Investigating the Postprandial Metabolome after Challenge Tests to Assess Metabolic Flexibility and Dysregulations Associated with Cardiometabolic Diseases
Abstract
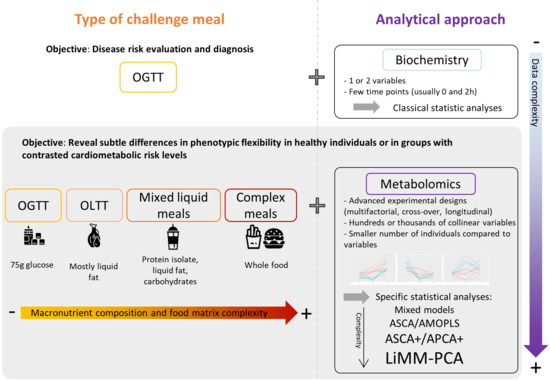
1. Introduction
2. The Postprandial Metabolism as a Window to Explore Phenotypic Flexibility
3. Metabolomics, Nutrition and Challenge Tests
4. Oral Glucose Tolerance Tests
4.1. OGTT in Healthy Individuals
4.2. OGTT in Insulin Resistance, Overweight and Hyperlipidemic Conditions
5. Mixed Challenge Meals
5.1. Main Metabolic Shifts Induced by Mixed Meals in Healthy Individuals
5.2. Main Metabolic Shifts Induced by Mixed Meals in Individuals with Increased Cardiometabolic Risk
6. Statistical Considerations: Which Statistical Tools for Metabolomics Postprandial Kinetics Analyses?
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Secor, S.M. Specific Dynamic Action: A Review of the Postprandial Metabolic Response. J. Comp. Physiol. B 2009, 179, 1–56. [Google Scholar] [CrossRef]
- Lloyd, D.; Aon, M.A.; Cortassa, S. Why Homeodynamics, Not Homeostasis? Sci. World J. 2001, 1, 133–145. [Google Scholar] [CrossRef]
- Van Ommen, B.; Van Der Greef, J.; Ordovas, J.M.; Daniel, H. Phenotypic Flexibility as Key Factor in the Human Nutrition and Health Relationship. Genes Nutr. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.; Burton, K.J.; Pralong, F.P.; Vionnet, N.; Portmann, R.; Vergères, G. The Postprandial Metabolome—A Source of Nutritional Biomarkers of Health. Curr. Opin. Food Sci. 2017, 16, 67–73. [Google Scholar] [CrossRef]
- Dimina, L.; Mariotti, F. The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances. Nutrients 2019, 11, 1963. [Google Scholar] [CrossRef]
- Jacome-Sosa, M.; Parks, E.J.; Bruno, R.S.; Tasali, E.; Lewis, G.F.; Schneeman, B.O.; Rains, T.M. Postprandial Metabolism of Macronutrients and Cardiometabolic Risk: Recent Developments, Emerging Concepts, and Future Directions. Adv. Nutr. 2016, 7, 364–374. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Owens, D.R. Integrating Glycaemic Variability in the Glycaemic Disorders of Type 2 Diabetes: A Move towards a Unified Glucose Tetrad Concept. Diabetes/Metab. Res. Rev. 2009, 25, 393–402. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Hill, J.O.; Wyatt, H.R. The Obesity Epidemic. Clin. Chest Med. 2009, 30, 415–444. [Google Scholar] [CrossRef]
- Poppitt, S.D. Postprandial Lipaemia, Haemostasis, Inflammatory Response and Other Emerging Risk Factors for Cardiovascular Disease: The Influence of Fatty Meals. Curr. Nutr. Food Sci. 2004, 1, 23–34. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- WHO. Preamble to the Constitution of the World Health Organization as Adopted by the International Health Conference, New York, 19–22 June, 1946; Signed on 22 July 1946 by the Representatives of 61 States (Official Records of the World Health Organization, No. 2, p. 100) and Entered into Force on 7 April 1948; WHO: Geneva, Switzerland, 1948. [Google Scholar]
- Huber, M.; van Vliet, M.; Giezenberg, M.; Winkens, B.; Heerkens, Y.; Dagnelie, P.C.; Knottnerus, J.A. Towards a ‘Patient-Centred’ Operationalisation of the New Dynamic Concept of Health: A Mixed Methods Study. BMJ Open 2016, 6, e010091. [Google Scholar] [CrossRef]
- Yu, E.A.; Le, N.-A.; Stein, A.D. Measuring Postprandial Metabolic Flexibility to Assess Metabolic Health and Disease. J. Nutr. 2021, 151, 3284–3291. [Google Scholar] [CrossRef]
- Stroeve, J.H.M.; van Wietmarschen, H.; Kremer, B.H.A.; van Ommen, B.; Wopereis, S. Phenotypic Flexibility as a Measure of Health: The Optimal Nutritional Stress Response Test. Genes Nutr. 2015, 10, 13. [Google Scholar] [CrossRef]
- Vis, D.J.; Westerhuis, J.A.; Jacobs, D.M.; van Duynhoven, J.P.M.; Wopereis, S.; van Ommen, B.; Hendriks, M.M.W.B.; Smilde, A.K. Analyzing Metabolomics-Based Challenge Tests. Metabolomics 2015, 11, 50–63. [Google Scholar] [CrossRef]
- Kardinaal, A.F.M.; van Erk, M.J.; Dutman, A.E.; Stroeve, J.H.M.; van de Steeg, E.; Bijlsma, S.; Kooistra, T.; van Ommen, B.; Wopereis, S. Quantifying Phenotypic Flexibility as the Response to a High-Fat Challenge Test in Different States of Metabolic Health. FASEB J. 2015, 29, 4600–4613. [Google Scholar] [CrossRef]
- Puiggròs, F.; Canela, N.; Arola, L. Metabolome Responses to Physiological and Nutritional Challenges. Curr. Opin. Food Sci. 2015, 4, 111–115. [Google Scholar] [CrossRef]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Lacueva, C.A.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol. Nutr. Food Res. 2019, 63, 1800384. [Google Scholar] [CrossRef]
- Ramautar, R.; Berger, R.; van der Greef, J.; Hankemeier, T. Human Metabolomics: Strategies to Understand Biology. Curr. Opin. Chem. Biol. 2013, 17, 841–846. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Rådjursöga, M.; Lindqvist, H.M.; Pedersen, A.; Karlsson, G.B.; Malmodin, D.; Brunius, C.; Ellegård, L.; Winkvist, A. The 1H NMR Serum Metabolomics Response to a Two Meal Challenge: A Cross-over Dietary Intervention Study in Healthy Human Volunteers. Nutr. J. 2019, 18, 25. [Google Scholar] [CrossRef]
- Fazelzadeh, P.; Hoefsloot, H.C.J.; Hankemeier, T.; Most, J.; Kersten, S.; Blaak, E.E.; Boekschoten, M.; Van Duynhoven, J. Global Testing of Shifts in Metabolic Phenotype. Metabolomics 2018, 14. [Google Scholar] [CrossRef]
- Sébédio, J.-L.; Polakof, S. Using Metabolomics to Identify Biomarkers for Metabolic Diseases: Analytical Methods and Applications. In Metabolomics as a Tool in Nutritional Research; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Amsterdam, The Netherlands, 2015; p. 268. [Google Scholar]
- Suárez, M.; Caimari, A.; del Bas, J.M.; Arola, L. Metabolomics: An Emerging Tool to Evaluate the Impact of Nutritional and Physiological Challenges. TrAC Trends Anal. Chem. 2017, 96, 79–88. [Google Scholar] [CrossRef]
- Brennan, L. 10—Metabolomics and Nutritional Challenge Tests: What Can We Learn? In Metabolomics as a Tool in Nutrition Research; Sébédio, J.-L., Brennan, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; pp. 197–202. ISBN 978-1-78242-084-2. [Google Scholar]
- Wopereis, S.; Wolvers, D.; van Erk, M.; Gribnau, M.; Kremer, B.; van Dorsten, F.A.; Boelsma, E.; Garczarek, U.; Cnubben, N.; Frenken, L.; et al. Assessment of Inflammatory Resilience in Healthy Subjects Using Dietary Lipid and Glucose Challenges. BMC Med. Genom. 2013, 6, 44. [Google Scholar] [CrossRef]
- LaBarre, J.L.; Singer, K.; Burant, C.F. Advantages of Studying the Metabolome in Response to Mixed-Macronutrient Challenges and Suggestions for Future Research Designs. J. Nutr. 2021, 151, 2868–2881. [Google Scholar] [CrossRef]
