Abstract
Inflammasomes are key intracellular multimeric proteins able to initiate the cellular inflammatory signaling pathway. NLRP3 inflammasome represents one of the main protein complexes involved in the development of inflammatory events, and its activity has been largely demonstrated to be connected with inflammatory or autoinflammatory disorders, including diabetes, gouty arthritis, liver fibrosis, Alzheimer’s disease, respiratory syndromes, atherosclerosis, and cancer initiation. In recent years, it has been demonstrated how dietary intake and nutritional status represent important environmental elements that can modulate metabolic inflammation, since food matrices are an important source of several bioactive compounds. In this review, an updated status of knowledge regarding food bioactive compounds as NLRP3 inflammasome modulators is discussed. Several chemical classes, namely polyphenols, organosulfurs, terpenes, fatty acids, proteins, amino acids, saponins, sterols, polysaccharides, carotenoids, vitamins, and probiotics, have been shown to possess NLRP3 inflammasome-modulating activity through in vitro and in vivo assays, mainly demonstrating an anti-NLRP3 inflammasome activity. Plant foods are particularly rich in important bioactive compounds, each of them can have different effects on the pathway of inflammatory response, confirming the importance of the nutritional pattern (food model) as a whole rather than any single nutrient or functional compound.
1. Introduction
1.1. The NLRP3 Inflammasome
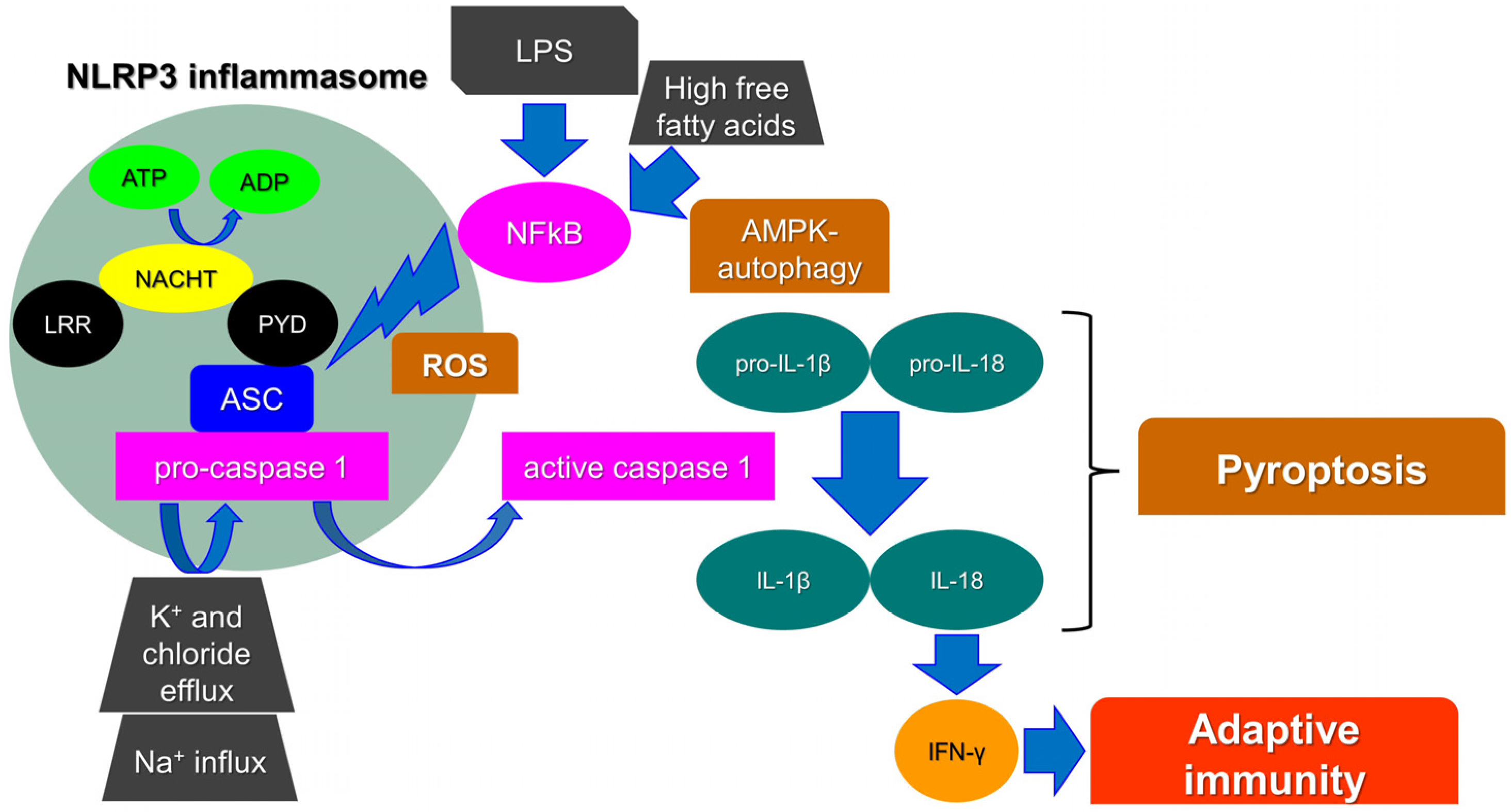
Inflammasomes are key intracellular multimeric protein complexes, able to initiate inflammatory signaling through sensor receptors, defined as pattern-recognition receptors (PPR). PPRs recognize pathogen-associated molecular patterns (PAMPs), or damage-associated molecular patterns (DAMPs) generated by endogenous stress stimuli. The signal transduction continues intracellularly through an adaptor protein and an effector enzyme that cause the maturation and the secretion of pro-inflammatory cytokines. As a result, the inflammasome activation ends up with the production of caspase-1, a major mediator in the inflammatory adaptive immune cell response [1]. Pro-interleukin 1β (pro-IL-1β) and pro-IL-18 are cleaved by active caspase-1 into their biologically active form to trigger endothelial cell responses, such as vasodilatation, which allows the extravasation of immune cells, or acts to control hypotension, fever, and pain threshold. In particular, IL-18 is crucial for interferon-gamma (IFN-γ) production and thus for adaptive immunity [2]. Finally, caspase-1 activation stimulates a unique pro-inflammatory cell death process, namely pyroptosis. Pyroptosis triggers intracellular pathogens to escape from their replicative environment, leading to their exposition to immune factors and thus allowing the immune system to face infections (Figure 1) [3].
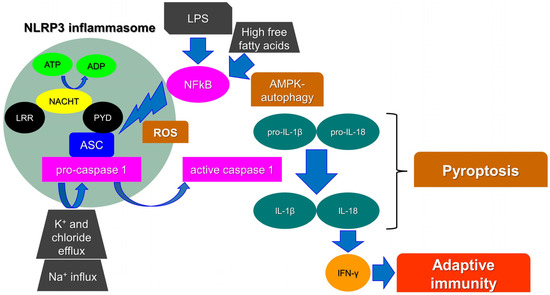
Figure 1.
NLRP3 activation and the main players involved in pyroptosis. Leucine-rich repeat (LRR), pyrin domain (PYD), interferon-gamma (IFN-γ), interleukin (IL), reactive oxygen species (ROS), lipopolysaccharide (LPS), NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3), caspase-recruitment domain (ASC), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), AMP-activated protein kinase (AMPK), adenosine diphosphate (ADP), adenosine triphosphate (ATP).
Among other PPRs, leucine-rich repeat (LRR)-containing proteins (NLR) family members (NLRP1 and 3) are widely known as components of inflammasomes. In particular, it has been reported that the NLRP3 inflammasome is crucial for adaptive cell responses towards viral, bacterial, and fungal infections. Nevertheless, when dysregulated, it has been associated with a variety of inflammatory or autoinflammatory disorders, such as diabetes, gouty arthritis, liver fibrosis, Alzheimer’s disease (AD), respiratory syndromes, atherosclerosis, and cancer initiation [4]. The NLRP3 protein consists of a C-terminal leucine-rich repeat (LRR) domain and an amino-terminal pyrin domain (PYD). The central domain is a nucleotide-binding, oligomerization domain (NACHT domain). The interaction between the PYD domain with the caspase-recruitment domain (ASC) facilitates the initiation of the inflammasome assembly. In parallel, the NACHT domain functions as an ATPase and the amount of ADP produced allows NLRP3 oligomerization and activation [5]. Finally, the LRR domain functions as a signal effector; although, its role is still not fully understood. It has been highlighted that the NLRP3, lacking the LRR domain, can be fully activated via the canonical inflammasome pathway. It has been therefore hypothesized that it may act as an auto-inhibitor, thus controlling aberrant signal amplification and participating in a protective mechanism, avoiding autoinflammation [6].
Many molecular mechanisms/compounds have been widely proposed for the activation of NLRP3, involving, among others, biological, physical, chemical, and metabolic agents, such as uric acid crystals, cholesterol, free fatty acids and lipids, β-amyloid (BA) protein, aluminum hydroxide, high intracellular glucose, and high levels of reactive oxygen species (ROS). Others factors, such as heme, ionic flux, lipopolysaccharide (LPS) toxin, pathogen-associated RNA, and ceramides, are also included [7]. Likewise, Ca2+-related signaling, Na+ influx, and K+ and chloride efflux have been reported as crucial events in the NLRP3 inflammasome [8]. As a matter of fact, a recently identified component of the NLRP3 inflammasome, namely NEK7 (NIMA-related kinase 7), requires K+ efflux for inflammasome assembly. It has been proposed that the activation of NLRP3 requires two steps. Firstly, the mitotic kinase NEK7 binds to NLRP3; although, this complex could not be sufficient for NLRP3 activation. Indeed, the inflammasome oligomerization requires ATP binding and thus the conversion of NACHT from an inactive to an active conformation [9]. Reactive oxygen species (ROS) production, mainly from mitochondria, has also been reported as an initiator of the activation of the NLRP3 inflammasome. Many studies have shown that NLRP3 agonists generate ROS in different cell types. As an example, fatty acid caused by a high-fat diet activates the NLRP3 inflammasome through the molecular axis AMPK–autophagy–NF-kB-ROS [7]. Moreover, Amyloid β, post-translational modifications of NLRP3, and non-canonical inflammasome activations have been reported as triggers for the NLRP3 inflammasome [10,11,12].
1.2. Food and Inflammation (Diet and Inflammation)
The emerging role of chronic inflammation as a determinant in the progress of the major degenerative diseases typical of modern society has promoted research to comprehend the influence of nutrition and dietary patterns on inflammatory markers. Dietary intake and nutritional status represent important environmental factors, which can modulate metabolic inflammation. In recent years, research has advanced significantly to achieve understanding of the impact of dietary components on metabolic inflammation, in the context of chronic infirmities, such as obesity, type-2 diabetes (T2D), cardiovascular diseases (CVD), and cancer [13]. Inflammatory and innate immune responses, provoked by pathogen-associated and other danger-associated signals, appearing during infections, result in the activation of cytosolic inflammasomes. Inflammasome signaling mainly furnishes a host innate immune defense against a broad range of microbial infections, including influenza virus [14,15]. Furthermore, NLRP3 inflammasome can also be activated by different endogenous risk agents, such as palmitic acid, amyloid β, and cholesterol crystals [16]. So, NLRP3 inflammasome intervening in such responses is associated with the development of several lifestyle-related chronic diseases, characterized by persistent inflammation [17].
In the mid-1900s, an important concept was emerging about the relationship between the immune and metabolic response systems, indicating that insulin resistance (IR), glucose intolerance, dyslipidemia, and other metabolic abnormalities occur in the course of an infection. Later, in the 1980s, a reduced binding capacity of insulin to its receptor in isolated blood cells was found in human patients affected by acute infection [18]. The network that connects metabolic and immune functions has been structured in relation to a lifestyle that was quite different from that of today, so that the current metabolic overload induces a low-grade chronic inflammatory state, for which some authors have recently proposed the term “metaflammation”. This term is used to describe the chronic low-grade inflammation orchestrated by metabolic cells in response to excess nutrients and energy. Metaflammation plays a pivotal role in the development and systemic expansion of the metabolic disease. White adipose tissue is likely to be the primary site of the metaflammation development; although, progressively, other metabolic tissues, such as liver, pancreas, and gut cells, get involved, affecting the metabolic homeostasis [13,19,20].
The strong link between nutrient sensing and immune signaling is justified by an evolutionarily conserved crosstalk pathway between immune and metabolic mediators. The functional units that control key metabolic and immune functions in higher organisms have evolved from common ancestral structures. Some invertebrates, such as the fruit fly Drosophila melanogaster or the nematode Caenorhabditis elegans, gave similar evidence of a conventional cellular crosstalk between immune and metabolic organs. Basically, cytokines act as metabolic hormones in the adaptation to nutrient fluctuations [13]. In Drosophila, for example, the body fat acts as the liver, adipose, and immune system, serving both the functions of nutrient storage and body defense [20]. During the course of evolution, a conservative, similar structure in an ancient mammalian ancestor differentiated into distinct metabolic organs and immune ones, as we essentially have in modern mammals, including humans. The principal framework of this translation from an adaptative to a maladaptive state is resumed by three integrated systems—Eiger, the Drosophila orthologue of TNF, and its receptor Wengen (in Drosophila) or TNFR (human); insulin and its receptors (dILP in Drosophila, insulin receptor insR in human); the TLR signaling pathways (Toll receptor in Drosophila). TNF and TLR signaling block insulin pathway or production through JNK activation and MyD88, from flies to humans, whereas activation results in abnormal metabolic homeostasis adaptation, which leads to a chronic metabolic inflammation. As a result, immunometabolic diseases often appear as clusters and promote ageing, disability, and premature death [13].
