros) | Muscle Molecular Adaptations (RNA Seq) | |
|---|---|---|
| N (#) | 19 (9 m/10 f) | 8 (4 m/4 f) |
| Age (years) | 32.2 ± 6.4 | 34.3 ± 8.4 |
| BMI (kg/m2) | 30.3 ± 3.0 | 29.9 ± 2.4 |
| Body Mass (kg) | 85.2 ± 10.9 | 84.8 ± 11.6 |
| FFM (kg) | 55.2 ± 10.6 | 55.9 ± 11.0 |
| FM (kg) | 30.0 ± 7.1 | 28.9 ± 8.3 |
| Fat mass (%) | 35.3 ± 7.7 | 34.2 ± 8.8 |
| Fasting Glucose (mg/dL) | 87.8 ± 4.6 | 86.6 ± 4.8 |
| Fasting Triglyceride (mg/dL) | 102.7 ± 52.8 | 115.9 ± 67.5 |
| HOMA-IR (SED day fasting) | 1.4 ± 0.7 | 1.2 ± 0.6 |
| IPAQ self-reported sitting time (h/day) | 10.6 ± 3.3 | 8.5 ± 3.0 |
| Dependent Variable | Independent Variable | Intervention | Slope Estimate | p-Value | p for Interaction |
|---|---|---|---|---|---|
| RCR fat | Fasting Insulin | SED | 0.08 | 0.96 | 0.003 ** |
| ONE | 0.65 | 0.008 ## | |||
| MICRO | 0.24 | 0.02 # | |||
| SED-ONE | −0.64 | 0.015 # | |||
| SED-BREAK | −0.24 | 0.05 # | |||
| ONE-BREAK | 0.40 | 0.03 # | |||
| RCR fat | Insulin/Glucose | SED | 4.27 | 0.58 | 0.005 ** |
| ONE | 58.11 | 0.001 ## | |||
| MICRO | 23.24 | 0.012 # | |||
| SED-ONE | −53.84 | 0.002 ## | |||
| SED-BREAK | −18.97 | 0.07 | |||
| ONE-BREAK | 34.87 | 0.03 # | |||
| CHO LEAK | Sleeping CHO (g/min) | SED | −8.63 | 0.56 | 0.077 * |
| ONE | 27.24 | 0.17 | |||
| MICRO | −36.06 | 0.03 # | |||
| SED-ONE | −35.87 | 0.11 | |||
| SED-BREAK | 27.45 | 0.31 | |||
| ONE-BREAK | 63.32 | 0.03 # | |||
| CHO OXPHOS | Sleeping CHO (g/min) | SED | 42.22 | 0.76 | 0.401 |
| ONE | −97.52 | 0.55 | |||
| MICRO | −320.65 | 0.15 | |||
| SED-ONE | 139.74 | 0.53 | |||
| SED-BREAK | 362.86 | 0.18 | |||
| ONE-BREAK | 223.13 | 0.42 | |||
| CHO ETS c1 + c2 | Sleeping CHO (g/min) | SED | −36.61 | 0.78 | 0.194 |
| ONE | 229.91 | 0.15 | |||
| MICRO | −200.32 | 0.33 | |||
| SED-ONE | −266.53 | 0.18 | |||
| SED-BREAK | 163.71 | 0.49 | |||
| ONE-BREAK | 430.24 | 0.09 | |||
| FAT LEAK | Sleeping FAT (g/min) | SED | −2.47 | 0.94 | 0.919 |
| ONE | −8.59 | 0.81 | |||
| MICRO | 16.99 | 0.75 | |||
| SED-ONE | 6.11 | 0.89 | |||
| SED-BREAK | −19.47 | 0.75 | |||
| ONE-BREAK | −25.59 | 0.68 | |||
| FAT OXPHOS | Sleeping FAT (g/min) | SED | −105.42 | 0.71 | 0.357 |
| ONE | −460.21 | 0.15 | |||
| MICRO | 344.18 | 0.46 | |||
| SED-ONE | 354.79 | 0.41 | |||
| SED-BREAK | −449.60 | 0.41 | |||
| ONE-BREAK | −804.40 | 0.16 | |||
| FAT ETS c1 + c2 | Sleeping FAT (g/min) | SED | −61.82 | 0.85 | 0.115 |
| ONE | −170.15 | 0.57 | |||
| MICRO | 936.09 | 0.04 | |||
| SED-ONE | 108.33 | 0.80 | |||
| SED-BREAK | −997.91 | 0.08 | |||
| ONE-BREAK | −1106.24 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jong, N.P.; Rudolph, M.C.; Jackman, M.R.; Sharp, R.R.; Jones, K.; Houck, J.; Pan, Z.; Reusch, J.E.B.; MacLean, P.S.; Bessesen, D.H.; et al. Short-Term Adaptations in Skeletal Muscle Mitochondrial Oxidative Capacity and Metabolic Pathways to Breaking up Sedentary Behaviors in Overweight or Obese Adults. Nutrients 2022, 14, 454. https://doi.org/10.3390/nu14030454
De Jong NP, Rudolph MC, Jackman MR, Sharp RR, Jones K, Houck J, Pan Z, Reusch JEB, MacLean PS, Bessesen DH, et al. Short-Term Adaptations in Skeletal Muscle Mitochondrial Oxidative Capacity and Metabolic Pathways to Breaking up Sedentary Behaviors in Overweight or Obese Adults. Nutrients. 2022; 14(3):454. https://doi.org/10.3390/nu14030454
Chicago/Turabian StyleDe Jong, Nathan P., Michael C. Rudolph, Matthew R. Jackman, Rachel R. Sharp, Ken Jones, Julie Houck, Zhaoxing Pan, Jane E. B. Reusch, Paul S. MacLean, Daniel H. Bessesen, and et al. 2022. "Short-Term Adaptations in Skeletal Muscle Mitochondrial Oxidative Capacity and Metabolic Pathways to Breaking up Sedentary Behaviors in Overweight or Obese Adults" Nutrients 14, no. 3: 454. https://doi.org/10.3390/nu14030454
APA StyleDe Jong, N. P., Rudolph, M. C., Jackman, M. R., Sharp, R. R., Jones, K., Houck, J., Pan, Z., Reusch, J. E. B., MacLean, P. S., Bessesen, D. H., & Bergouignan, A. (2022). Short-Term Adaptations in Skeletal Muscle Mitochondrial Oxidative Capacity and Metabolic Pathways to Breaking up Sedentary Behaviors in Overweight or Obese Adults. Nutrients, 14(3), 454. https://doi.org/10.3390/nu14030454