- Krug, S.; Kastenmüller, G.; Stückler, F.; Rist, M.J.; Skurk, T.; Sailer, M.; Raffler, J.; Römisch-Margl, W.; Adamski, J.; Prehn, C.; et al. The Dynamic Range of the Human Metabolome Revealed by Challenges. FASEB J. 2012, 26, 2607–2619. [Google Scholar] [CrossRef]
- Wilkerson, H.L.C.; Remein, Q.R. Studies of Abnormal Carbohydrate Metabolism in Pregnancy: The Significance of Impaired Glucose Tolerance. Diabetes 1957, 6, 324–329. [Google Scholar] [CrossRef]
- Wopereis, S.; Rubingh, C.M.; van Erk, M.J.; Verheij, E.R.; van Vliet, T.; Cnubben, N.H.P.; Smilde, A.K.; van der Greef, J.; van Ommen, B.; Hendriks, H.F.J. Metabolic Profiling of the Response to an Oral Glucose Tolerance Test Detects Subtle Metabolic Changes. PLoS ONE 2009, 4, e4525. [Google Scholar] [CrossRef]
- Zhao, X.; Peter, A.; Fritsche, J.; Elcnerova, M.; Fritsche, A.; Häring, H.-U.; Schleicher, E.D.; Xu, G.; Lehmann, R. Changes of the Plasma Metabolome during an Oral Glucose Tolerance Test: Is There More than Glucose to Look At? Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E384–E393. [Google Scholar] [CrossRef]
- Shaham, O.; Wei, R.; Wang, T.J.; Ricciardi, C.; Lewis, G.D.; Vasan, R.S.; Carr, S.A.; Thadhani, R.; Gerszten, R.E.; Mootha, V.K. Metabolic Profiling of the Human Response to a Glucose Challenge Reveals Distinct Axes of Insulin Sensitivity. Mol. Syst. Biol. 2008, 4, 214. [Google Scholar] [CrossRef]
- Spégel, P.; Danielsson, A.P.H.; Bacos, K.; Nagorny, C.L.F.; Moritz, T.; Mulder, H.; Filipsson, K. Metabolomic Analysis of a Human Oral Glucose Tolerance Test Reveals Fatty Acids as Reliable Indicators of Regulated Metabolism. Metabolomics 2010, 6, 56–66. [Google Scholar] [CrossRef]
- Ho, J.E.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; Cheng, S.; Rhee, E.P.; Florez, J.C.; Clish, C.B.; Gerszten, R.E.; Wang, T.J. Metabolite Profiles During Oral Glucose Challenge. Diabetes 2013, 62, 2689–2698. [Google Scholar] [CrossRef]
- Wang, Q.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Keinänen-Kiukaanniemi, S.; Järvelin, M.-R.; Kettunen, J.; Mäkinen, V.-P.; Ala-Korpela, M. Insulin Resistance and Systemic Metabolic Changes in Oral Glucose Tolerance Test in 5340 Individuals: An Interventional Study. BMC Med. 2019, 17, 217. [Google Scholar] [CrossRef]
- Beyene, H.B.; Hamley, S.; Giles, C.; Huynh, K.; Smith, A.; Cinel, M.; Mellet, N.A.; Morales-Scholz, M.G.; Kloosterman, D.; Howlett, K.F.; et al. Mapping the Associations of the Plasma Lipidome with Insulin Resistance and Response to an Oral Glucose Tolerance Test. J. Clin. Endocrinol. Metab. 2020, 105, e1041–e1055. [Google Scholar] [CrossRef]
- Rämö, J.T.; Kaye, S.M.; Jukarainen, S.; Bogl, L.H.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Rissanen, A.; Kaprio, J.; Matikainen, N.; et al. Liver Fat and Insulin Sensitivity Define Metabolite Profiles During a Glucose Tolerance Test in Young Adult Twins. J. Clin. Endocrinol. Metab. 2017, 102, 220–231. [Google Scholar] [CrossRef]
- Huo, S.; Sun, L.; Zong, G.; Shen, X.; Zheng, H.; Jin, Q.; Li, H.; Yin, H.; Lin, X. Changes in Plasma Metabolome Profiles Following Oral Glucose Challenge among Adult Chinese. Nutrients 2021, 13, 1474. [Google Scholar] [CrossRef]
- Nowak, C.; Hetty, S.; Salihovic, S.; Castillejo-Lopez, C.; Ganna, A.; Cook, N.L.; Broeckling, C.D.; Prenni, J.E.; Shen, X.; Giedraitis, V.; et al. Glucose Challenge Metabolomics Implicates Medium-Chain Acylcarnitines in Insulin Resistance. Sci. Rep. 2018, 8, 8691. [Google Scholar] [CrossRef]
- Müllner, E.; Röhnisch, H.E.; von Brömssen, C.; Moazzami, A.A. Metabolomics Analysis Reveals Altered Metabolites in Lean Compared with Obese Adolescents and Additional Metabolic Shifts Associated with Hyperinsulinaemia and Insulin Resistance in Obese Adolescents: A Cross-Sectional Study. Metabolomics 2021, 17, 11. [Google Scholar] [CrossRef]
- Geidenstam, N.; Spégel, P.; Mulder, H.; Filipsson, K.; Ridderstråle, M.; Danielsson, A.P.H. Metabolite Profile Deviations in an Oral Glucose Tolerance Test—A Comparison between Lean and Obese Individuals. Obesity 2014, 22, 2388–2395. [Google Scholar] [CrossRef]
- Liu, L.; Feng, R.; Guo, F.; Li, Y.; Jiao, J.; Sun, C. Targeted Metabolomic Analysis Reveals the Association between the Postprandial Change in Palmitic Acid, Branched-Chain Amino Acids and Insulin Resistance in Young Obese Subjects. Diabetes Res. Clin. Pract. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Geidenstam, N.; Danielsson, A.P.H.; Spégel, P.; Ridderstråle, M. Changes in Glucose-Elicited Blood Metabolite Responses Following Weight Loss and Long Term Weight Maintenance in Obese Individuals with Impaired Glucose Tolerance. Diabetes Res. Clin. Pract. 2016, 113, 187–197. [Google Scholar] [CrossRef]
- LaBarre, J.L.; Hirschfeld, E.; Soni, T.; Kachman, M.; Wigginton, J.; Duren, W.; Fleischman, J.Y.; Karnovsky, A.; Burant, C.F.; Lee, J.M. Comparing the Fasting and Random-Fed Metabolome Response to an Oral Glucose Tolerance Test in Children and Adolescents: Implications of Sex, Obesity, and Insulin Resistance. Nutrients 2021, 13, 3365. [Google Scholar] [CrossRef]
- Li, Q.; Gu, W.; Ma, X.; Liu, Y.; Jiang, L.; Feng, R.; Liu, L. Amino Acid and Biogenic Amine Profile Deviations in an Oral Glucose Tolerance Test: A Comparison between Healthy and Hyperlipidaemia Individuals Based on Targeted Metabolomics. Nutrients 2016, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, J.; Zhao, J.; Guo, J.; Zhang, M.; Liu, L. Glucose Challenge Metabolomics Implicates the Change of Organic Acid Profiles in Hyperlipidemic Subjects. Biomed. Chromatogr. 2020, 34, e4815. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A.; Goldfine, I.D.; Rosen, M.S.; Taplitz, R.A.; Williams, J.A. Cholecystokinin Bioactivity in Human Plasma. Molecular Forms, Responses to Feeding, and Relationship to Gallbladder Contraction. J. Clin. Investig. 1985, 75, 1144–1152. [Google Scholar] [CrossRef]
- Watford, M. The Urea Cycle: Teaching Intermediary Metabolism in a Physiological Setting. Biochem. Mol. Biol. Educ. 2003, 31, 289–297. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of Uric Acid Metabolism and Excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Yoshikawa, H.; Itakura, M.; Yamashita, K. Increased de Novo Purine Synthesis by Insulin through Selective Enzyme Induction in Primary Cultured Rat Hepatocytes. Am. J. Physiol.-Cell Physiol. 1990, 258, C841–C848. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell. Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin Action, Type 2 Diabetes, and Branched-Chain Amino Acids: A Two-Way Street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Arany, Z.; Neinast, M. Branched Chain Amino Acids in Metabolic Disease. Curr. Diab. Rep. 2018, 18, 76. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef]