It has been shown that an excess of fat mass, especially the visceral type, associated with a diet rich in saturated fats and simple sugars and low in fiber and micronutrients (minerals and vitamins), which is the current Western diet, is able to promote a chronic low-grade inflammatory state, which, albeit involving molecules and signals common to the classic inflammatory response, recognizes the primary trigger in the metabolic overload [21]. Conversely, obesity, by inducing chronic low-grade activation of inflammatory pathways, is linked to the development of IR and T2D [22]. In relation to the different organ meiopragia, genetically determined, the metaflammation can manifest itself in pathologies, such as tumors, neuro- and cardio-vascular diseases, neurodegenerative diseases (AD), or metabolic diseases (metabolic syndrome, T2D, non-alcoholic fatty liver disease (NAFLD)). These pathologies are characterized by a variable increase in serum biomarkers level of inflammation, especially TNF-α, IL-1β, IL-6, PCR, fibrinogen, intercellular (ICAM1), and vascular (VCAM1) adhesion molecules, etc. These biomarkers were also shown to be significantly related to the risk of T2D, cardiovascular disease and cancers in healthy subjects [23].
Moreover, particular interest has been directed to IL-1β-mediated inflammation because of the strong inter-relationship between dietary fats, metabolic stressors, and NLRP3-mediated inflammation [24]. Metabolic stress, in the form of obesity, saturated fatty acids (SFAs), cholesterol, reactive oxygen species, and/or uric acid promote IL-1β signaling [25]. IL-1β disrupts cellular metabolism by interrupting a range of signaling pathways, including insulin sensitivity, lipid metabolism, and adipogenesis [26]. It is now widely accepted that IL-1β signaling plays a key role in the development of obesity, IR, and T2D risk [27,28,29,30,31].
1.3. Role of Diet in Inflammatory Response
During the feeding/fasting cycle, in physiological conditions, an intermittent, non-specific, low-grade inflammatory response occurs at the level of “metabolic tissues” (adipose, muscle, and liver tissue), which results in a transient increase in some inflammatory proteins/cytokines in the serum. This increment reaches its maximum peak during the absorption phase (post-prandial) and then gradually decreases in about 2 h, when the nutrients have been distributed, metabolized, and/or accumulated in the respective cellular sites [32]. The inflammatory response is amplified in overeating conditions (hyperlipidic diet, excess of saturated fats and simple carbohydrates, and low intake of fiber, vitamins, and antioxidant compounds), in obesity, and in diabetes—when the metabolic overload generates a “traffic jam” of the physiological metabolic pathways, progressive recruitment and activation of immune-competent cells, such as macrophages, mast cells, and T lymphocytes, occurs [33]. This results in the establishment of a vicious circle in which the physiological quiescent phase of the inflammatory response is incomplete and generates a pro-inflammatory milieu that impairs the metabolic functions [34]. On the contrary, a food pattern adherent to the Mediterranean dietary model is associated with a reduction in serum concentration of inflammatory biomarkers [35]. The complex mechanisms through which this inflammatory response is induced by the diet are gradually emerging. It was found that both stages of IL-1β priming and NLRP3-mediated IL-1β activation may be affected by the nutritional environment. One of these mechanisms appears to be associated with the quality of the diet, that is, the excess or lack of specific nutrients intake [28,36].
1.4. Fatty Acids and Inflammatory Response
Experimental data showed that the quantity and quality of fats influences the acute inflammatory response to a single meal. The study revealed that SFAs content and the ratio among polyunsaturated fatty acids (PUFAs) from ω-3 to ω-6 have proved to be the major determinants of the extent of postprandial inflammatory response [36,37]. In particular, it has been observed that high meal SFA increased inflammatory indices; contrarily, high ω-3/ω-6 PUFA ratio decreased them [36,37].
Fatty acids can affect the inflammatory response either through the synthesis of eicosanoids that regulate the transduction of signals at cell membrane or cytoplasmic level, by modulating the activity of transcription factors involved in the inflammatory process.
An examination of data from the National Health and Nutrition Examination Survey (NHANES 99-00) found that levels of SFAs in serum phospholipids of male civil servant workers (40–69 years) positively correlated with some inflammatory markers, such as HS-CRP (high sensitivity C-reactive protein) and fibrinogen; by contrast, phospholipid PUFA levels were inversely associated with HS-CRP [32,36]. It has been recently shown in a C57BL/6 male mice model that consuming a high-fat diet, enriched with SFAs, induces especially adipose IL-1β inflammation and insulin resistance. However, if SFAs are replaced with monounsaturated fatty acids (MUFAs), an attenuation of NLRP3-mediated inflammation is observed [16,28].
In vitro studies have shown that SFAs, such as lauric, myristic, and palmitic acids, are able to activate the Toll-like receptors (TLR) of adipocytes and macrophages [28]. The SFA-TLR interaction determines the activation of c-jun N-terminal kinase (JNK), the inhibitor of kappa B kinase (IKK), and protein kinase R (PKR), to which it follows that:
- -
- Phosphorylation of insulin receptor substrate-1 (IRS-1) in serine and its degradation and elimination through the ubiquitin pathway, thus blocking insulin signal transmission;
- -
- Activation of the transcription factor NF-kB, by its dissociation from the cytoplasmic inhibitor (IkB) and transfer to the nucleus, with over-regulation of the pro-inflammatory gene expression.
In conclusion, SFAs impair insulin signaling through TLR4, activating the NF-κB pathway and NLRP3 inflammasome that promotes the conversion of pro-IL-1β into mature IL-1β [16,31,32]. In a clinical study, the relationship between SFAs and inflammation biomarkers has been demonstrated in subjects with excess weight, in which the ω6:ω3 ratio is directly related to the concentrations of IL-6, CRP, and adhesion molecules; in addition, the inhibition of IRS-1 and the reduction in adiponectin levels induce IR, thus increasing the risk of the metabolic syndrome [21]. Additionally, trans-fatty acid (TFA) consumption has been found to be positively associated with markers of systemic inflammation. In the Harvard Nurses’ Health Study, it emerged that TFA intake was positively associated with IL-6 and HS-CRP in women with higher BMI (body mass index) and serum HS-CRP level [38]. Partially hydrogenated oils (PHO) are the main source of industrially produced TFA. PHO is an ingredient in different foods, including margarine and vegetable shortening, but also in baked foods (crackers, biscuits, pies), or in those fried in semi-hydrogenated oils/fats. However, there are also many foods that naturally contain TFA, such as dairy products and meat from ruminant animals. A review of observational and interventional data concluded that the TFA pro-inflammatory effects (increased TNF-α, IL-6, and HS-CRP) were associated with markers of vascular endothelial dysfunction and were most evident when compared with the effects of cis-unsaturated fatty acids [38]. Controlled trials and observational studies provide concordant demonstration that the TFA intake from PHO adversely affects multiple cardiovascular risk factors and contributes significantly to increased risk of CHD events [39]. Several experimental and observational studies in humans have demonstrated the potential benefits of replacing SFAs with unsaturated fatty acids, such as oleic acid and PUFAs. These studies have shown an inverse association between consumption of ω-3 PUFA and systemic markers of inflammation, such as TNFs and ILs [40]. ω-6 PUFA consumption shows variable effects on inflammation. Both anti-inflammatory and pro-inflammatory effects have been described [41], suggesting how the interaction of ω-6 PUFAs and their lipid mediator derivatives in the context of inflammation is complex and still not properly understood. For example, in overweight subjects, the serum concentration of ω-6 PUFAs (α-linoleic acid (ALA), AL, and AA) has been shown to be inversely related to IL-6 levels [41]. ω-3 PUFAs (ALA, eicosapentaenoic acid (EPA) and especially docosahexaenoic acid (DHA)) have much more marked effects than ω-6 PUFAs in reducing the expression of numerous pro-inflammatory cytokines, such as IL-6, TNF-α, IL-18, sICAM-1, sVCAM-1, sE-selectin, and HS-CRP. ω-3 PUFAs suppress NLRP3 inflammasome in obese subjects through downregulation of inflammasome gene expression in adipocytes and macrophages [28]. It has been shown that stimulation of macrophages with ω-3 PUFAs (at a dose of 20 μM) was able to suppress NLRP3 inflammasome activation and to inhibit subsequent caspase-1 activation and IL-1β secretion [42,43].
Furthermore, the ω-3 PUFAs, by binding to the G-protein-coupled receptor 120 (GRP120—a membrane receptor sensitive to fatty acids, expressed at the level of macrophages), block the NFκB activation pathway. Finally, EPA would be able to inhibit the activity of the Δ-6 desaturase enzyme, thus reducing AA formation [42,44]. Some studies indicate that dietary consumption of MUFAs, oleic acid in particular, may have anti-inflammatory effects. Studies suggest that dietary MUFAs can reduce IL-1β-mediated adipose dysfunction and insulin resistance via preservation of AMP-activated protein kinase (AMPK) activity which, in turn, quenches NLRP3 inflammasome activation [30,45,46].
1.5. Carbohydrates and Inflammatory Response
Diets with relatively high glycemic index (GI) and glycemic load (GL) have been linked to elevated risk of T2D, coronary heart disease, and stroke, particularly among overweight individuals [47]. GI and GL are associated with an increase in the plasma concentration of inflammatory biomarkers, such as IL-6, HS-CRP, and IL-18 [36]. There are convincing data suggesting that NLRP3 inflammasome is fundamental in the deleterious effect observed in chronic hyperglycemia and oxidative stress. Hyperglycemia is associated with both conditions of oxidative stress and inflammatory state and promotes mitochondrial metabolism in β cells which enhances the production of ROS, thus inducing NLRP3 inflammasome activation and then IL-1β production [48].
Recent clinical trials indicate that targeting the prototypic pro-inflammatory cytokine IL-1β ameliorates the outcomes of cardiovascular disease, which is the first cause of death in T2D patients. Several T2D-related metabolic factors, including reactive oxygen species, glyco/lipoxidation end products, and cholesterol crystals, have been involved in the pathogenesis of diabetes complications (diabetic kidney disease and diabetic retinopathy) and in the progression of atherosclerosis and NAFLD [49].
High levels of glucose and non-esterified fatty acids (NEFAs) can cause oxidative stress due to both the decoupling of oxidative phosphorylation and to the increased mitochondrial β-oxidation. Lee et al. found that hyperglycemia-induced elevated mitochondrial reactive oxygen species in myeloid cells of T2D patients are associated with increased production of inflammasome-dependent cytokines IL-1β and IL-18 [50]. Some data also showed that inhibition of AMP-activated protein kinase can exacerbate ROS-dependent NLRP3 activation [50]. It was recently pointed out that NLRP3 might directly sense the presence of increased ROS production (mitochondrial superoxide anion radical (O2•−), hydrogen peroxide (H2O2)) by normal or malfunctioning mitochondria, or indirectly by other activators of NLRP3. In particular, it was suggested that increased ROS are sensed by a complex of redox-dependent inhibition of thioredoxin (TRX) and TRX-interacting protein (TXNIP) and cause the dissociation of the complex described above. Following an increase in cellular ROS concentration, this complex dissociates and TXNIP binds to the LRR region of NLRP3, with the consequent NLRP3 activation [51].
Chronic hyperglycemia (associated or not with increased triglycerides and NEFAs) promotes the formation of advanced glycation end-products (AGEs). The interaction between AGEs and receptors for advanced glycation end-products (RAGEs), the activation of the polyols pathway and the auto-oxidation of glucose lead to increased production of ROS which, in turn, induce the activation of transcription factors, such as NF-kB [52]. The result is an increase in inflammatory biomarkers, such as HS-CRP, IL-6, IL-1β, IL-8, IL-18, and TNF-α, matrix metalloproteinases, and markers of endothelial dysfunction (VCAM-1, ICAM-1 and E-selectin). Redox signaling molecules, such as ROS, mediate NLRP3 inflammasome activation, while ROS inhibitors suppress NLRP3 inflammasome-mediated inflammation [52], indicating the vital role of oxidative stress on inflammasome activation that can be prevented by supplementation with antioxidants, such as vitamin C, E, lipoic acid, phenolic compounds, and other bioactive compounds found in plant foods [48,53].
Consumption of whole grains, through increased fiber intake, is associated with a reduction in serum concentrations of inflammation biomarkers (HS-CRP, IL-6, Il-1 β); although, the mechanisms still need to be elucidated, and a synergistic effect of fiber, minerals, vitamins, or phytochemicals, such as lignans and phenolic acids, could take place [21,36].
Several studies in recent years have demonstrated the role of specific nutrients in the primary or secondary prevention of inflammation-related diseases, such as metabolic syndrome and diabetes. In this regard, some nutraceuticals and bioactive compounds (polyphenols, flavonoids, carotenoids, curcumin, resveratrol, etc.) have been widely studied and have been recognized for their ability to inhibit the synthesis of TNF-α in monocytes and macrophages and downregulate the expression of TLR2 and TLR4 in human monocytes [1].