- Yu, E.A.; Yu, T.; Jones, D.P.; Martorell, R.; Ramirez-Zea, M.; Stein, A.D. Macronutrient, Energy, and Bile Acid Metabolism Pathways Altered Following a Physiological Meal Challenge, Relative to Fasting, among Guatemalan Adults. J. Nutr. 2020, 150, 2031–2040. [Google Scholar] [CrossRef]
- Adamska-Patruno, E.; Samczuk, P.; Ciborowski, M.; Godzien, J.; Pietrowska, K.; Bauer, W.; Gorska, M.; Barbas, C.; Kretowski, A. Metabolomics Reveal Altered Postprandial Lipid Metabolism After a High-Carbohydrate Meal in Men at High Genetic Risk of Diabetes. J. Nutr. 2019, 149, 915–922. [Google Scholar] [CrossRef]
- Bastarrachea, R.A.; Laviada-Molina, H.A.; Nava-Gonzalez, E.J.; Leal-Berumen, I.; Escudero-Lourdes, C.; Escalante-Araiza, F.; Peschard, V.-G.; Veloz-Garza, R.A.; Haack, K.; Martínez-Hernández, A.; et al. Deep Multi-OMICs and Multi-Tissue Characterization in a Pre- and Postprandial State in Human Volunteers: The GEMM Family Study Research Design. Genes 2018, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Li-Gao, R.; de Mutsert, R.; Rensen, P.C.N.; van Klinken, J.B.; Prehn, C.; Adamski, J.; van Hylckama Vlieg, A.; den Heijer, M.; le Cessie, S.; Rosendaal, F.R.; et al. Postprandial Metabolite Profiles Associated with Type 2 Diabetes Clearly Stratify Individuals with Impaired Fasting Glucose. Metabolomics 2018, 14, 13. [Google Scholar] [CrossRef]
- Yu, E.A.; Yu, T.; Jones, D.P.; Ramirez-Zea, M.; Stein, A.D. Metabolomic Profiling After a Meal Shows Greater Changes and Lower Metabolic Flexibility in Cardiometabolic Diseases. J. Endocr. Soc. 2020, 4. [Google Scholar] [CrossRef]
- Yu, E.A.; He, S.; Jones, D.P.; Sun, Y.V.; Ramirez-Zea, M.; Stein, A.D. Metabolomic Profiling Demonstrates Postprandial Changes in Fatty Acids and Glycerophospholipids Are Associated with Fasting Inflammation in Guatemalan Adults. J. Nutr. 2021, 151, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, T.J.; Bakker, G.C.M.; Rubingh, C.M.; Bijlsma, S.; Stroeve, J.H.M.; van Ommen, B.; van Erk, M.J.; Wopereis, S. Ranges of Phenotypic Flexibility in Healthy Subjects. Genes Nutr. 2017, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Fiamoncini, J.; Rundle, M.; Gibbons, H.; Thomas, E.L.; Geillinger-Kästle, K.; Bunzel, D.; Trezzi, J.; Kiselova-Kaneva, Y.; Wopereis, S.; Wahrheit, J.; et al. Plasma Metabolome Analysis Identifies Distinct Human Metabotypes in the Postprandial State with Different Susceptibility to Weight Loss-mediated Metabolic Improvements. FASEB J. 2018, 32, 5447–5458. [Google Scholar] [CrossRef]
- Wopereis, S.; Stroeve, J.H.M.; Stafleu, A.; Bakker, G.C.M.; Burggraaf, J.; van Erk, M.J.; Pellis, L.; Boessen, R.; Kardinaal, A.A.F.; van Ommen, B. Multi-Parameter Comparison of a Standardized Mixed Meal Tolerance Test in Healthy and Type 2 Diabetic Subjects: The PhenFlex Challenge. Genes Nutr. 2017, 12, 21. [Google Scholar] [CrossRef]
- Morris, C.; O’Grada, C.M.; Ryan, M.F.; Gibney, M.J.; Roche, H.M.; Gibney, E.R.; Brennan, L. Modulation of the Lipidomic Profile Due to a Lipid Challenge and Fitness Level: A Postprandial Study. Lipids Health Dis. 2015, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Lautt, W.W. Postprandial Insulin Resistance as an Early Predictor of Cardiovascular Risk. Ther. Clin. Risk Manag. 2007, 3, 761–770. [Google Scholar] [PubMed]
- Shrestha, A.; Müllner, E.; Poutanen, K.; Mykkänen, H.; Moazzami, A.A. Metabolic Changes in Serum Metabolome in Response to a Meal. Eur. J. Nutr. 2017, 56, 671–681. [Google Scholar] [CrossRef]
- Moriya, T.; Satomi, Y.; Kobayashi, H. Metabolomics of Postprandial Plasma Alterations: A Comprehensive Japanese Study. J. Biochem. 2018, 163, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Krug, S.; Skurk, T.; Halama, A.; Stank, A.; Artati, A.; Prehn, C.; Malek, J.A.; Kastenmüller, G.; Römisch-Margl, W.; et al. Metabolomics of Ramadan Fasting: An Opportunity for the Controlled Study of Physiological Responses to Food Intake. J. Transl. Med. 2014, 12, 161. [Google Scholar] [CrossRef]
- Kumar, A.A.; Satheesh, G.; Vijayakumar, G.; Chandran, M.; Prabhu, P.R.; Simon, L.; Kutty, V.R.; Kartha, C.C.; Jaleel, A. Postprandial Metabolism Is Impaired in Overweight Normoglycemic Young Adults without Family History of Diabetes. Sci. Rep. 2020, 10, 353. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Maukonen, J.; Mattila, I.; Rissanen, A.; Saarela, M.; Kaprio, J.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Hyötyläinen, T.; et al. Metabolome and Fecal Microbiota in Monozygotic Twin Pairs Discordant for Weight: A Big Mac Challenge. FASEB J. 2014, 28, 4169–4179. [Google Scholar] [CrossRef]
- Fazelzadeh, P.; Hangelbroek, R.W.J.; Joris, P.J.; Schalkwijk, C.G.; Esser, D.; Afman, L.; Hankemeier, T.; Jacobs, D.M.; Mihaleva, V.V.; Kersten, S.; et al. Weight Loss Moderately Affects the Mixed Meal Challenge Response of the Plasma Metabolome and Transcriptome of Peripheral Blood Mononuclear Cells in Abdominally Obese Subjects. Metabolomics 2018, 14. [Google Scholar] [CrossRef]
- Pellis, L.; van Erk, M.J.; van Ommen, B.; Bakker, G.C.M.; Hendriks, H.F.J.; Cnubben, N.H.P.; Kleemann, R.; van Someren, E.P.; Bobeldijk, I.; Rubingh, C.M.; et al. Plasma Metabolomics and Proteomics Profiling after a Postprandial Challenge Reveal Subtle Diet Effects on Human Metabolic Status. Metabolomics 2012, 8, 347–359. [Google Scholar] [CrossRef]
- Peach, J.T.; Wilson, S.M.; Gunderson, L.D.; Frothingham, L.; Tran, T.; Walk, S.T.; Yeoman, C.J.; Bothner, B.; Miles, M.P. Temporal Metabolic Response Yields a Dynamic Biosignature of Inflammation. iScience 2021, 24, 102817. [Google Scholar] [CrossRef]
- Bütikofer, U.; Burnand, D.; Portmann, R.; Blaser, C.; Schwander, F.; Kopf-Bolanz, K.A.; Laederach, K.; Badertscher, R.; Walther, B.; Vergères, G. Serum Metabolites Responding in a Dose-Dependent Manner to the Intake of a High-Fat Meal in Normal Weight Healthy Men Are Associated with Obesity. Metabolites 2021, 11, 392. [Google Scholar] [CrossRef]
- Schwander, F.; Kopf-Bolanz, K.A.; Buri, C.; Portmann, R.; Egger, L.; Chollet, M.; McTernan, P.G.; Piya, M.K.; Gijs, M.A.M.; Vionnet, N.; et al. A Dose-Response Strategy Reveals Differences between Normal-Weight and Obese Men in Their Metabolic and Inflammatory Responses to a High-Fat Meal. J. Nutr. 2014, 144, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Calder, P.C. Metabolic and Inflammatory Responses to Different Caloric Loads of a High-Fat Meal Are Distinct between Normal-Weight and Obese Individuals. J. Nutr. 2014, 144, 1493–1494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boullart, A.C.I.; de Graaf, J.; Stalenhoef, A.F. Serum Triglycerides and Risk of Cardiovascular Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Moazzami, A.A.; Shrestha, A.; Morrison, D.A.; Poutanen, K.; Mykkänen, H. Metabolomics Reveals Differences in Postprandial Responses to Breads and Fasting Metabolic Characteristics Associated with Postprandial Insulin Demand in Postmenopausal Women. J. Nutr. 2014, 144, 807–814. [Google Scholar] [CrossRef]
- Adamska, E.; Kretowski, A.; Goscik, J.; Citko, A.; Bauer, W.; Waszczeniuk, M.; Maliszewska, K.; Paczkowska-Abdulsalam, M.; Niemira, M.; Szczerbinski, L.; et al. The Type 2 Diabetes Susceptibility TCF7L2 Gene Variants Affect Postprandial Glucose and Fat Utilization in Non-Diabetic Subjects. Diabetes Metab. 2018, 44, 379–382. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines—Old Actors Auditioning for New Roles in Metabolic Physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or Inflicting Insulin Resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut Microbiota and Intestinal FXR Mediate the Clinical Benefits of Metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Ross, A.; Willson, V.L. Paired Samples T-Test. In Basic and Advanced Statistical Tests: Writing Results Sections and Creating Tables and Figures; Ross, A., Willson, V.L., Eds.; SensePublishers: Rotterdam, The Netherlands, 2017; pp. 17–19. ISBN 978-94-6351-086-8. [Google Scholar]
- Vonesh, E.; Chinchilli, V.M. Linear and Nonlinear Models for the Analysis of Repeated Measurements; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-18019-4. [Google Scholar]
- St»hle, L.; Wold, S. Analysis of Variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Multiple Significance Tests: The Bonferroni Method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Govaerts, B.; Francq, B.; Marion, R.; Martin, M.; Thiel, M. 1.16—The Essentials on Linear Regression, ANOVA, General Linear and Linear Mixed Models for the Chemist. In Comprehensive Chemometrics, 2nd ed.; Brown, S., Tauler, R., Walczak, B., Eds.; Elsevier: Oxford, UK, 2020; pp. 431–463. ISBN 978-0-444-64166-3. [Google Scholar]
- Guisset, S.; Martin, M.; Govaerts, B. Comparison of PARAFASCA, AComDim, and AMOPLS Approaches in the Multivariate GLM Modelling of Multi-Factorial Designs. Chemom. Intell. Lab. Syst. 2019, 184, 44–63. [Google Scholar] [CrossRef]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.-J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA-Simultaneous Component Analysis (ASCA): A New Tool for Analyzing Designed Metabolomics Data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Thissen, U.; Wopereis, S.; van den Berg, S.A.; Bobeldijk, I.; Kleemann, R.; Kooistra, T.; Willems van Dijk, K.; van Ommen, B.; Smilde, A.K. Improving the Analysis of Designed Studies by Combining Statistical Modelling with Study Design Information. BMC Bioinform. 2009, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Rutledge, D.N. A Consensus Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) Strategy for Multiblock Omics Data Fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Thiel, M.; Féraud, B.; Govaerts, B. ASCA+ and APCA+: Extensions of ASCA and APCA in the Analysis of Unbalanced Multifactorial Designs. J. Chemom. 2017, 31, e2895. [Google Scholar] [CrossRef]
- Martin, M.; Govaerts, B. LiMM-PCA: Combining ASCA+ and Linear Mixed Models to Analyse High-Dimensional Designed Data. J. Chemom. 2020, 34, e3232. [Google Scholar] [CrossRef]
- Smilde, A.; Bro, R.; Geladi, P. Multi–Way Analysis: Applications in the Chemical Sciences; Wiley–Blackwell: Chichester, UK, 2004; ISBN 978-0-471-98691-1. [Google Scholar]
- Skantze, V.; Wallman, M.; Sandberg, A.-S.; Landberg, R.; Jirstrand, M.; Brunius, C. Identifying Metabotypes from Complex Biological Data Using PARAFAC. Curr. Dev. Nutr. 2021, 5, 882. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Li-Gao, R.; Hughes, D.A.; van Klinken, J.B.; de Mutsert, R.; Rosendaal, F.R.; Mook-Kanamori, D.O.; Timpson, N.J.; van Dijk, K.W. Genetic Studies of Metabolomics Change After a Liquid Meal Illuminate Novel Pathways for Glucose and Lipid Metabolism. Diabetes 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Krassowski, M.; Das, V.; Sahu, S.K.; Misra, B.B. State of the Field in Multi-Omics Research: From Computational Needs to Data Mining and Sharing. Front. Genet. 2020, 11, 1598. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathé, E.A. Metabolomics and Multi-Omics Integration: A Survey of Computational Methods and Resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Wörheide, M.A.; Krumsiek, J.; Kastenmüller, G.; Arnold, M. Multi-Omics Integration in Biomedical Research—A Metabolomics-Centric Review. Anal. Chim. Acta 2021, 1141, 144–162. [Google Scholar] [CrossRef]


| OGTT Response in Healthy Individuals | |||||
| Subjects | Samples after OGTT | Analytical Platform | Response to the OGTT vs. Fasting | Refs. | |
| Men and women (n = 16) | Plasma at 0, 30, 60, 90 and 120 min | UPLC-qTOF-MS | ↓ saturated and monounsaturated FFA ↓ C10:0, C12:0 and C14:1 acylcarnitines ↑ PUFA ↑ bile acids ↑ lysophosphatidylcholines. | [31] | |
| Men and women (n = 6) | Plasma at 0, 15, 30, 45, 60, 75, 90, 105 and 120 min | GC-MS | ↓ fatty acids ↓ AA and related metabolites (isoleucine, valine, tyrosine, phenylalanine, methionine, threonine, lysine, arginine, glycine, ornithine, 4-hydroxyproline) and threonic acid ↑ alpha-tocopherol, cystine | [33] | |
| OGTT Response Depending on IR Status | |||||
| Subjects | Samples | Analytical Platform | Response to the OGTT vs. Fasting | Comparison vs. Healthy Individuals | Refs. |
| Healthy men and women (n = 22): Young adults from Metabolic Abnormalities in College Student study, aged 18–30 year old Validation cohort (n = 25): Participants from Framingham Offspring study, aged 40–49 year-old Subjects with impaired glucose tolerance (n = 25): Participants from Framingham Offspring study, aged 40–50 year old, 2 h glucose concentration between 140 and 199 mg/100 mL | Plasma at 0, 30, 60, 90 and 120 min | LC-MS | 18 metabolites changed during the OGTT: ↓ AA and related metabolites (valine, leucine/isoleucine, phenylalanine, tyrosine, histidine, lysine, arginine, methionine, ornithine and citrulline) ↓ urea cycle intermediates: ornithine and citrulline ↓ glycerol ↓ ketogenesis product (β-hydroxybutyrate) ↓ hypoxanthine ↑ glycolysis products (pyruvate and lactate) ↑ bile acids ↑ hippuric acid | Higher fasting insulin vs. lower fasting insulin groups: Smaller ↑: lactate and bile acid Smaller ↓: AA (leucine/isoleucine, valine and methionine), β-hydroxybutyrate and glycerol | [32] |