However, due to the reciprocal synergistic or antagonistic interactions between nutrients and/or other food compounds (antinutrients and/or phytochemicals), it is clear that, in order to understand the role of diet in the modulation of the inflammatory state and oxidative stress, it is necessary to evaluate the nutritional pattern in its entirety. It was found that diet characterized by an abundant consumption of vegetables, fruit, nuts, olive oil, legumes, and fish, with moderate amounts of alcohol and low intake of red meat, processed meats, refined grains, and dairy products, has a protective effect against immunometabolic diseases [49]. These aspects correspond to the characteristics of the Mediterranean dietary pattern. Indeed, several studies have shown an inverse association between the degree of adherence to the diet Mediterranean and serum levels of IL-6, CRP, and TNF-α [35].
2. Materials and Methods
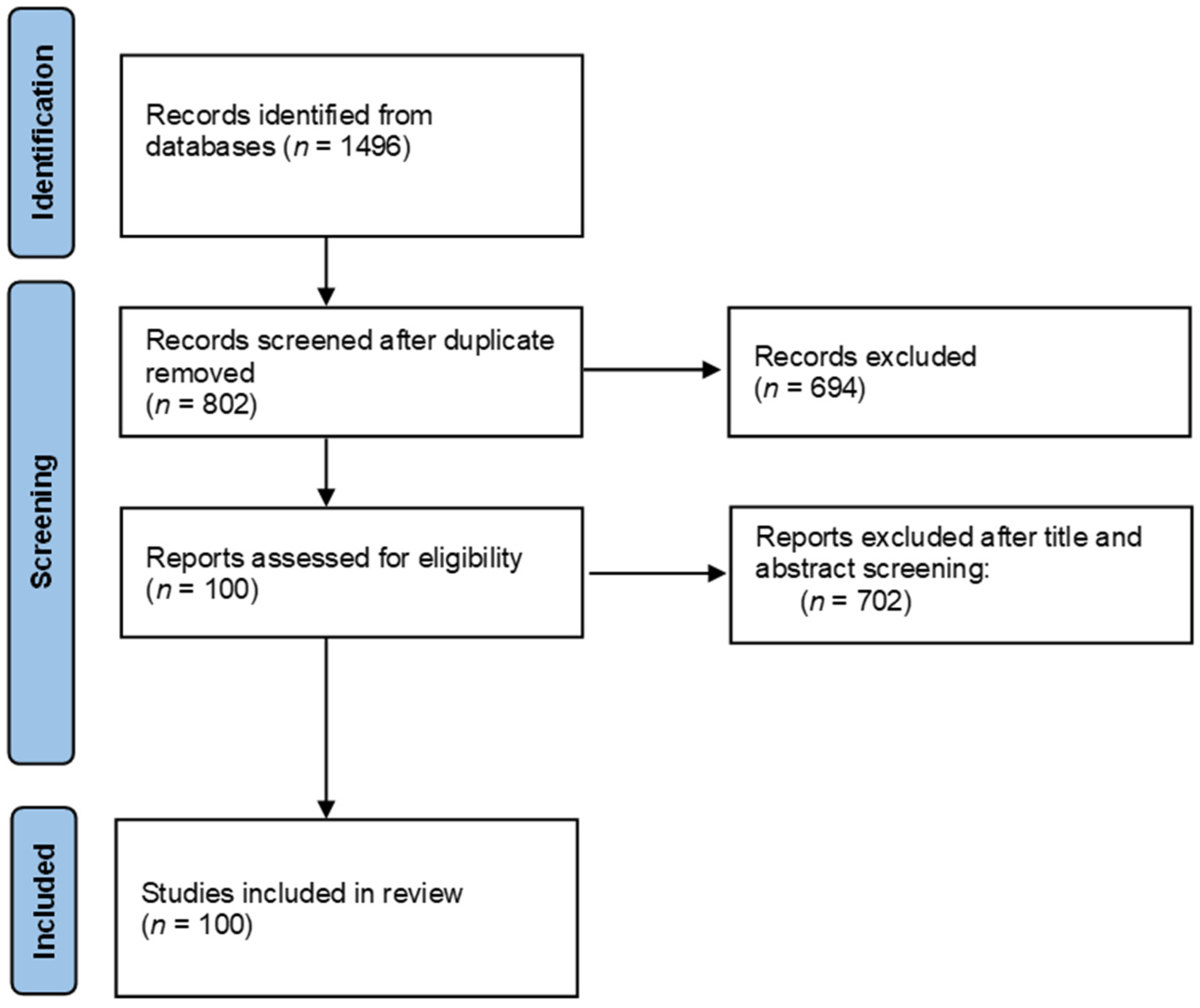
The literature search was carried out in August 2021 in official scientific databases, using combinations of keywords “NLRP3”, “NLRP3 inflammasome”, “food”, “nutrients”, and “bioactive compound” in the literature published during the last five years. Study selection and data were collected according to the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [54], Figure 2.
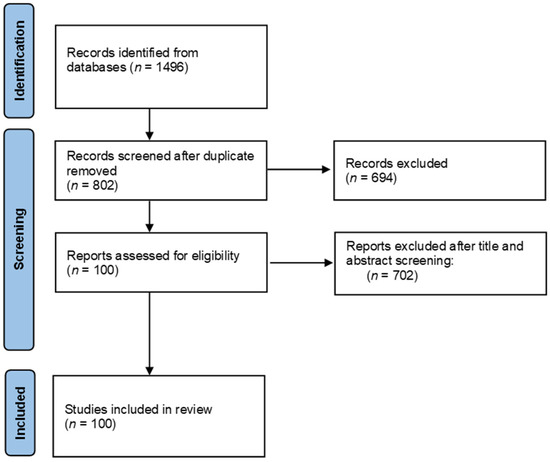
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) flow chart.
3. Food Components as NLPR3 Modulators
3.1. Polyphenols
Polyphenols are secondary metabolites produced by the metabolic processes of plants. Although more than 8000 polyphenols have been identified up to now, all these compounds arise from a common intermediate—phenylalanine or shikimic acid. Generally, they occur in conjugated forms, with one or more sugar residues, organic acids, amines, lipids, and other phenols [55]. Polyphenols may be classified into different groups based on their phenol rings number or on the structural elements that bind these rings to one another. The main classes include phenolic acids, flavonoids, stilbenes, and lignans. In recent years, a great interest on the potential health effects of polyphenols has been developed, since these molecules have shown to be mainly characterized by antioxidant and anti-inflammatory activities, proving to be very effective in the prevention and reduction in tumors, chronic inflammations, diabetes, aging, and infections [56]. The anti-inflammatory activity of polyphenols has also been demonstrated with the inhibition of the NLRP3 inflammasome activation. In the present paragraph, the anti-NLRP3 inflammasome activity of several polyphenols is discussed, by dividing the discussed compounds basing on their polyphenol class. A summary list of all the discussed food derived molecules is presented in Table 1.

Table 1.
Summary list of all the discussed food-derived molecules with a NLRP3 inflammasome modulatory activity. Compound name, food source, considered experimental model, dose, treatment duration, and main outcome are described.
3.1.1. Phenolic Acids
Phenolic acids are one of the main compounds of polyphenol class. Phenolic acids are characterized by a phenolic ring with a carboxylic acid function and can be further divided into two main groups, benzoic acid or cinnamic acid derivatives, based on the C1–C6 or C3–C6 backbones [132]. Several compounds belonging to the phenolic acids family have shown to possess interesting antioxidative and anti-inflammatory properties [56,133]. The modulating activity against the NLRP3 inflammasome complex has also been widely studied [1].
Sinapic acid is a hydroxycinnamic acid widely found in many foodstuffs, such as vegetables, spices, citrus, berry fruits, cereals, oilseed crops, wine, and vinegar. Sinapic acid has shown antioxidant and anti-inflammatory properties [134], as well as a modulating activity against the NLRP3 inflammasome complex in mouse models with chemically induced colitis [57]. The monitoring of NLRP3 inflammasome protein levels in the inflamed colon tissue of Kunming mice (colitis model), by Western blot analysis, after sinapic acid treatment for 7 days, have shown a reduction in the amounts of NLRP3, ASC, IL-1β, and caspase-1 proteins. In particular, the administration of 50 mg/kg of sinapic acid had a higher effect than 10 mg/kg.
Ferulic acid is an another hydroxycinnamic acid commonly found in fruits, vegetables, grains, beans, leaves, seeds, nuts, grasses, flowers, and in some plants, such as corn and wheat, and in the spice turmeric [135]. A total of 24 adult male Wistar rats with kidney injury induced by methotrexate were treated with 25 or 50 mg/kg of ferulic acid for 15 days [58]. Both doses of ferulic acid have produced an amelioration of NLRP3 and caspase-1 proteins expression, and subsequently a reduction in the IL-1β levels in the rat kidneys.
Chlorogenic acid is another phenolic acid, widely studied for its anti-inflammatory properties [136]. Chlorogenic acid can be found in foods, such as apples, coffee beans, eggplants, grapes, kiwi fruits, pears, plums, potatoes, tea, and tomatoes. This molecule has demonstrated an inhibitory effect against the NLRP3 signaling pathway, decreasing the protein levels of NLRP3, ASC, caspase-1 p45, and caspase-1 p20 in the colon tissue of BALB/c mice with induced colitis [59]. The NLRP3 expression reduction in RAW264.7 cells stimulated by LPS and adenosine triphosphate, resulting in the decreased secretion of IL-1β and IL-18, was also observed.
Lastly, among cinnamaldehyde-related compounds, only cinnamaldehyde and 2-methoxycinnamaldehyde limited the expression of NLRP3 and pro-IL-1β at 25–100 µM, whereas cinnamic acid, cinnamyl alcohol, cinnamyl acetate, and α-methylcinnamaldehyde were ineffective, thus demonstrating the importance of the propenal group in the side chain [137,138].
3.1.2. Flavones and Flavanones
Among flavones, apigenin, largely present in common fruits and vegetables, such as parsley, onions, oranges, tea, etc., has shown to be effective in reducing NLRP3 inflammasome activation in two different studies. In the first one, in vivo and in vitro models of high-fat diet-induced non-alcoholic fatty liver disease (HFD-induced NAFLD) have been treated with apigenin [60]. In particular, mice were administered by gavage with 50 mg/kg apigenin (4 mg/kg HED); whereas, for in vitro studies, Hepa1–6 cells were exposed for 24 h with 16 and 32 µM apigenin. In both assays, an important liver reduction in NLRP3, ASC, pro-caspase-1, and caspase-1 (measured by PCR), together with a reduction in ROS production was obtained by inhibiting xanthine oxidase. In the second study [61], 30 and 50 µM apigenin showed to be effective in reducing NLRP3 protein expression in ISO-HAS human endothelial cells treated with the pro-inflammatory agent TMAO (trimethylamine N-oxide). Isoorientin, a flavone identified in Gentiana roots, has shown to possess anti-NLRP3 inflammasome activation in in vivo and in vitro models of hyperuricemia by using 5 and 10 mg/kg (0.4 and 0.8 mg/kg HED) dosages in male ICR mice for in vivo studies and 25, 50, 100, 200, and 400 µM isoorientin for in vitro assays (MXC207 cells). In both cases, a dose-dependent reduction in inflammasome expression was observed, through the inhibition of xanthine oxidase activity and interleukin release [62]. In a very similar way, the anti-inflammasome activity in hyperuricemia male Sprague Dawley mice model has also been demonstrated for chrysin flavone, used at 50, 100, and 150 mg/kg oral doses (4, 8, and 12 mg/kg HED) for 4 weeks [63]. Luteolin preventive effects in spinal cord ischemia–reperfusion injury (SCII) have been demonstrated in an in vivo study where the treatment of male Sprague Dawley mice with intragastric injections of 50 and 100 mg/kg doses (4 and 8 mg/kg HED) of the considered flavone for 14 days has shown to reduce, with respect to non-treated mice, the incoming of induced SCII. In particular, the reduction in this event has been associated with the reduction in NLRP3, IL-1β, and IL-18 expression by ELISA assay [64].
Regarding flavanones, hesperidin methylchalcone and naringin have been demonstrated to be effective in reducing NLRP3 inflammasome activation. In particular, hesperidin methylchalcone, particularly present in citrus fruits, has shown to reduce the NLRP3, ASC, pro-caspase-1, and pro-IL-1β mRNA expression in induced gout arthritis Swiss mice models when orally administrated at 30 mg/kg (2.4 mg/kg HED) [65]. In a model of DSS-induced ulcerative colitis in male C57BL/6 mice [66], naringin has shown to reduce NLRP3, ASC, caspase-1, and IL-1β expression in the colon tissue in a dose-dependent way, when administrated at 25, 50, and 100 mg/kg (2, 4, and 8 mg/kg HED) for 7 days.
3.1.3. Flavonols
Among polyphenols, quercetin, largely present in several vegetables and fruits, has been the most considered compound for the study of its anti-NLRP3 inflammasome activity. In particular, in an alcohol-induced acute liver injury in male SPF-Wistar mice, the oral administration of 100 mg/kg quercetin (8 mg/kg HED) for 14 days caused a reduction in the injury evolution, thanks to a lower expression of inflammasome factors including ROS, NF-κB, NLRP3 inflammasome, IL-1β, and IL-18, measured by ELISA assays in the liver tissue [67]. The same quercetin dosage described in the previous study has been applied for 16 days in high fat treated ApoE−/− mice to observe the potential reduction in atherosclerotic inflammation, where NLRP3 inflammasome is largely involved. In the study, an important decrease in pro-IL-1β and IL-1β was registered [68]. The effect of quercetin in reducing inflammasome activation has also been demonstrated for neurodegenerative models [69], since aging mice treated with 35 and 70 mg/kg (2.8 and 5.6 mg/kg HED) of quercetin for 4 weeks have shown a dose-dependent increase in the cognitive functions, together with an important reduction in NLRP3 activation factors expression by Western blot analysis. An in vitro assay on E. coli-infected cells has also been carried out to demonstrate the anti-inflammasome activity of quercetin during infection. In particular, the 12 h treatment with 200 µM quercetin before infection has shown to strongly reduce NLRP3, caspase-1, and IL-1β expression, together with an enhanced ROS scavenger activity [70]. Similarly, a quercetin saturated derivative, dihydroquercetin, has shown to be active against NLRP3 inflammasome in alcoholic liver steatosis in vivo (male C57BL/6 mice) and in vitro (human hepatoma cells HepG2) models [71]. Other two flavonols largely present in tea, vegetables, oranges and wine, namely kaempferol and myricetin, have been shown to be effective in reducing NLRP3 inflammasome. In particular, kaempferol has been used in an in vivo induced hepatotoxicity male C57BL/6 mice model, causing a dose-dependent decrease in NLRP3 inflammasome activation factors (IL-1β, TNF-α, IL-6) in the liver and in the blood when administrated at 30 and 60 mg/kg (2.4 and 4.8 mg/kg HED) dosages for 7 days [72]. It is noteworthy that, in this work, kaempferol has been extracted from a Chinese medical plant but, since its purity has been measured >98%, the activity can be asserted to the single molecule as it is. The same inflammasome inhibition has been observed for myricetin, when its activity has been considered in a high-fat diet model for the simulation of human T2D symptoms in male Wistar mice treated with oral doses of 20 mg/kg (1.6 mg/kg HED) myricetin for 4 weeks [73].
3.1.4. Other Phenolics
Curcumin is the main active compound of turmeric and its anti-inflammatory activities have been largely demonstrated in many inflammatory diseases, in which the inhibition of NLRP3 inflammasome represents an important mechanism of action [139]. This activity has also been observed in a chronic unpredictable mild stress (CUMS) mice model for the simulation of depression pathology, that also involves inflammatory process. In particular, during inducing CUMS in mice, the treatment with 100 mg/kg (8 mg/kg HED) curcumin daily dosages for 4 weeks has caused a reduction in CUMS symptoms in curcumin-treated mice, with respect to untreated ones. This symptom reduction has been accompanied by a strong decrease in NLRP3 inflammasome factors in the hippocampus, namely IL-1β, IL-6, and TNF-α, measured with Western blotting analysis [74]. Interestingly, curcumin supplementation has also been studied in a randomized double-blind clinical study to investigate its anti-inflammatory effects in hemodialysis patients [75]. In this study, half of the considered hemodialysis patients have received, 3 times a week for 12 weeks, a beverage containing 2.5 g of turmeric (95% curcumin) after dialysis. At the end of the trial, patients who received curcumin supplementation have shown lower expression levels of blood NLRP3 inflammasome markers (NF-kB, NLRP3, and IL-1β) with respect to non-curcumin-treated ones, underlining the potential anti-inflammasome activity. 6-Shogaol is one of the main pungent chemical constituents of ginger roots and its anti-NLRP3 activation has been demonstrated in an in vitro assay where cell calcification has been induced in human artery smooth muscle cells with high dosage of glucose. The 14-day treatment of cell cultures with 6-shogaol drastically reduced the expression of NLRP3, caspase-1, and IL-1β [76]. The potent anti-inflammatory activity of 6-shogaol has also been demonstrated when the NLRP3 inflammasome has been activated in human THP-1 monocytes [77]. In this study, 5, 10, 20, and 40 µM of 6-, 8-, and 10-shoagol/gingerol (all present in ginger roots) have been used to treat cell cultures, resulting in a higher dose-dependent decrease in NLRP3 and IL-1β levels when 6-shoagol was used. Pterostilbene, a stilbenoid compound largely present in grapes and blueberries, has shown to be largely effective in inhibiting inflammasome in acute liver failure, allergic contact dermatitis, and hyperuricemia models. In particular, in an acute liver failure model obtained by treating female BALB/c mice with lipopolysaccharide and D-galactosamine, the concurrent intraperitoneal administration of 50 mg/kg/12 h (4 mg/kg HED) pterostilbene for 1 day determined an important decrease in IL-1β, IL-6, caspase-1, TNF-α, and NLRP3 protein [78]. Similar effects, together with ROS reduction, have been observed when 500 mg/kg (40 mg/kg HED) dosage of pterostilbene were injected for 2 weeks and then orally for 3 weeks in female C57BL/6 mice with chromium-induced allergic contact dermatitis [79]. The anti-inflammasome activity of pterostilbene in hyperuricemia model has been demonstrated by an in vitro assay where NLRP3 inflammasome and epithelial–mesenchymal transition in renal cells have been stimulated by TGF-β [80]. The cells treatment with 2 µM pterostilbene caused a reduction in NLRP3 inflammasome by inducing autophagy, a cellular process that is activated when cellular stress events, such as inflammatory factors release, occur [140]. Hyperuricemia disease has also been considered to demonstrate the anti-inflammasome activity of the stilbenoid glucoside polydatin present in grape juice. In the considered in vivo model [81], male Sprague Dawley mice with potassium oxidate-induced hyperuricemia and daily treated with oral 25 and 50 mg/kg (2 and 4 mg/kg) polydatin for 7 days have shown a dose-dependent decrease in IL-1β, TNF-α, IL-6, NLRP3, and caspase-1, measured in the kidney tissues, with respect to non-treated mice. Polydatin has also shown to be effective in reducing NLRP3 inflammasome in both in vivo and in vitro dry-eye disease models when used at 0.05/0.5% ocular solution and 0.1/1/10 µM solution, respectively [82]. Again, an eye inflammation event has been simulated for the anti-inflammasome activity study of cyanidin-3-O-β-glucoside (C3G), a phenolic molecule mainly present in red–violet fruits. In particular, 4-hydroxyhexenal-induced inflammation in human retinal pigment epithelial cells has shown to be less severe when cells have been pre-treated for 2 h with 50 and 100 µM C3G, showing a dose-dependent decrease in NLRP3, IL-18, IL-β, and caspase-1 [83]. C3G has also been effective in reducing NLRP3 inflammasome activation in an in vivo hepatic inflammation model in male C57BL/6 J mice, where the administration of a 200 mg/kg/day (16 mg/kg HED) C3G dosage for 8 weeks caused a decrease in NLRP3, IL-18, IL-1β, and caspase-1 expression in serum and liver, together with blocking the NF-κB signaling pathway [84]. Green tea is characterized by the presence of several bioactive polyphenols, of which epigallocatechin-3-gallate is the most abundant. This molecule has shown to be very effective in attenuating NLRP3 inflammasome in both in vivo and in vitro lung injury models [85]. In particular, a male Balb/C mice model of acute pancreatitis induced lung injury has been treated with several epigallocatechin-3-gallate dosages (5, 10, 20, 40, and 80 mg/kg) (0.4, 0.8, 1.6, and 3.2 mg/kg HED) for 4 weeks, and a notably dose-dependent decrease in IL-1β inflammation factor has been observed after treatment. Similarly, the in vitro treatment of injured adherent cells with 2.5, 5, and 10 µM epigallocatechin-3-gallate has shown a dose-dependent decrease in caspase-1, IL-1β, and ROS. The same results have been observed in both in vivo and in vitro models of microglial inflammation and neurotoxicity where the use of 2 mg/kg/day (0.16 mg/kg HED) and 10 µM of epigallocatechin-3-gallate has reduced the NLRP3 inflammasome factors expression [86]. Resveratrol has shown to be very effective in reducing NLRP3 inflammasome when encapsulated in poly(lactic-co-glycolic acid) nanoparticles (Res NPs), in both in vitro and in vivo kidney injury models [87]. In particular, kidney cells with LPS/ATP-induced inflammation have shown a dose-dependent reduction in NLRP3, pro-caspase-1, cleaved-caspase-1, and IL-1β expression when treated with 25, 50, and 100 µM Res NPs. The same dose-dependent results have been observed by treating mice injured kidney with 2 and 4 mg/kg 2 times a week for 4 weeks.
3.1.5. Polyphenols Mixtures
Food extracts characterized by the presence of polyphenols mixtures have also shown to be effective in the inhibition of NLRP3 inflammasome. In this case, it is not possible to have an idea about the contribute of single molecules that are present in the mixture, also in consideration of the synergistic, additive, and antagonistic effects that can occur [141]. Anyway, the study of food-derived mixtures is also important, considering that whole foods, food supplements, and nutraceutical products are generally a mixture of several compounds.
Fermented non-digestible fraction (FNDF) of baked corn (Zea mays L.) and common bean (Phaseolus vulgaris L.) snacks are food products, rich in polyphenols, such as gallic acid and other compounds, such as butyric acid and verbascose, that present an in vitro anti-inflammatory activity. Regarding the NLRP3 inflammasome complex, the FNDF pure components inhibited the NLRP3 assemblage, decreasing caspase-1 activity, IL-1β, and apoptosis in THP-1 cells and differentiated Caco-2 cells after NLRP3 inflammasome activation [88].
Green tea is a plant with well-known antioxidant and anti-inflammatory activities based on the high polyphenol contents, with epigallocatechin-3-gallate (EGCG) being the most abundant one. In a recent work, the green tea polyphenols (GTPs) have reported a modulating activity against the NLRP3 inflammasome activation [89]. In particular, GTPs and EGCG were administered at ICR mice with induced liver damage and the expression levels of NLRP3 inflammasome proteins were determined by Western blot analysis. The results showed a significant down-regulation of NLRP3, ASC, caspase-1, and IL 1β protein expressions, indicating a reduction in NLRP3 signaling in mice.
In another study, the GTPs were administrated to the same mice, in a dose (100–200 mg/kg) (8 and 16 mg/kg HED) comparable with the normal drinking tea levels consumed by humans, in order to determine their protective effects against the inflammasome activation. The results confirmed that GTPs inhibited the NLRP3, ASC, and caspase-1 p20 expression in a dose-dependent manner [90].
Soy isoflavones represent an interesting flavonoid choice for the treatment of many inflammation disorders. A recent study conducted on dextran sodium sulphate (DSS)-treated mice reported the inhibiting capacity of soy isoflavones on NLRP3 inflammasome complex expression, reducing the NLRP3, caspase-1 p20, and ASC protein levels and suppressing the release of IL-1β and IL-18 [91].
Red raspberry polyphenol extracts are rich in anthocyanins, ellagic acid, myricetin, (−)-epicatechin, and (+)-catechin. The extract was administrated to C57BL/6 mice revealing the attenuation of NLRP3 inflammasome activation in adipose tissue macrophages and epididymal white adipose tissue. The downregulation of NLRP3 inflammasome activation, leading to reduced expression levels of IL-1β, IL-18, and NLRP3 proteins, was also observed in vitro on C3H10T1/2 cells [92].
3.2. Organosulfur Compounds
Organosulfur compounds (OSCs) are a class of molecules usually present in several food sources, such as cereals, legumes, vegetables, and fruits. However, the main food sources of OSCs belong to the Allium (garlic, onion) and Brassica (broccoli, cabbage, cauliflower) genera [142] and are responsible for the typical flavor of these matrices. This group, that mainly includes isothiocyanates, indoles, allylic sulfur compounds, and sulfones, is well known to possess several biological activities, such as antioxidant, anticancer, antimicrobial, and anti-inflammatory [143,144]. Among the possible mechanisms considered to be involved in the anti-inflammatory activity, inhibition of NLRP3 activation has been demonstrated to be the action mode of some OSCs. Allicin, an isothiocyanate compound very abundant in garlic, has shown to reduce the acrylamide induced inflammation in both Kupffer and Sprague Dawley rat liver cells by inhibiting several inflammation pathways that activate the NLRP3 inflammasome in liver [93]. In both in vitro (1 mM acrylamide and 3.75, 7.5, 15 μM allicin) and in vivo (30 mg/kg/d acrylamide and 25 or 50 mg/kg/d of allicin) (2 or 4 mg/kg HED) studies, by comparing the cells treated with acrylamide and allicin/acrylamide, a decrease in several inflammation factors involved in the activation of NLRP3 inflammasome was observed in allicin groups. In particular, allicin has shown to reduce the release of ROS and ERS (endoplasmic reticulum stress) factors that represent the activation signals for NLRP3 inflammasome. Another study has been carried out on Kupffer and liver cells in order to observe the effect of benzyl isothiocyanate, an isothiocyanate compound present in cruciferous vegetables, in reducing the inflammation events in diet-induced NASH [94]. When benzyl isothiocyanate was administrated to Kupffer cell cultures (2.5 or 5.0 μM benzyl isothiocyanate) or male C57BL/6 J mice with induced NASH (1 g/kg/d benzyl isothiocyanate for 9 weeks) (80 mg/kg HED), a reduction in NLRP3 inflammasome activation was observed, through a reduction in NLRP3, p20 caspase-1, and IL1-β expression in blood and liver; moreover, benzyl isothiocyanate has shown to reduce the release of cathepsin β, an inflammasome assembler, and the interaction of cathepsin B with NLRP3. Similar to what described in the previous study, sulforaphane, an allylic sulfur molecule typical of broccoli and other cruciferous vegetables, has shown to reduce the expression of NLRP3, p20 caspase-1, and IL1-β in cerulean-induced acute pancreatitis in BALB/c mice, through a 3-day treatment with 5 mg/kg (0.4 mg/kg HED) of sulforaphane [95]. Finally, another OSC typical of Allium genus vegetables, methylsulfonylmethane, has shown to have anti-NLRP3 inflammasome activity in in vitro assays on human and mouse macrophages by using different methylsulfonylmethane solution concentrations (0.3, 0.5, 1.0, 2.0, 4.0, and 8.0%) [96]. In particular, the inhibition of NLRP3 inflammasome was demonstrated to occur at several steps, namely by blocking the NF-κB signaling and pro IL1-β expression and by reducing IL-1β production and inhibiting mitochondrial ROS production.