| Nondiabetic individuals (n = 377): Framingham Offspring cohort participants, mean age 57 years, Mean BMI = 30 kg/m2 IR was defined as the top quartile of HOMA-IR from the entire cohort free of diabetes at the 5th examination cycle. | Plasma at 0 and 120 min | LC-MS | 91 metabolites significantly changed with OGTT: ↓ AA (including BCAA and AAA) ↓ β-hydroxybutyrate ↓ TCA cycle intermediates, ↓ unconjugated bile acids ↓ urea cycle metabolites (citrulline, ornithine and arginine) ↓ nucleic acids derivatives (hypoxanthine, xanthine etc.) ↓ serotonin derivatives and B vitamins ↑ glycolysis products (lactate, phosphoenolpyruvic acid and phosphoglycerate) ↑ conjugated bile acids | IR vs. IS groups: Smaller ↓ for β-hydroxybutyrate, isoleucine and pyridoxate Smaller ↑ for lactate Higher ↓ for orotate Changes in taurodeoxycholates, niacinamide and ornithine associated with HOMA-IR with a blunted response for individuals with greater IR | [34] |
| Non-diabetic, IR individuals (n = 470): Men, 70.6 years old | Plasma at 0, 30 and 120 min | UPLC-TOF-MS | Not applicable | 35 metabolites associated with IR: 7 glycerophospholipids, 6 glycerolipids, 4 glycerophosphoethanolamines, 6 unsaturated FAs, 4 acylcarnitines, 2 bile acids and one each of monosaccharide, peptide, SFA, steroids, imidazopyrimidine and propranolol IR vs. IS individuals: Smaller ↓: oleate, palmitoleate, C10-carnitine and C12-carnitine Lack of ↓: LPE 18:1, LPE 18:2 and LPE 20:4 ↑ Deoxycholate-glycine ↑ for both IR and IS subjects followed by a smaller ↓ to baseline in IR subjects: hexoses | [39] |
| Healthy individuals (n = 4745): middle-aged Finnish Replication cohort (n = 595): senior Finnish participants IS-NGT group: fasting insulin at the bottom quartile of NGT and fasting glucose < 5.6 mmol/L and 2-h glucose < 7.8 mmol/L) IR-NGT group: fasting insulin at the top quartile of NGT and fasting glucose < 5.6 mmol/L and 2-h glucose < 7.8 mmol/L) | Serum at 0, 30, 60 and 120 min | NMR | ↑ glycolysis intermediates (pyruvate and lactate) ↑ TCA intermediate (citrate) ↓ BCAA (isoleucine, leucine an and valine) and AAA (phenylalanine and tyrosine) ↓ ketone bodies (beta-hydroxybutyrate and acetoacetate). ↓ Acetate, TG and glycerol | IR-NGT vs. IS-NGT groups: Smaller ↑: glycolysis products (pyruvate, lactate and alanine) Smaller ↓ for ketone bodies (β-hydroxybutyrate and acetoacetate), BCAA, glycerol and VLDL and HDL TG Similar findings were reported in individuals with prediabetes and diabetes compared to the IR-NGT group. | [35] |
| Healthy men and women (n = 246): Non-obese (BMI < 30 kg/m2), young adults aged 18–35 | Plasma at 0 and 120 min | LC-MS | 405 lipids significantly perturbated following the OGTT. ↓ sphingolipids and SM ↓ acylcarnitines, especially C14:2 ↓ LPC, LPE and lysophosphatidylinositol ↓ DG and TG species containing MUFA or SFA ↑ lysoetherphospholipid species | Association with HOMA-IR: Positive association: 12 ceramides, 10 deoxyceramides, 22 PE, 6 PS, 19 DG and 42 TG. Negative association: 3 alkylphosphatidylcholine, 16 alkenylphosphatidylcholine, 6 medium- to long-chain acylcarnitine, 9 cholesteryl ester containing omega-3 and omega-6 polyunsaturated fatty acids and 9 hexosylceramide species. Cholesteryl ester (20:4) displayed the strongest association with 17.8% decrease per HOMA-IR unit Association with Insulin AUC: 5 Medium chain acylcarnitines, 25 TG, 10 DG, 15 LPC, 10 PE and 4 PS | [36] |
| NGT group (n = 234): fasting glucose < 5.6 mmol/L and OGTT 2 h glucose < 7.8 mmol/L Prediabetes group (n = 281): 5.6 ≤ fasting glucose < 7.0 mmol/L or 7.8 ≤ OGTT 2 h glucose < 11.1 mmol/L Newly diagnosed T2D group (n = 66): fasting glucose ≥ 7.0 mmol/L or OGTT 2 h glucose ≥ 11.1 mmol/L | Plasma at 0 and 120 min | LC-MS | 35 increased metabolites (NGT: 18, prediabetes: 23, T2D: 13): ↑ tauropine ↑ methane metabolism related metabolites ↑ glycolysis related metabolites (3-phospho-D-glycerate) 45 decreased metabolites (NGT: 36, prediabetes: 29, T2D: 18) ↓ AA-related metabolites (glutamate and citrulline) ↓ purine metabolism metabolites: xanthosine, AMP and hypoxanthine ↓ fatty acids oxidation metabolites | T2D vs. NGT groups: 22 metabolites significantly different ↑ AA: glutamate and homocysteine ↑ TCA cycle and glycolysis intermediates: succinate, malate, pyruvate Smaller ↓ pentose phosphate pathway (D-glycerate) Smaller ↓ galactose metabolism (alpha-D-galactosyl-(1-3)-1D-myo-inositol) | [38] |
| Adolescent aged 10–18 year-old Group 1: Lean, NGT, IS group (n = 21) Group 2: Obese, NGT, IS group (n = 18) Group 3: Obese, NGT, IR group (n = 20) Group 4: Obese, IGT, IR group (n = 23) | Plasma at 0, 15, 30, 60,90 and 120 min | NMR | IR groups (group 3–4) vs. IS groups (1–2): ↑ BCAA (valine, leucine, isoleucine), AAA (tyrosine, phenylalanine) and lysine, 2-oxoisocaproic acid ↓ serine, glycine, myo-inositol and dimethylsulfone In obese groups (2–3-4) compared to the lean group (1): ↑ glutamate, alanine, pyruvate and O-acetylcarnitine ↓ acetate | [40] | |
| OGTT Response Depending on Obesity and BMI Status | |||||
| Subjects | Samples after OGTT | Analytical Platform | Response to the OGTT vs. Fasting | Comparison vs. Healthy Individuals | Refs. |
| Obese group (n = 14): BMI 43.6 kg/m2 Lean group (n = 6): BMI of 22.4 kg/m2 | Serum at 0, 30 and 120 min | GC-MS | In the obese group: 52 metabolites affected by OGTT No change at 30 min and ↓ at 120 min: β-hydroxybutyrate, glycerol, hypoxanthine and fatty acids Heterogeneity in AA and fatty acids profiles with overall ↓at 120 min | Obese vs. lean group: 16 metabolites significantly different (out of 59) 30 min delayed ↓: palmitic acid, lauric acid, oleic acid, pentadecanoic acid and stearic acid. 30 min ↑ asparagine, glutamate, taurine, tyrosine, isoleucine and leucine. 30 min lack of ↑: pyrophosphate, threonic acid, phenylalanine, serine, glyceric acid and aspartate. | [41] |