3.3. Terpenes and Terpenoids
Terpenes are a class of volatile hydrocarbon compounds derived from two or more isoprene units and represent the main components of vegetable essential oils. In recent years, a paramount interest for these molecules has emerged, since several biological activities, such as antitumoral, anti-inflammatory, antibacterial, antiviral, antimalarial, and cardiovascular modulation have been demonstrated [145]. In particular, among the different proposed anti-inflammatory modes of action, the inhibition of NLRP3 inflammasome has shown to be the typical mechanism of action of these compounds. Carnosic acid, a diterpenoid mainly present in Rosmarinus and Salvia plants, has been demonstrated to reduce the evolution of DSS-induced colitis in male Balb/c mice daily gavaged with 50 or 100 mg/kg (4 and 8 mg/kg HED) of carnosic acid for 10 days by inhibiting the activation of caspase-2 and the release of pro-inflammatory cytokines and ROS in the colon tissue [97]. Moreover, in this study, the anti-NLRP3 inflammasome activity of carnosic acid has shown to be comparable with those of 5-aminosalicylic acid, a standard of care for the treatment of colitis disease. The anti-NLRP3 inflammasome effect of geranylgeraniol, a diterpenoid naturally occurred in vegetable oils such as flax, sunflower, and olive, has been studied in an in vitro model of programmed cell death induced in Daoy cell lines after treatment with 10 μM statins or mevalonate for 24 h [98]. The addition of 50 μM geranylgeraniol, following the treatment with statins or mevalonate, has shown to be effective in the reduction in cell death by inhibiting the expression of NLRP3 gene after 24 h. Conversely to what observed in the previous studies, in the following work the beneficial effect of a terpene (kaurenoic acid) has been associated with a stimulation of the NLRP3 inflammasome activation. In particular, kaurenoic acid, a diterpenoid found in several natural spices, such as the fruits of X. aethiopica, has shown to possess a dose-dependent (at 10, 30, 50, 70, and 90 μM) triggering effect of NLRP3 inflammasome in BALB/c mice macrophages infected with L. amazonensis promastigotes by increasing the production of the inflammatory mediators NO and IL-1β [99].
Although not present in natural sources used as foods, other diterpenoids, sesquiterpenes, iridoids, phytocannabinoids, and derivatives typical of plants used in Eastern and Western medicine, namely α-bisabolol, aucubin, abscisic acid (phytohormone), cannabidiol, phytanyl amine, triptolide, tanshinone IIA, sodium tanshinone IIA sulfonate, paclitaxel, phorbol myristate acetate, andrographolide, oridonin, glaucocalyxin A, and teuvincenone F have shown to be effective in the prevention of NLRP3 inflammasome activation by blocking the release of inflammasome enhancers, mainly IL-1β, IL-4, IL-6, IL-12, IL-18, caspase-1, and ROS [146,147,148,149,150,151,152,153]. Similarly, other terpenoids, such as the triterpenoid celastrol [154] and the sesquiterpenoids of Ainsliaea yunnanensis [155], have shown to be involved in the modulation of NLRP3.
3.4. Fatty Acids
In general, most of the evidence highlighted that SFAs acted as priming signals of inflammasome activation, whereas MUFAs and PUFAs have been shown to impair this activation, but these assumptions have frequently been challenged [31]. To better understand the FA implication in NLRP3 inflammasome, data reported in the literature will be described according two categories: SFAs and UFAs.
3.4.1. Saturated Fatty Acids
Different research groups attempted to investigate the mechanisms underlying SFA-mediated NLRP3 inflammasome activation. Wen et al. demonstrated palmitate acid inhibits AMP-activated protein kinase in LPS-primed bone marrow-derived macrophages followed by the accumulation of mitochondrial ROS, thus activating the NLRP3-ASC inflammasome and causing caspase-1, IL-1β, and IL-18 production [16]. According to Robblee et al., palmitate and stearate acids could induce activation of one of the three endoplasmic reticulum stress sensors, IRE1α (inositol-requiring enzyme 1-α), through the saturated phosphatidylcholine accumulation, and mediate the NLRP3 inflammasome activation in LPS-primed bone marrow-derived dendritic cells [100]. More recently, Gianfrancesco et al. suggested that the accumulation of saturated phosphatidylcholine induced by SFAs led to the loss of membrane fluidity and the disruption of Na+, K+-ATPase transmembrane protein, resulting in an increase in K+ efflux, which is considered a NLRP3 activator [156].
However, few studies reported the anti-inflammatory effects displayed by different SFAs. As an example, virgin coconut oil (VOC), mainly composed of medium chain saturated fatty acids (6–12 carbons), reduced IL-1β protein, caspase-1, and NLRP3 genes expression at different doses in AD (receiving Amyloid-β) and in high-fat diet (HFD) models both in vitro and in vivo [101]. The anti-inflammatory properties of VOC might be ascribed to lauric acid (which represents up to 55% of the total) that is considered the precursor of monolaurin, which has been shown to modulate immune cell proliferation and, through this, it can control inflammasome [157].
3.4.2. Unsaturated Fatty Acids
In the literature, most of the studies on NLRP3 inflammasome and fatty acids are focused on PUFAs. In particular, fish oil, a matrix particularly enriched in ω-3 DHA and EPA, has been extensively studied. All data reported the anti-inflammatory properties of EPA and DHA resulting in a downregulation of NLRP3 gene expression and in a decreased secretion of pro-inflammatory cytokines (IL-1β and IL-18) in obesity models [102,103], in DSS-induced colitis in mice [104], in LPS-induced Kupffer cells [105], and in both prefrontal cortex and hippocampus of rats [106]. Nevertheless, numerous studies have explored the anti-inflammatory mechanisms of PUFAs. Dang et al. suggested fish oil could attenuate the LPS-induced neuroinflammation and oxidative through modulation of P2X7R/NLRP3 inflammasome axis [106]. Miao et al. underlined that the mechanisms involve inhibition of ROS production, mediation of NLRP3/ASC/caspase-1 signaling pathway, regulation of gut microbiota, and SCFAs levels [104]. More recently, Quingyao et al. assumed that DHA-mediated NLRP3 inflammasome inhibition was due to the blockade of high glucose-induced TXNIP via the PI3K/Akt pathway in pre-adipocytes [103]. Other mechanisms through which ω-3 PUFAs reduce metabolic inflammation may include the G protein-coupled receptor 120 (GPR120) and GPR40, which interact with NLRP3 and inhibited the NLRP3 inflammasome complex assembly [42,105].
Interestingly, different properties have been reported between ω-3 and ω-6 PUFAs. Schuster et al. investigated the anti-inflammatory activity of different fatty acids (SFAs, MUFAs and PUFAs) in macrophages, blood monocytes, and hepatocytes, confirming the data described above. Nevertheless, when compared with ω-6 PUFAs, ω-3 were more potent in inhibiting ATP-mediated NLRP3 inflammasome [158]. Conversely, Yan et al. found out that ω-6 PUFAs failed to block IL-1β secretion induced by nigericin [42]. All these findings suggest that fatty acids should be considered individually in terms of defining potential differences with respect to metabolic or inflammatory properties, or both.
3.5. Carotenoids
Recently, only two carotenoids were studied in vivo for the modulation of NLPR3 inflammasome. Zeaxanthin dipalmitate, a lipophilic antioxidant whose primary presence in some functional fruits (e.g., Lycium barbarum) has been confirmed, demonstrated the ability to counteract ethanol-induced hepatic damage in a murine model of AFLD targeting the converged AMPK-FoxO3a mitophagy and NPLR3 pathway combined to P2X7 and adiponectin receptor 1 on the hepatocyte membrane [159]. Since the role of NLRP3 in virtually all liver diseases has been demonstrated, this compound or foods containing it can have a positive impact or a preventive effect against a large range of human pathologies. Conversely, astaxanthin, which is poorly represented in typical Western diet but is rich in seafood, exerted an indirect NPLR3 modulation (−23%) via the modification of the gut microbiota in a murine model of inflammation and metabolic homeostasis when administered as a supplementation (0.04% w/w) to the normal diet [160].
3.6. Proteins and Amino Acid Derivatives
Proteins, peptides, and amino acids appear to be involved in the inhibition of the inflammasome pathway. Two short peptides, RDP2, RDP3 (rice-derived-peptide-2, AAAAGAMPK-NH2, 785,97 Da), and RDP3 (rice-derived-peptide-3, AAAAMAGPK-NH2, 785,97 Da), were identified and isolated from the aqueous extract of the shelled fruits of Oryza sativa and studied to verify the antigout effects [107,108]. The RDP2 peptide [107] study was conducted on hyperuremic mice, that were injected intraperitoneally once a day for 7 days, and divided into 7 groups: control, model, allopurinol (Allo, 10 mg/kg, 0.8 mg/kg HED), benzbromarone (Benz, 8 mg/kg, 0.6 mg/kg HED), and 3 RDP2 groups (5, 10, and 100 μg/kg, 0.4, 0.8, and 8 μg/kg HED). The RDP2 groups induced a reduction in serum uric acid levels by decreasing renal inflammation. Indeed, the content of serum IL-1β, the production of which depended on NLRP3 inflammasome, was significantly decreased in hyperuricemic mice treated with the RDP2 peptide, and the expression of NLRP3, ASC, and caspase-1 in the kidneys was reduced. A model of hyperuremic mice was established to explore the mechanism and function of the RDP3 peptide [108] and animal tests were performed by dividing mice into various groups: control, model, allopurinol, benzbromarone, and RDP3. Mice were injected with uric acid intraperitoneally to induce hyperuricemia; then, for 7 days, the groups were treated with intraperitoneal injection of standard drugs allopurinol (10 mg/kg, 0.8 mg/kg HED) or with benzbromarone (8 mg/kg, 0.6 mg/kg HED); instead, the RDP3 groups were treated with intraperitoneal injection of different doses of RDP3 (100 μg/kg, 500 μg/kg and 1 mg/kg, 8 μg/kg, 40 μg/kg, and 0.08 mg/kg). The results showed that serum uric acid concentrations in the RDP3 group were significantly lower than in the other treatments. Furthermore, Western blot analysis was performed to detect NLRP3 inflammasome expression in the kidneys of mice and it was observed that NLRP3 contents in the model group kidneys were higher than those of the allopurinol group, suggesting that NLRP3 was activated. Contrariwise, the expression of the NLRP3 inflammasome was significantly decreased in the RDP3 group, demonstrating that RDP3 reduced inflammation by inhibiting the expression of the NLRP3 inflammasome.
Regarding the hyperuricemia and its associated kidney inflammation, the effects and biological mechanisms of tuna meat oligopeptides (TMOP) were investigated in mice [109]. The conducted experiments indicated that TMOP relieved hyperuricemia dose-dependently and regulated uric acid metabolism in mice with diet-induced hyperuricemia. The administration of TMOP inhibited the activation of the NLRP3 inflammasome complex, and intestinal microbiota-mediated beneficial effects of TMOP were explored by fecal microbiota transplantation.
Concerning the intestinal diseases, the ability of an isoflavone-free soy protein concentrate (SPC) to prevent inflammation and loss of intestinal barrier function have been examined [110]. The cytoprotective effects of soy protein concentrate were analyzed in vitro, and its anti-inflammatory effects in the mouse model of acute ulcerative colitis treated with DSS. In particular, the experiments were conducted on mice fed in the basic diet with the components present in the SPC powder for 7 days a randomized based on weight into control (water and AIN93G diet), DSS (1.5% DSS in drinking fluid and AIN93G), DS6 (1.5% DSS and 6% dietary SPC), and DS12 (1.5% DSS and 12% dietary SPC). SPC mice had lower NLRP3 mRNA levels than DSS-treated control mice, demonstrating that SPC is able to prevent increased pro-inflammatory signaling and thereby moderate colitis severity. Overall, the findings support the efficacy of dietary SPC as a means of preventing colonic inflammation and loss of gut barrier function.
Otherwise, a gluten-derived peptide with high content of glutamine and proline residues, α-gliadin 31–43, has been identified, which appears to induce an innate immune response in the gut [111]. Mice intestinal samples, treated for 4 or 16 h with 200 μL of p31–43 using a curved oral gavage needle, were collected for mRNA evaluation and for histological analysis. The results showed that p31–43 gliadin has an inherent propensity to form oligomers that activate NLRP3 inflammasome and that this pathway is required for intestinal inflammation and pathology when p31–43 is administered orally to mice.
By considering that the redox state of the cell can mediate the production of placental cytokines, which are responsible for the function and development of the placenta and that the NLRP3 inflammasome represents the first line of innate immunity [161], the NLRP3 placental inflammasome was investigated in a study regarding the integration of N-acetyl-cysteine (NAC) in the late gestational diet of sows [112]. A total of 16 sows at day 85 of gestation were selected based on body weight and assigned into 2 groups: the control group (7 sows) and NAC group (9 sows; the basal diet supplemented with 500 mg/kg NAC, 40 mg/kg HED). The effects of NAC on placental redox status, function, inflammasome, and fecal microbiota in sows were explored to elucidate the relationship between the fecal microbiota and the placenta. The results obtained showed that NAC significantly reduced maternal and placental inflammatory cytokines through inhibition of the NLRP3 inflammasome in sows during late pregnancy; suppression of oxidative stress and inflammatory response in sows and placenta can therefore reduce fetal exposure to inflammatory mediators and improve fetal growth.
NLRP3 inflammasome has been reported to play an essential role in the inflammation responses during acute lung injury [162]. Regarding this point, glycine supplementation in lipopolysaccharide-induced acute lung injury in mice was investigated [113]. Indeed, after being treated with aerosolized glycine (1000 mg in 5 mL of 0.9% saline) or vehicle (0.9% saline) once daily for 7 continuous days, the male mice were exposed to aerosolized lipopolysaccharide to induced lung injury in mice. The glycine prevents mucin reduction and upregulation of pro-inflammatory cytokines in lung tissue; this beneficial effect of glycine was associated with modulation of the NLRP3 inflammasome and NRF2 signaling. Carnosine (β-alanyl-histidine) and L-homocarnosine (γ-aminobutyryl-histidine) are the major endogenous constituents of excitable tissues—the brain and skeletal muscles [163]. Their supplementation was also studied in terms of modulation of NLRP3. The neuroprotective and anti-aging carnosine was shown to improve cognitive dysfunction in SAMP8 mice after a 6-week oral administration (100–200 mg/Kg/day) via NLRP3 inactivation and amelioration of the oxidative stress [114]. The effects of L-Homocarnosine against inflammation induced by cerebral ischemia in male albino rats were also analyzed [115]. In this study rats were grouped into control, middle cerebral artery occlusion (MCAO), 0.5 mM L-Homocarnosine + MCAO, and 1 mM L-Homocarnosine + MCAO treatment groups and treated for 45 days. NLRP3 inflammasome levels were substantially elevated in MCAO rats, whereas supplementation with 1 mM L-homocarnosine significantly reduced NLRP3 inflammasome levels to near normal levels.
Since cholinergic degeneration plays a major role in the pathophysiology of AD, it has been investigated whether choline supplementation during adulthood delays the progression of this disease [116]. The study was conducted on the APP/PS1 model of double transgenic mice expressing a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and a mutant human presenilin 1 (PS1-dE9), both directed to CNS neurons; APP/PS1 transgenic mice were divided into a control group (1.10 g choline chloride per kg, 0.09 mg/kg) and a group supplemented with choline (4.95 g choline chloride kg, 0.4 mg/kg HED). Firstly, it was shown that choline supplementation improves the cognitive and non-cognitive behavioral effects of AD; furthermore, from the results, both an increase in the formation of the synaptic membrane and the inhibition of the NLRP3 inflammasome were observed. Inhibition of inflammasome activation explained the reduced Aβ deposition, microgliosis, and pro-inflammatory cytokine production observed in the brains of APP/PS1 mice after 9 months of choline supplementation.
3.7. Saponins and Sterols
Ginseng is considered as a highly valued herb and widely used in dietary supplements and herbal medicines, as it exerts a regulation on endocrine system, nerves, metabolism, and other physiological functions [164,165]. The effectiveness of ginseng is attributable to its main active components, ginsenosides, a class of triterpene saponins with a steroid structure.
It has been confirmed that 25-OCH3-PPD (20S,25-methoxyldammarane-3β,12β,20-triol), a ginsenoside isolated from Panax ginseng, relieves liver damage, by inducing apoptosis of activated hepatic stellate cells [166]. Considering the correlation between inflammation and liver fibrosis, a study evaluated the regulation of 25-OCH3-PPD (5, 10, or 20 mg/kg for 5 weeks, 0.4, 0.8, or 1.6 mg/kg HED) against hepatic fibrosis and inflammation in thioacetamide (TAA)-stimulated mice by activating the LXRs (liver X receptors) pathway [117]. Study results demonstrated that 25-OCH3-PPD has a hepatoprotective effect against liver fibrosis and reduces inflammation by regulating P2X7R-mediated NLRP3 inflammasome.
Wang et al. [118] analyzed the effects of saponins isolated from Panax notoginseng in ameliorating NLFD through inhibition of inflammasome activation. The saponin extract, compared with conventional red ginseng, contained significantly increased amounts of ginsenosides Rh1 (10.34-fold) and Rg2 (7.1-fold), which are the main components highlighting the pharmacological activity of ginseng. In this study mice were fed a fast-food diet for 16 weeks to induce NLFD and then treated with saponin extract (50 or 150 mg/kg) for 9 weeks to determine the effects of the saponin. Particularly, it has been observed that Rh1 and Rg2 ginsenosides exerted anti-inflammatory effects and inhibited NLRP3 inflammasome by promoting mitophagy and alleviating mtROS production.
The characteristics of the ginsenoside compound K (CK) are peculiar, a final metabolite of panaxadiol ginsenosides. CK seems to be involved in the stimulation of insulin secretion and its protective effect against diabetic nephropathy has been elucidated in inhibiting the oxidative stress, NLRP3 inflammasome, and NF-κB/p38 [167,168].
Numerous studies have demonstrated the neuroprotective action and antidiabetic properties of CK, indicating its potential in the treatment of memory disorders and cognitive dysfunctions related to diabetes mellitus; in particular, a study tried to assess the effects of CK against memory impairment and cognitive dysfunction in diabetic db/db mice treated with 10 mg/kg of CK for 12 weeks [119]. The results showed that the CK treatment improved insulin resistance, alleviated cognitive dysfunctions, relieved oxidative stress, attenuated inflammatory responses in the hippocampus, and inhibited NLRP3 activation. Specifically, CK downregulates inflammatory cytokines and mediator production by suppressing the NLRP3 inflammasome pathway.
Magnesium isoglycyrrhizinate, a magnesium salt of 18α-glycyrrhizic acid stereoisomer extracted from the roots of the plant Glycyrrhiza glabra, acts as a hepatoprotective agent in the immune and anti-inflammatory modulation of liver disease [169]. The effects of magnesium isoglycyrrhizinate on the characteristics of the metabolic syndrome in fructose fed rats were investigated [170]. Rats were given 100 mL of water containing 10% fructose for 6 weeks, followed by treatment with saline injection, 10, 20, and 40 mg/kg (0.8, 1.6, and 3.2 mg/kg HED) of magnesium isoglycyrrhizinate (by intraperitoneal injection) or 4 mg/kg (0.3 mg/kg HED) of pioglitazone (for intragastric administration) for an additional 11 weeks. The data showed that magnesium isoglycyrrhizinate inhibited the activation of the NF-κB/NLRP3 and thus reduced the immunological–inflammatory response, preventing hepatic lipid metabolic disorder and accumulation in high fructose conditions [120].
Physalin B, one of the main active withanolides existing in Physalis alkekengi L. var. franchetii, displayed anti-inflammatory activity in intestinal ischemia [171]. The Physalin B antiulcer effects in mice with ulcerative colitis DSS-induced were evaluated [121]; mice were injected intraperitoneally with Physalin B (250 μL) once daily for 7 days. Body weight, colonic length, disease activity index, pathological changes in colonic tissue, cytokine levels, NF-κB pathway, protein levels of related pathways, and NLRP3 activation were measured. The results of this study provided evidence that Physalin B could significantly inhibit the production and secretion of various inflammatory factors: Physalin B reduced the pro-inflammatory cytokine levels of mice with colitis, suppressed the NF-κB cascade, STAT3-arrestin1 signaling, and inhibited NLRP3 inflammasome activation.
A plant phytosterol isolated from Moringa oleifera, β-sitosterol, was studied to evaluate its anti-inflammatory activity in two cell lines [122]. Tween 80 surfactant was used to produce a dispersion of small particles of β-sitosterol dissolved in dimethyl sulfoxide and applied to macrophages to evaluate the anti-inflammatory activity of β-sitosterol. Cells were incubated with BSS (7.5–60 μM). An increase in the solubility of water-insoluble phytosterols has been reported thanks to the formation of nanoparticles, the formulation of which is able to inhibit the signal transduction pathways of inflammation in macrophages. The results indicated that β-sitosterol dispersed well in the medium as nanoparticles, suppressed the secretion of inflammatory factors from keratinocytes and macrophages induced by PGN, TNF-α, or LPS (such as TNF-α, IL-1β, IL-6, IL-8, and ROS), significantly reduced NLRP3 expression and inhibited caspase-1 activation.
3.8. Polysaccharides
Polysaccharides from different sources (mushroom, herbs, plant, seaweed) have proved to exert an anti-inflammatory role via affecting the NLRP3 inflammasome pathway. However, a detailed mechanistic study on how polysaccharides modulate NLRP3 activation remained to be explored. In particular, several polysaccharides involved in the modulation of NLRP3 inflammasome belong to traditional Chinese medicine, but they are also present in dietary plants. As an example, the major bioactive component of Trametes orientalis, used for the treatment of pulmonary disease, is a polysaccharide composed of galactose, glucose, mannose, and arabinose with molar ratios of 5.79:5.77:3.45:1.20 (average MW 63000), which proved to inhibit the activation of NLRP3 inflammasome and the release of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, by the detection of expression levels of proteins in lung tissue involved in the NLRP3 inflammatory pathway [123]. Liang et al. found out the anti-inflammatory activity of polysaccharides extracted from Dendrobium officinale (DOPS) was likely to via suppressing mRNA expression of NLRP3, ASC, caspase-1, IL-1β, and IL-18 both in vivo, in DSS-induced acute ulcerative colitis mice, and in vitro, in LPS-stimulated NCM460 cells model, through a possible downregulation of β-arrestin1 expression (which could positively regulate NLRP3) [124]. A recent work by Li et al. reported CYP-1, a newly characterized mannoglucan from Chinese yam was able to suppress the expression of several key genes involved in colonic inflammatory signaling pathways (such as NF-κB and NLRP3) in DDS-induced colitis mice [125]. Ganoderma lucidum polysaccharides (GLPS), isolated from a Chinese medicinal mushroom, proved to markedly inhibit liver inflammatory factors via suppression of NLRP3 in liver tissue. Indeed, GPLS-treated acute liver injury mice exhibited a significantly decreased protein expression levels of NLRP3, ASC, and caspase-1 [126]. Armillariella tabescens (AT), that belongs to the family of Tricholomataceae, is a well-known traditional medicinal mushroom, characterized by 86.52% of polysaccharides (mannose, arabinose, and fucose at a molar ratio of 1.6:1.0:2.7.). AT possesses potent antioxidant and anti-inflammatory properties by possibly involving the repression of the TXNIP/NLRP3 inflammasome pathway in the liver of T2D mice [127]. Among the plant-derived polysaccharides, low methoxyl pectin (LMP) showed an important role in the autoimmune diabetes through suppression of NLRP3 and associated proteins expression in cecum (NLRP3, caspase-1-p20, cleaved IL-1𝛽, and cleaved IL-18) [128]. The NLRP3 inhibition could be ascribed to the increase in the SCFAs (short chain fatty acids) by gut microbiota, induced by LMP supplementation, which act as histone-deacetylase (HDAC) inhibitors towards the NLRP3 inflammasome. Furthermore, Wu et al. pointed out that LMP supplementation also suppressed NLRP3 activation in pancreas, but a detailed mechanistic study on how LMP modulates NLRP3 inflammasome activation in pancreas has not yet been performed [129].
Recently, Castro-Alves et al. investigated the activity of non-digestible carbohydrates (NDCs) from chayote fruit, which consist mainly of pectic homogalacturonan and highly branched rhamnogalacturonan-II, as well as hemicellulosic material including glucomannan, xyloglucan, and glucurono(arabino)xylan, in human THP-1 macrophage-like cells. Results showed that NDCs indirectly inhibit NLRP3 activation through the interaction between the NDCs and other pattern-recognition receptors that are essential to induce priming signals required for NLRP3 activation [130].