| Young college students aged 18–23 years Lean group (n = 15): BMI > 18.5 and <23 kg/m2 Obese group (n = 15): BMI > 27.6 kg/m2 | Serum at 0, 30, 60, 90 and 120 min | UPLC–TQ–MS GC-MS | In lean subjects: ↓ fatty acids (C18:3, C18:2, C16:1 and C16:0) ↓ amino acids (BCAA, phenyalanine, tryptophan, alanine, proline, glycine, methionine, serine, arginine, threonine, asparagine and lysine) ↓ biogenic amines (taurine, creatine and GABA) ↑ niacinamide, tyrosine, histidine and glycerophophorylcholine | In obese vs. lean group: Smaller ↓ for most AA and fatty acids BMI, waist circumference, body fat and fat mass were positively associated with arginine, histidine and GABA OGTT postprandial changes. OGTT postprandial changes in palmitic acid, BCAA, phenylalanine and lysine were positively associated with fasting insulin and HOMA-IR in the obese group. | [42] |
Obese individuals (n = 14) compared at:
| Serum at 0, 30 and 120 min | GC-MS | Not applicable | After weight loss and weight-maintenance phases compared to baseline in obese individuals: Higher ↓: AAA (tyrosine and phenylalanine), BCAA (leucine, isoleucine), FFA and glycerol Suppressed ↑: glutamate and glutamine | [43] |
| Young healthy twins (n = 274): monozygotic twin pairs (n = 64) and dizygotic twin pairs (n = 73) with either concordant BMI or discordant BMI | Serum at 0, 30 60 and 120 min | NMR | Response in the whole population: ↓ AA (BCAA, tyrosine, phenylalanine, histidine, glycine and glutamine) ↓ ketone bodies (β-hydroxybutyrate, acetoacetate and acetate) ↓ glycerol, fatty acids, total TG and VLDL TG ↑ glycolysis products (lactate and pyruvate) ↑ very large HDL-TG and small HDL-TG | Individuals with higher BMI and liver fat content vs. cotwins: Smaller ↓ isoleucine, SFA and MUFA, TG, small VLDL and small LDL | [37] |
| Adolescents (8–17 year-old) clinically healthy Lean group (n = 55): BMI percentile < 85th for sex and age) Overweight or obese group (n = 228): BMI percentile ≥ 85th for sex and age | Plasma at 0 and 60 min | LC-MS | Response in the whole population: ↓ medium and long-chain acylcarnitines, FFA, lipids, such as SMs, PCs and DGs ↑ hippurate | Obese or overweight vs. lean groups: Smaller ↑: medium and long-chain acylcarnitines, FA oxidation intermediates and FAs | [44] |
| OGTT Response Depending on Lipidemic Status | |||||
| Subjects | Samples after OGTT | Analytical Platform | Response to the OGTT vs. Fasting | Comparison vs. Healthy Individuals | Refs. |
| Healthy group (n = 35) HLP group (n = 35): TG > 1.7 mmol/L Total cholesterol >5.7 mmol/L | Serum at 0 and 120 min | UPLC-TQ-MS | Healthy group: ↓ methionine, aminobutyric acid, niacinamide, 4-hydroxy-l-proline, valine, GABA, glutamic acid, asparagine, tyrosine and allantoin) ↑ serine, taurine, cysteine and creatine In the HLP group: ↑ leucine, isoleucine, serine, histidine, lysine, γ-aminobutyric acid, taurine, cysteine and creatine ↓ methionine, dimethylglycine, aminobutyric acid, niacinamide, allantoin and creatinine | HLP vs. healthy groups: Higher ↑: cysteine, taurine, lysine, histidine and leucine. ↑ instead of ↓: GABA, tyrosine, asparagine, isoleucine and valine ↓ instead of ↑: dimethylglycine and creatine Smaller ↓: methionine Within the HLP group, IR vs. non IR subjects: Higher ↑: GABA, tyrosine, taurine, isoleucine, leucine and valine. Correlation within HLP group with IR: Isoleucine and GABA postprandial OGTT changes positively correlated to HOMA-IR | [45] |
| Healthy group (n = 50) HLP group (n = 38): TG > 1.7 mmol/L Total cholesterol > 5.7 mmol/L | Serum at 0 and 120 min | GC-MS | Healthy group: ↓ glycolysis (lactic acid) ↓ TCA cycle intermediates (citric acid and malic acid) ↓ ketogenesis product (β-hydroxybutyrate) ↓ pyroglutamic acid, α-hydroxybutyrate, pimelic acid and suberic acid ↑ cis-aconitic acid | Hyperlipidemic vs. healthy groups at 120 min: Smaller ↑: glycolysis products (pyruvate, phosphoenol pyruvate), TCA cycle intermediates (oxalic acid, isocitric acid, fumaric acid) and orotic acid ↓ instead of ↑: glycolysis product (lactate), TCA cycle intermediates (malonic acid, citric acid), sebacic acid, suberic acid, pyroglutamic acid, glycolic acid, α-hydroxybutyrate and caprylic acid ↑ instead of ↓: 2-hydroxyisocaproic acid | [46] |
| Healthy Population | ||||||
|---|---|---|---|---|---|---|
| Subjects | Samples | Analytical Platform | Response to Challenge Meal (vs. Fasting) | Main Meal Ingredients | Meal Macronutrient Composition (kcal, %E) | Refs. |
| Healthy men and women (n = 123) | Plasma at 0 and 2 h after meal intake | LC-MS | 1130 features significantly different Key pathways affected in C18 analyses:
| Mixed liquid meal Incaparina (vegetable protein mixture): 12 g, skim milk (lactose-free): 170 mL, safflower oil: 25 g, sugar: 52 g | 520 kcal Carbohydrate: 52%E Lipid: 42%E Protein: 6%E | [55] |
| Healthy men and women (n = 100) age range: 19–71 years-old Classification in 10 groups according to gender, age and body fat percentage Optimal phenotypic flexibility group: 20–29-year-old men and women with low to normal body fat (<20%) Reduced phenotypic flexibility group: 60–70-year-old men and women with normal to high body fat (>20%) | Plasma at 0, 0.5, 1, 2, 4, 6 and 8 h after meal intake | GC-MS | “reduced phenotypic flexibility” group vs. “optimal phenotypic flexibility group”: ↑ 4-methyl-2-oxovalericacid, 3-methyl-2-oxovaleric acid, tyrosine, isoleucine | High fat liquid meal 400 mL beverage Palm olein: 12.4% (weight/weight), dextrose: 17.25%, protifar (Nutricia): 4.13%, vanilla flavor: 0.10%, trisodium citrate: 0.12%, sodium hydroxide: 0.08%, water: 66.12% | 950 kcal Carbohydrate: 33%E Lipid: 59%E Protein: 8%E | [61] |
| Healthy men and postmenopausal women (n = 72): Mean age: 59.2 ± 4.2 y; Mean BMI: 29.7 ± 2.7 kg/m2 | Plasma at 0, 1, 2, 4, 6 and 8 h after meal intake | LC-MS/MS | Key metabolites for the identification of two distinct metabotypes: Lipolysis: glycerol, FFA Ketegogenesis: 3-OH-butyric acid, β-oxidation: acetylcarnitine (C2), hexanoylcarnitine (C6), octanoylcarnitine (C8), decanoylcarnitine (C10), dodecanoylcarnitine (C12), miristoylcarnitine (C14) and the ratio of medium-to-long chain acylcarnitines In metabotype B (considered as prediabetic with decreased insulin sensitivity and higher visceral fat) vs. metabotype A: smaller ↑ after an initial ↓: lipolysis products (FFA), ketogenesis products (3-OH-butyric acid) and β-oxidation products (acylcarnitines) | High fat liquid meal 400 mL beverage Palm olein: 12.4% (weight/weight), dextrose: 17.25%, protifar (Nutricia): 4.13%, vanilla flavor: 0.10%, trisodium citrate: 0.12%, sodium hydroxide: 0.08%, water: 66.12% | 950 kcal Carbohydrate: 33%E Lipid: 59%E Protein: 8%E | [62] |