Several papers highlighted the anti-inflammatory activity of different polysaccharides probably ascribed to NLRP3 inhibition by decreased protein levels of NLRP3, ASC, caspase-1, and IL-1β [172,173,174]; however, few works assessed the pro-inflammatory efficacy of some polysaccharides towards the NLRP3 inflammasome. In particular, Liu et al. found out that the polysaccharide SF-2, a mannoglucan sulfate isolated from starfish (A. rollestoni), could improve the release of cytokines and the expression of NLRP3 in primary macrophages, confirmed by the elevated expression of NLRP3, cleaved caspase-1, and ASC proteins [131].
3.9. Vitamins and Derivatives
The importance of lipophilic vitamin E and its derivatives, which mainly occur as abundant nutrients in oily nuts and seeds, in inflammation and oxidation processes has been elegantly reviewed by Wallert and collaborators [175]. Different forms of vitamin E or their long-chain metabolites can interfere with ROS production, NF-kB priming, or NLRP3 activation. Recently, further studies confirmed these results in two different murine models of HFD and alloxan-induced diabetes, especially regarding γ-tocopherol supplementation, as such, or in more complex extracts (e.g., Rosa mosqueta oil contains about 74 g/100 g oil of α-tocopherol and 359 g/100 g oil of γ-tocopherol, stripped corn oil) [176,177].
Vitamin D and its derivatives were also studied, alone or in association with pro-biotics and microelements as food supplements, for their effects on the modulation of inflammasome halting the priming step required for NLRP3 (and NLRP1) activation in immune cells, in placental explants from preeclamptic and normotensive pregnant women, in clinical trials involving COVID-19 patients [178], in the proliferative diabetic retinopathy pathogenesis, in diabetic corneal wound healing and reinnervation, in non-alcoholic fatty liver disease, and in acute kidney injury.
3.10. Probiotics, Symbiotics, and Their Main Components
Lactic acid bacteria (LAB) are currently used as food supplements for human health as probiotics due to their modulatory effects on intestinal conditions, immunomodulatory function, and host metabolism. The effect of each probiotic is dependent on the cell line and species used. To test the anti-inflammatory action of LAB or their main components (e.g., butyrate at 200 mg/kg, 16.3 mg/kg HED) via the inhibition of inflammasome activation, Lactobacillus paracasei KW3110 (1 × 106 cells/mL), Bifidobacterium infantis (2 × 108 CFU/mL) in combination with the prebiotic xylooligosaccharide (XOS, 230 mg/kg, 18.7 mg/kg HED), Enterococcus faecium NCIMB 10415 (1 × 107 CFU/mL), and heat-killed cells of Enterococcus faecalis (17 mg/kg, 1.4 mg/kg HED) were recently proposed in different in vitro and in vivo disease models of DSS-induced ulcerative colitis [179], HFD-induced T2D [180], cerulein-induced acute pancreatitis [181], and colitis-associated colorectal cancer [182], as well as on porcine dendritic cells [183]. The beneficial effects of a direct modulation of NLRP3 were also confirmed by a downregulation of pro-inflammatory cytokines, upregulation of anti-inflammatory players. These results have been also described feeding animals or treating cells derived from livestock with probiotics (1 × 105 CFU of Lactobacillus rhamnosus GR-1 and 3 × 105–2 × 107 CFU of Lactobacillus johnsonii L531) with the aim of limiting the inflammatory damage of bacterial infections induced by Escherichia coli in porcine mammary epithelial cells, MAC-T cells and in a mouse mastitis model, or Salmonella typhimurium in IPEC-J2 cells [184,185,186]. The administration of these probiotics allowed limited bacterial adhesion to cells and bacterial alteration of cellular morphology, thus removing the first cause of ROS production and immune system activation. The NLRP3 attenuation downregulated ILs, TNF-α, and chemokine Cxcl2 expression and, concurrently, decreased the expression of autophagic receptor SQSTM1/p62 and induced tight junction injury.
4. Food and Nutraceuticals Components Bioavailability and Toxicity
Apart from table sugar, foods are “complex matrices” made up of different components, divided into macronutrients (proteins, carbohydrates, and fats), micronutrients (vitamins and minerals), and water. The presence of other minority components also increases the complexity of food matrices. Some of them are defined as “bioactive compounds” because, based on scientific evidence, it is assumed that they can have a positive effect on our organism and can play a fundamental and important role in modulating inflammasomes [12,30,35].
The “bioactive compounds” belong to many different categories based on their chemical nature and on the type of positive effect they could exert on human health. Commonly, they are not considered nutrients and are referred as “essential and non-essential compounds” that occur in nature, are part of the food chain, and can help to protect ourselves from illnesses and improve our quality life [1,187]. Although the most known and studied “bioactive compounds” are of plant origin, it is wrong to think that they are not present in foods of animal origin. Conjugated linoleic acid, taurine, carnitine, carnosine, glutathione, and lipoic acid are some examples, together with bioactive peptides, which confirm the importance of all food types in our diet [188,189].
Certainly, the most numerous classes of “bioactive compounds” are represented by phytochemicals: molecules of various kinds such as carotenoids, tocopherols, phytosterols, phenolic compounds, etc., but, in any case, these are of vegetable origin. Among all these different metabolites that are important for their health benefits, the phenolic compounds have been studied most extensively [190,191]. These constituents, with more than 5000 structurally diverse molecules of various types, are the most numerous, abundant, and widely distributed “bioactive compounds”, very important for their potential beneficial effects, which are also associated with inflammasome disorder in diseases [1,17,53,190,192]. Many of these positive effects on safety and efficacy have been studied in vitro and on animal models and have yet to be confirmed with absolute certainty on humans with clinical trials [193,194,195]. It is well known that the “bioactive compounds”, whatever their origin (from animals or plants source), due to the food complexity and in order to exert their beneficial health action, need to be released from the food matrix after ingestion, and then metabolized [196]. As different researchers highlighted, the global food matrix composition, with the presence of nutrient interaction, has a crucial role on the health benefit of “bioactive compounds”; however, some other specific characteristics can determine the biological activity of these metabolites [17,197]. The factors that influence the absorption and bioavailability of “bioactive compounds” are numerous, one of these is represented by the cell wall structure and properties, since, often, they are resistant to degradation in the upper gut [196]. In addition, the in vivo biological activity of many high molecular weight compounds, such as flavonoids, is influenced considerably by molecular structure, binding to other molecules (i.e., by esterification or glycosylation), different isomeric configuration and their interaction with macromolecules, such as proteins and dietary fibers [196,198,199,200,201]. Among these factors, we must include different transport and diffusion mechanisms of ingested food “bioactive compounds” because their bioavailability can be blocked and/or modified significantly by certain nutrients [202]. Moreover, as many drugs, also bioactive food compounds are subjected to several metabolic and enzymatic processes that modify the structure and could be responsible for molecular forms, which are different from the original constituent of the ingested food and can show a different effect (increase, decrease, or toxic) in presence of specific nutrients (i.e., lipids) [196].
The intake of “bioactive compounds” in the human diet varies enormously in relation to the type, quantity, and quality of foods consumed. Generally, the most important food sources of bioactive nutrients are vegetable, fruits, chocolate, tea, wine, olives, and spices; however, their concentration in the same food varies, often significantly, in relation to the cultivation techniques and food processing, the degree of ripeness, and the time elapsed between harvesting and consumption [198,203]. Thus, because different foods contain a huge variety of bioactive compounds and a lot of them often are poorly absorbed in our intestine, there is great difficulty in establishing the “effective dose”, which is the amount of this substance that we must take to have the expected effect. Furthermore, the “bioactive compounds” stability and, consequently, their antioxidant, anti-inflammatory, and immunomodulatory properties, may be lost during digestion [1,190].
Usually, to improve the sensory attributes and make a product more attractive for taste, consistency, and appearance, many foods are submitted to culinary treatments. Different domestic practice and cooking techniques (i.e., boiling, roasting, stir-frying, microwaving, and streaming) have been compared to define the optimal process to reduce the degradation of the “bioactive compounds”. It was observed that food nature, cooking time, temperature, addition of acidulant, spices, or other condiments might influence both the amount of water-soluble “bioactive compounds” and of those fixed to the lipidic matrix [204]. Each food contains a characteristic composition, and the chemical structure of each bioactive metabolite strongly influences the loss extent of individual component. Generally, food processing and even storage frequently cause losses in phenolic compounds [205]. Overall, high-temperature food processing (i.e., roasting, blanching, drying, and pasteurization) considerably reduces the concentration of phenolic compounds in foods. To ensure the greatest phenolic amounts, it is recommended to consume fresh food or use steam cooking with almost no osmotic processes. Other methods, such as microwave cooking, that imply low water and short cooking time, are more recommended to maintain high levels of these compounds inside foods. In fact, when subject to boiling (commonly used for vegetable) or too high temperatures, foods suffer considerable losses of most phenolic compounds, especially flavonol glucosides [204,205,206]. For other bioactive compounds that are part of lipidic membrane or oil fraction of foods (i.e., carotenoids and tocopherols), cooking methods involving water are less aggressive than those using oils for stir-frying [204]. Innovative non-thermal procedures (UV, high hydrostatic pressure, and pulsed electric fields treatments) do not significantly deteriorate bioactive compounds, preserve their antioxidant activity, and provide promising alternative to thermal processing commonly used for food disinfection or to inactivate microbes and enzymes in foods [206].
Although there is evidence showing the beneficial effects of “bioactive compounds” present in foods, more studies suggest that their health benefits instead depend on the synergy and interactions among different molecules [207]. Therefore, in drafting the anti-inflammatory diet it must be considered that bioactive nutrients cannot be from a single food but rather it is the result from the synergy between foods that provide different antioxidant molecules to counteract the inflammatory processes that occur. In addition, as stated above, it is not only important to know how much a nutrient is present in a specific food, but it is even more important to know how much of it is bioavailable.
Despite the growing amounts of studies, definitive conclusions supporting the bioavailability of most “bioactive compounds” from a wide variety of food for optimal nutrition and health well-being are difficult to obtain. Consumers’ demand for well-being pushes towards an increasing request for food supplements and innovative new market segments have been developed to meet their needs. Alternatively, dietary supplements, also differentiated into nutraceutical or functional foods, can be supplied to consumers to deliver a specific bioactive compound or a group of them [1,189]. Generally, nutraceuticals are bioactive compounds derived from food sources. They can include one or more substances that are purported to provide extra health benefits and are considered as a pharmaceutical alternative [208]. They are supplied to consumers in a concentrated form and the ingredients are in higher dose than in normal foods. Therefore, as a drug, the food supplement should be used as a necessary complement in diets that are poor in nutrients and foods, in order to provide more energy and nutrients to our body to properly support its physiological processes. On the other hand, functional foods are foods fortified with an extensive array of bioactive compounds which provide a clinically proven health benefit, beyond their natural properties, when consumed in a regular and consistent manner through diet [189,209]. Therefore, as a drug, nutritional supplementation of nutrients in order to increase the functionality of the immune system has no indication in case of adequate intake; on the contrary, it can be even harmful if it involves exceeding the maximum daily doses.
Nowadays, a great variety of nutraceuticals and functional foods are provided to the consumers; however, their use is largely unregulated, or they are treated differently according to local jurisdictions. Generally, they are more the subject of marketing hype, and for many of these “supplements”, it is not even yet known whether they provide more benefits than risks to consumers. Currently the term “bioactive compound” is not defined in the European regulations; however, the European Food Safety Authority (EFSA) is carrying out scientific evaluations to assess the safety and the toxicity of “bioactive compounds” that can be found in foods [210]. Due to the great number of compounds that can have a potentially positive effect on health, the European Commission has not produced a list of authorized ones, but it has tried to put order for “borderline” products, by identifying the criteria to define a dietary supplement. At present, among food supplement phytochemicals, EFSA claims for hydroxytyrosol, its derivatives (i.e., oleuropein complex and tyrosol), and olive oil polyphenols as substances able to protect the blood lipids from the harmful effects of oxidative stress [211]. With this background, we highlighted that the “bioactive compounds” present in foods can possess a potential therapeutic effect on different diseases associated with the activation of inflammasomes. Anyhow, due to different food matrix effects and compositions, the outcome obtained from concentrations used in in vitro tests are often not achievable through a targeted diet. Currently there is insufficient information available on the safety of supplements or functional foods. They cannot be considered as the emergency remedy and are not intended as a substitute for a varied and balanced diet and a correct lifestyle.
Finally, food consumed as such, or after cooking processes, as well as water, may also contain internal or accidental components with a stimulating effect on NLRP3 inflammasome. Among these, mycotoxins (zearalenone, patulin, deoxynivalenol), trace metals (arsenic, cadmium), environmental and food contaminants (acrolein, 3-monochloropropane-1,2-diol, glycidol, and its esters), and acrylamide are few examples of NLRP3 activators, endowed with harmful inflammatory effects [212,213,214,215,216,217,218,219,220].
5. Conclusions
Eating is something we have always done and which, from birth to old age, strongly affects life quality and physical and psychological well-being. Nowadays, consumers are increasingly interested in lifestyles and nutritional habits that can prevent diseases since different studies have evidenced that a relationship occurs between these factors and pathological consequences based on inflammatory processes. In this regard, it should be emphasized that these effects are related, not only to the quantity, but, above all, to the variety of food choices and, in particular, to the inclusion of fruit and vegetables. Plant foods are particularly rich in important bioactive compounds, each of them can produce different effects on the pathway of inflammatory response, confirming the importance of the nutritional pattern (food model) as a whole, rather than the single nutrient or functional compound.