| Healthy men and women (n = 40): aged 18–60 years | Plasma at 0, 1, 2, 3, 4 and 5 h after meal intake | LC-MS/MS-ESI | Metabolite with a fold change > 1.5 between 0 min and following time-point: At 60 min: N-C10:0(OH)-Cer(2H) and N-C26:0-Cer(2H) At 120 min: LPE a C18:2, LPE a C18:1, PE aa C36:2, PE aa C36:3 and N-C16:1-Cer, PG aa C36:2 At 180 min: PE aa C36:1, PG aa C34:1 and N-C24:0(OH)-Cer(2H), PG aa C36:2 At 300 min: N-C25:0(OH)-Cer LPE a C18:2, PE aa C36:2 and PE aa C36:3 were predictive of fasting and peak plasma TG concentrations following the challenge test. | High fat liquid meal Calogen (Nutricia): 100 mL, liquid Duocal (SHS Nutrition): 50 mL | 533 kcal Carbohydrate: 8%E Lipid: 92%E Protein: 0%E | [64] |
| Healthy postmenopausal women (n = 19): Divided on 2 subgroups (A and B) based on insulin response. Subgroup B compared to subgroup A: Higher postprandial insulin response for similar glucose response | Serum at 0, 30, 45, 60, 90 and 180 min after meal intake | LC-MS NMR | 73 metabolites significantly different ↓ acylcarnitines ↓ ketone bodies (3-hydroxybutyrate, acetone) ↑ and ↓ below baseline: leucine, isoleucine (and catabolic products), phenylalanine, methionine and threonine catabolic products ↑ and ↓ to baseline: alanine and proline, 2-hydroxyisovalerate (originates from ketogenesis and BCAA) ↑ and ↓: glycolysis products (pyruvate, lactate) ↑ and ↓: TCA cycle intermediates (succinate, citrate) Subgroup B vs. subgroup A at 180 min after meal intake: Higher ↑ just after meal intake: leucine ↑ followed by smaller ↓: AA (lysine, serine), creatinine Smaller ↓ acylcarnitines (C3, C4, C5, C16) No ↓ PCs (PC aa C28:1, PC ae C38:1, PC ae C40:1, PC ae C42:3) | Complex meal Refined wheat bread, cucumber: 40 g, noncaloric orange drink: 300 mL | For bread alone: 281 kcal Carbohydrate: 71.1%E Lipid: 16.6%E Protein: 12.8%E | [66] |
| Healthy men and women (n = 10) Aged 25–50 years old | Plasma at 1 h before meal and 1 h after meal intake | UPLC-MS/MS GC-MS | ↑ glycolysis related products (pyruvate) ↑ primary and secondary bile acids (conjugated form) ↑ AA ↑ TCA cycle related products (malate, citrate) ↓ succinate ↓ lipolysis and β-oxidation related products (glycerol, fatty acids and 3-hydroxybutyrate) ↓ endocannabinoids | Complex meal Broiled salmon, pork cutlet, shao mai, Japanese omelet, kamaboko, ganmodoki, rice, tomato, lemon, vegetable mix, ginger, heavenly bamboo, carrot, taro, snow pea and bamboo shoot | 763 kcal Carbohydrate: 53.2%E Lipid: 28.8%E Protein: 15.5%E | [67] |
| Healthy men (n = 11) Muslim volunteers of varying BMI and age performing Ramadan fasting | plasma at 0 and 2 h after meal intake | LC-MS/MS FIA-MS/MS | 48 metabolites were significantly changed ↑ AA (asparagine, arginine, alanine, glutamate, proline and phenylalanine) ↑ methionine sulfoxide (degradation product of methionine with reactive oxygen species, potential marker of oxidative stress) ↑ glycine/taurine conjugated bile acid ↓ long-chain acyl-carnitine ↓ polyamine (spermidine and putrescine) | Complex meal Meal week 1: white rice: 100 g, egg pasta: 50 g, chicken meat: 150 g, bell pepper: 50 g, avocado: 50 g, whipping cream 30% fat: 20 g, orange juice: 200 mL, Italian salad: 100 g, rice pudding: 100 g, pita bread: 40 g, vegetable soup: 200 mL Meal week 4: white rice: 120 g, raisin: 15 g, hazelnuts: 15 g, yoghurt 3.5% fat: 150 mL, pita bread: 40 g, orange juice: 200 mL, lamb muscular meat with intermuscular fat: 200 g, lentil soup: 200 mL, white bread with grains: 13 g and Italian salad: 100 g | Meal week 1: 1097 kcal Carbohydrate: 38.5 E% Lipid: 38.2E% Protein: 23.3E% Meal week 4: 1322 kcal Carbohydrate: 37.8 E% Lipid: 42.3E% Protein: 19.9E% | [68] |
| Subjects | Samples | Analytical Platform | Observed Response to Challenge Meal (vs. Fasting) | Comparison vs. Healthy Controls | Meal Ingredients | Meal Macronutrient Composition (kcal, %E) | Refs. |
|---|---|---|---|---|---|---|---|
| Healthy overweight men (n = 36): BMI: 25.6–34.7 kg/m2 Mildly elevated C-reactive protein (CRP) levels: 1.0–8.1 µg/L | Plasma at 0, 1, 2, 3, 4 and 6 h after meal intake | GC-MS | 106 metabolites significantly modified Late ↑ lipolysis related products (long chain FFA and glycerol) Late ↑ ketogenesis related products (3-hydroxybutanoic acid and acetonacetate) Late ↑: succinate ↑ followed by ↓ below baseline: Glycolysis and TCA intermediates related products (pyruvate, citrate, α-keto-glutaric acid) ↑ followed by ↓ to baseline: most amino acids ↓ lactate ↓ uric acid | Effects of an anti-inflammatory dietary mix in a cross-over, double-blind intervention. 31 plasma features (metabolites and proteins) were significantly different between groups with 17 uniquely identified at the fed state compared to the fasting state. | Complex meal Dairy shake 500 mL: Custard: 300 mL, cream cheese: 150 mL and whipping cream: 50 mL | 706 kcal Carbohydrate: 29.6 E% Lipid: 58.7E% Protein: 11.7E% | [72] |
| healthy women Low BMI group (n = 8): BMI = 25 High BMI group (n = 8): BMI = 32 | Plasma at 0, 0.5, 3 and 5 h after meal intake | HPLC-ESI-MS | High BMI vs. low BMI group: Smaller ↑: alanine, arginine, asparagine, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, proline, serine, threonine, tryptophan and valine Higher ↑: aspartic acid, cysteine, glutamic acid and phenylalanine ↓ acylcarnitine C14:3 (30 min) ↓ acylcarnitine (300 min) | Mixed liquid meal Ensure Plus® (Abbott Nutrition, Lake Buff, Illinois, USA) | 30% of the participant’s daily energy requirement based on total body weight and fat free mass (kg) Carbohydrate: 57%E Lipid: 28%E Protein: 15%E | [57] | |
| Healthy men and women (n = 50) Monozygotic twins discordant for weight (n = 32): BMI difference >3 kg/m2 Monozygotic twins concordant for weight (n = 18): BMI difference < 3 kg/m2 | Serum at 0, 0.5, 1 and 2 h after meal intake | GC-GC-TOFMS UPLC-QTOFMS UPLC-QqQMS | 20 metabolites with a converging time profile (≠ at fasting but = at fed state): ↑ at fasting state in heavier co-twins: isoleucine and valine ↓ at fasting state in heavier co-twins: fatty acids 22 metabolites with diverging time profile (= at fasting but ≠ at fed state) ↑ at fed state in heavier co-twins: sugar derivatives (arabinitol) and organic acids (acetic acid) ↓ at fed state in heavier co-twins: lipids and bile acids | Complex meal McDonald’s Big Mac MealTM (Chicago, Illinois, USA) 1 Big Mac hamburger, French fries: 100 g, sucrose-sweetened Coca-ColaTM: 400 g | 979 kcal Carbohydrate: 50 E% Lipid: 37 E% Protein: 13 E% | [70] | |
| Lean group (n = 15): BMI: 23.0 Abdominally obese men group (n = 29): BMI = 30.3 This group underwent an 8-wk weight loss intervention or control intervention | Plasma at 0, 0.5, 1, 2, 3 and 4 h after meal intake | UPLC UPLC-MS/MC GC-MS ESI-MS NMR | Lean vs. obese: alanine, proline, threonine, histidine, methionine, 2-hydroxyisovalerate (degradation product of BCAA), phosphocholine and methylmalonic acid significantly different. Effect of the weight-loss intervention in the obese group: At the fed state, 11 metabolites were significantly changed before and after intervention: Oxylipins, glutamine, histidine, creatine, pyroglutamic acid, glucose and choline | Complex meal 2 muffins, 0% fat milk: 300 mL | 1100 kcal Carbohydrate: 44%E Fat: 46.3%E Protein: 9.6%E | [71] | |
| nondiabetic men with high T2D risk genotypes at the rs7901695 locus (n = 8): age: 31.2 ± 6.3 y, BMI (kg/m2): 28.5 ± 8.1) or Low T2D risk genotypes at the rs7901695 locus (n = 13): age: 35.2 ± 10.3 y, BMI: 28.1 ± 6.4 | Plasma at 0, 0.5, 1 and 2 h after meal intake | UHPLC-Q-TOF | AUC from 0 to 120 min after challenge meal. In High Risk group compared to low risk group: Following High Carbohydrate meal: ↓ AUC phospholipids, lysophospholipids, sphingolipids, arachidonic and oleic acids, their metabolites: keto- and hydoxy-fatty acids, leukotrienes, uric acid and pyroglutamic acid. Following Normal Carbohydrate meal: ↑ AUCs of postprandial sphingosine Following both meal type: ↓ AUCs of acylcarnitines ↑ AUCs of fatty acid amides. | Mixed liquid meal Normal carbohydrate meal: Cubitan (Nutricia): 360 mL High carbohydrate meal: Nutridrink Juice Style, Fat Free, (Nutricia): 300 mL | 450 kcal Normal carbohydrate meal: Carbohydrate: 45%E Lipid: 25%E Protein 30%E High carbohydrate meal: Carbohydrate: 89%E Lipid: 0%E Protein: 11% | [56] | |
| Healthy men and women (n = 123) Mean and women with cardiometabolic disease (n = 226): Either obesity, diabetes, hypertension or metabolic syndrome | Plasma at 0 and 2 h after meal intake | LC-MS | In healthy participants: 1383 features were significantly changed In cardiometabolic disease group: 1756 features were significantly changed | 22 metabolites differed after meal challenge and had different response depending on cardiometabolic disease status. Examples includes:
| Mixed liquid meal Incaparina (vegetable protein mixture): 12 g, skim milk (lactose-free): 170 mL, safflower oil: 25 g, sugar: 52 g | 520 kcal Carbohydrate: 52%E Lipid: 42%E Protein: 6%E | [59] |
| Healthy men and women (n = 110) aged 18–40, divided in 4 groups: Normal healthy control (n = 30) 1st degree relatives of patients with T2D (n = 30) Overweight group (n = 30): BMI: 23–30 kg/m2 Prediabetes group (n = 20): Fasting glucose levels: 100–125 mg/dL | Plasma at 0, 1 and 2 h after meal intake | LC-QToF-MS | In Overweight group compared to control and T2D relative group: ↓ (fasting and fed state): MG(22:2(13Z,16Z)/0:0/0:0) and LPC (15:0) In prediabetes group compared to control group: ≠ LPE (0:0/18:2(9Z, 12Z)), LPE (0:0/20:4(5Z, 8Z,11Z,14Z)), 10, 11-dihydro-leukotriene B4 and 3-Oxocholic acid In men: Association with ↓ IS: triglycerides, VLDL, C-peptide, uric acid, xanthine and GCDC-3-glucuronide In women: Association with ↑ IS: HDL, leptin, adiponectin, glutathione-conjugate, phytosphingosine and lysophospholipids | Complex meal Idli, chutney (coconut and Bengal gram), milk tea, skimmed milk powder, table sugar and coconut oil | 25% of the total daily energy required per day—calculated with ideal body weight Carbohydrate: 55% Lipid: 30% Protein: 15% | [69] | |
| Three groups of men and women (45–65 years old) without any history of IFG or T2D stratified by fasting glucose concentrations: NGT (n = 176): Fasting glucose ≤ 6.0 mmol/L IFG (n = 186): Fasting glucose ≥ 6.1 and < 7.0 mmol/L T2D (n = 171)): Fasting glucose ≥ 7.0 mmol/L | Plasma 0 and 2.5 h after meal intake | FIA-ESI-MS/MS | NGT vs. T2D: At 150-min after challenge meal: a profile of four metabolites (acylcarnitine C16:1 and C4:1, glycine and LPC a C17:0) was able to distinguish the T2D group from the NGT group. Response profile (value at 150 min—value at fasted state): A profile of 16 metabolites (50% short-chain acylcarnitines) distinguished T2D and NGT groups. | Mixed liquid meal 400 mL Ingredients details not available | 600 kcal Carbohydrate: 50%E Lipid: 34%E Protein: 16%E | [58] | |
| Men aged 30–70 years old Healthy group (n = 20): BMI: 20.0–25.0 kg/m2 T2D group (n = 20): BMI: 25.1–34.9 kg/m2 | plasma at 0, 0.5, 1, 2, 4, 6 and 8 h after meal intake | GC-MS | In healthy group: (selected examples) Late ↑: lipolysis (glycerol, several FFA) Late ↑: ketogenesis (3-hydroxybutanoicacid, acetoacetate, 2-hydroxybutanoic acid). ↑ followed ↓ to baseline: most AA ↑ followed ↓ to baseline: glycolysis (pyruvate and, glycerol-3-phosphate) ↓ followed ↑ to baseline: TCA cycle intermediates (succinate, malate and citrate) | T2D vs. healthy: 58 features different between groups: ↓ lipolysis (blunted glycerol and non-essential FFA response) ↓ ketogenesis (delayed ↓ and smaller ↑ for 3-hydroxybutanoic acid and acetoacetate) Higher ↑ for most AA (BCAA and derivatives, serine, lysine, threonine, glutamate and tyrosine) | High fat liquid meal 400 mL beverage, palm olein: 12.4% (weight/weight), dextrose: 17.25%, protifar (Nutricia): 4.13%, vanilla flavor: 0.10%, trisodium citrate: 0.12%, sodium hydroxide: 0.08%, water: 66.12% | 950 kcal Carbohydrate: 33%E Lipid: 59%E Protein: 8%E | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lépine, G.; Tremblay-Franco, M.; Bouder, S.; Dimina, L.; Fouillet, H.; Mariotti, F.; Polakof, S. Investigating the Postprandial Metabolome after Challenge Tests to Assess Metabolic Flexibility and Dysregulations Associated with Cardiometabolic Diseases. Nutrients 2022, 14, 472. https://doi.org/10.3390/nu14030472
Lépine G, Tremblay-Franco M, Bouder S, Dimina L, Fouillet H, Mariotti F, Polakof S. Investigating the Postprandial Metabolome after Challenge Tests to Assess Metabolic Flexibility and Dysregulations Associated with Cardiometabolic Diseases. Nutrients. 2022; 14(3):472. https://doi.org/10.3390/nu14030472
Chicago/Turabian StyleLépine, Gaïa, Marie Tremblay-Franco, Sabrine Bouder, Laurianne Dimina, Hélène Fouillet, François Mariotti, and Sergio Polakof. 2022. "Investigating the Postprandial Metabolome after Challenge Tests to Assess Metabolic Flexibility and Dysregulations Associated with Cardiometabolic Diseases" Nutrients 14, no. 3: 472. https://doi.org/10.3390/nu14030472
APA StyleLépine, G., Tremblay-Franco, M., Bouder, S., Dimina, L., Fouillet, H., Mariotti, F., & Polakof, S. (2022). Investigating the Postprandial Metabolome after Challenge Tests to Assess Metabolic Flexibility and Dysregulations Associated with Cardiometabolic Diseases. Nutrients, 14(3), 472. https://doi.org/10.3390/nu14030472