Author Contributions
All authors have participated in the elaboration of the manuscript, have read the final version, and agreed with the contents. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Grants: Italian Ministry of Education, Universities and Research-Dipartimenti di Eccellenza-L. 232/2016.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Castejón-Vega, B.; Giampieri, F.; Alvarez-Suarez, J.M. Nutraceutical compounds targeting inflammasomes in human diseases. Int. J. Mol. Sci. 2020, 21, 4829. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Paola, R. Di Focus on the role of NLRP3 inflammasome in diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.; Bergstralh, D.T.; Wang, Y.; Willingham, S.B.; Ye, Z.; Zimmermann, A.G.; Ting, J.P.Y. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 8041–8046. [Google Scholar] [CrossRef] [Green Version]
- Hafner-Bratkovič, I.; Sušjan, P.; Lainšček, D.; Tapia-Abellán, A.; Cerović, K.; Kadunc, L.; Angosto-Bazarra, D.; Pelegrίn, P.; Jerala, R. NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat. Commun. 2018, 9, 5182. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, T.; Yang, Y.; Jin, T.; Jiang, W.; Zhou, R. Orchestration of NLRP3 Inflammasome Activation by Ion Fluxes. Trends Immunol. 2018, 39, 393–406. [Google Scholar] [CrossRef]
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Núñez, G.; Mao, Y.; et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 2019, 570, 338–343. [Google Scholar] [CrossRef]
- Kuwar, R.; Rolfe, A.; Di, L.; Blevins, H.; Xu, Y.; Sun, X.; Bloom, G.S.; Zhang, S.; Sun, D. A novel inhibitor targeting NLRP3 inflammasome reduces neuropathology and improves cognitive function in alzheimer’s disease transgenic mice. J. Alzheimer’s Dis. 2021, 82, 1769–1783. [Google Scholar] [CrossRef]
- Liang, Z.; Damianou, A.; Di Daniel, E.; Kessler, B.M. Inflammasome activation controlled by the interplay between post-translational modifications: Emerging drug target opportunities. Cell Commun. Signal. 2021, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Assanga, S.B.I.; Luján, L.L.; O’keefe, J.H.; Dinicolantonio, J.J. Nutraceutical strategies for suppressing nlrp3 inflammasome activation: Pertinence to the management of covid-19 and beyond. Nutrients 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P.Y. The NLRP3 Inflammasome Mediates In Vivo Innate Immunity to Influenza A Virus through Recognition of Viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Son, S.; Hwang, I.; Hyeokhan, S.; Shin, J.S.; Shin, O.S.; Yu, J.W. Advanced glycation end products impair NLRP3 inflammasome-mediated innate immune responses in macrophages. J. Biol. Chem. 2017, 292, 20437–20448. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.H.; Brickey, W.J.; Ting, J.P.Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, L.; Cardozo, L.F.M.F.; Borges, N.A.; Lindholm, B.; Stenvinkel, P.; Shiels, P.G.; Fouque, D.; Mafra, D. Can nutritional interventions modulate the activation of the NLRP3 inflammasome in chronic kidney disease? Food Res. Int. 2020, 136, 109306. [Google Scholar] [CrossRef]
- Drobny, E.C.; Abramson, E.C.; Baumann, G. Insulin receptors in acute infection: A study of factors conferring insulin resistance. J. Clin. Endocrinol. Metab. 1984, 58, 710–716. [Google Scholar] [CrossRef]
- Caputo, T.; Gilardi, F.; Desvergne, B. From chronic overnutrition to metaflammation and insulin resistance: Adipose tissue and liver contributions. FEBS Lett. 2017, 591, 3061–3088. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S1–S78. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Tack, C.J.; Stienstra, R.; Joosten, L.A.B.; Netea, M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012, 249, 239–252. [Google Scholar] [CrossRef]
- Lagathu, C.; Yvan-Charvet, L.; Bastard, J.P.; Maachi, M.; Quignard-Boulangé, A.; Capeau, J.; Caron, M. Long-term treatment with interleukin-1β induces insulin resistance in murine and human adipocytes. Diabetologia 2006, 49, 2162–2173. [Google Scholar] [CrossRef] [Green Version]
- Stienstra, R.; Van Diepen, J.A.; Tack, C.J.; Zaki, M.H.; Van De Veerdonk, F.L.; Perera, D.; Neale, G.A.; Hooiveld, G.J.; Hijmans, A.; Vroegrijk, I.; et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, H.M. Dietary modulation of energy homoeostasis and metabolic-inflammation. Proc. Nutr. Soc. 2019, 78, 313–318. [Google Scholar] [CrossRef]
- McGillicuddy, F.C.; Harford, K.A.; Reynolds, C.M.; Oliver, E.; Claessens, M.; Mills, K.H.G.; Roche, H.M. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes 2011, 60, 1688–1698. [Google Scholar] [CrossRef] [Green Version]
- Kirwan, A.M.; Lenighan, Y.M.; O’Reilly, M.E.; McGillicuddy, F.C.; Roche, H.M. Nutritional modulation of metabolic inflammation. Biochem. Soc. Trans. 2017, 45, 979–985. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome-Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst-Trigg, R.; Hulston, C.J.; Markey, O. The effect of quantity and quality of dietary fat intake on subcutaneous white adipose tissue inflammatory responses. Proc. Nutr. Soc. 2020, 79, 542–556. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncero-Ramos, I.; Rangel-Zuñiga, O.A.; Lopez-Moreno, J.; Alcala-Diaz, J.F.; Perez-Martinez, P.; Jimenez-Lucena, R.; Castaño, J.P.; Roche, H.M.; Delgado-Lista, J.; Ordovas, J.M.; et al. Mediterranean Diet, Glucose Homeostasis, and Inflammasome Genetic Variants: The CORDIOPREV Study. Mol. Nutr. Food Res. 2018, 62, 1700960. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Margioris, A.N. Fatty acids and postprandial inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 129–137. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63, S5–S21. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Nutrition: Trans Fat. Available online: https://www.who.int/news-room/questions-and-answers/item/nutrition-trans-fat (accessed on 29 November 2021).
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A Systemic Review of the Roles of n-3 Fatty Acids in Health and Disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.R.; Midgette, Y.; Shah, R. Fish oil derived omega 3 fatty acids suppress adipose NLRP3 inflammasome signaling in human obesity. J. Endocr. Soc. 2019, 3, 504–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sears, B. Anti-inflammatory diets for obesity and diabetes. J. Am. Coll. Nutr. 2009, 28, 482S–491S. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Devaraj, S.; Jialal, I. Dietary factors that promote or retard inflammation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; Mcallan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef] [Green Version]
- Levitan, E.B.; Cook, N.R.; Stampfer, M.J.; Ridker, P.M.; Rexrode, K.M.; Buring, J.E.; Manson, J.A.E.; Liu, S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 2008, 57, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–226. [Google Scholar] [CrossRef]
- Pavillard, L.E.; Marín-Aguilar, F.; Bullon, P.; Cordero, M.D. Cardiovascular diseases, NLRP3 inflammasome, and western dietary patterns. Pharmacol. Res. 2018, 131, 44–50. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.J.; Lin, J.H.; Yen, G.C. Beneficial Properties of Phytochemicals on NLRP3 Inflammasome-Mediated Gout and Complication. J. Agric. Food Chem. 2018, 66, 765–772. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H. Bin Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Qian, B.; Wang, C.; Zeng, Z.; Ren, Y.; Li, D.; Song, J. Le Ameliorative effect of sinapic acid on dextran sodium sulfate-(DSS-) induced ulcerative colitis in kunming (km) mice. Oxid. Med. Cell. Longev. 2020, 2020, 8393504. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Abd El-Twab, S.M.; Hozayen, W.G. Ferulic acid protects against methotrexate nephrotoxicity: Via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019, 10, 4593–4607. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, D.; Wan, X.; Bai, Y.; Yuan, C.; Wang, T.; Yuan, D.; Zhang, C.; Liu, C. Chlorogenic Acid Suppresses miR-155 and Ameliorates Ulcerative Colitis through the NF-κB/NLRP3 Inflammasome Pathway. Mol. Nutr. Food Res. 2020, 64, e2000452. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, X.; Luo, Y.; Fan, W.; Shen, T.; Ding, C.; Yao, M.; Song, S.; Yan, L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J. Nutr. Biochem. 2019, 71, 110–121. [Google Scholar] [CrossRef]
- Yamagata, K.; Hashiguchi, K.; Yamamoto, H.; Tagami, M. Dietary Apigenin Reduces Induction of LOX-1 and NLRP3 Expression, Leukocyte Adhesion, and Acetylated Low-Density Lipoprotein Uptake in Human Endothelial Cells Exposed to Trimethylamine-N-Oxide. J. Cardiovasc. Pharmacol. 2019, 74, 558–565. [Google Scholar] [CrossRef]
- An, M.F.; Wang, M.Y.; Shen, C.; Sun, Z.R.; Zhao, Y.L.; Wang, X.J.; Sheng, J. Isoorientin exerts a urate-lowering effect through inhibition of xanthine oxidase and regulation of the TLR4-NLRP3 inflammasome signaling pathway. J. Nat. Med. 2021, 75, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chiang, Y.F.; Chen, H.Y.; Huang, Y.J.; Wang, K.L.; Hong, Y.H.; Ali, M.; Shieh, T.M.; Hsia, S.M. Anti-inflammatory and anti-hyperuricemic effects of chrysin on a high fructose corn syrup-induced hyperuricemia rat model via the amelioration of urate transporters and inhibition of nlrp3 inflammasome signaling pathway. Antioxidants 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sun, H.; Zhang, Y.; Xu, W.; Wang, C.; Fang, Y.; Zhao, J. Neuroprotective Effects of Luteolin Against Spinal Cord Ischemia-Reperfusion Injury by Attenuation of Oxidative Stress, Inflammation, and Apoptosis. J. Med. Food 2018, 21, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Staurengo-Ferrari, L.; Fattori, V.; Amaral, F.A.; Teixeira, M.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; et al. Hesperidin Methylchalcone Suppresses Experimental Gout Arthritis in Mice by Inhibiting NF-κB Activation. J. Agric. Food Chem. 2018, 66, 6269–6280. [Google Scholar] [CrossRef]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, 2000746. [Google Scholar] [CrossRef]
- Li, H.; Chen, F.J.; Yang, W.L.; Qiao, H.Z.; Zhang, S.J. Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2021, 12, 717–725. [Google Scholar] [CrossRef]
- Xue, Y.; Du, M.; Zhu, M.J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157:H7. Free Radic. Biol. Med. 2017, 108, 760–769. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Q.; Li, X.; Jiang, M.; Cui, B.W.; Xia, K.L.; Wu, Y.L.; Lian, L.H.; Nan, J.X. Amelioration of Alcoholic Liver Steatosis by Dihydroquercetin through the Modulation of AMPK-Dependent Lipogenesis Mediated by P2X7R-NLRP3-Inflammasome Activation. J. Agric. Food Chem. 2018, 66, 4862–4871. [Google Scholar] [CrossRef]
- Du, Y.C.; Lai, L.; Zhang, H.; Zhong, F.R.; Cheng, H.L.; Qian, B.L.; Tan, P.; Xia, X.M.; Fu, W.G. Kaempferol from: Penthorum chinense Pursh suppresses HMGB1/TLR4/NF-?B signaling and NLRP3 inflammasome activation in acetaminophen-induced hepatotoxicity. Food Funct. 2020, 11, 7925–7934. [Google Scholar] [CrossRef]
- Lalitha, N.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. Anti-hyperglycemic activity of myricetin, through inhibition of DPP-4 and enhanced GLP-1 levels, is attenuated by co-ingestion with lectin-rich protein. PLoS ONE 2020, 15, e0231543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.-Y.; Guo, Y.-J.; Han, W.-X.; Yang, M.-Q.; Wen, L.-P.; Wang, K.-Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.M.F.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef]
- Chen, T.C.; Yen, C.K.; Lu, Y.C.; Shi, C.S.; Hsieh, R.Z.; Chang, S.F.; Chen, C.N. The antagonism of 6-shogaol in high-glucose-activated NLRP3 inflammasome and consequent calcification of human artery smooth muscle cells. Cell Biosci. 2020, 10, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Chang, Y.H. Comparison of inhibitory capacities of 6-, 8- and 10-gingerols/shogaols on the canonical NLRP3 inflammasome-mediated IL-1β secretion. Molecules 2018, 23, 466. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Huang, J.; Wang, K.; Yu, Q.; Zhu, C.; Ren, H. Pterostilbene Protects against Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Upregulating the Nrf2 Pathway and Inhibiting NF-κB, MAPK, and NLRP3 Inflammasome Activation. J. Med. Food 2020, 23, 952–960. [Google Scholar] [CrossRef]
- Wang, B.J.; Chiu, H.W.; Lee, Y.L.; Li, C.Y.; Wang, Y.J.; Lee, Y.H. Pterostilbene attenuates hexavalent chromium-induced allergic contact dermatitis by preventing cell apoptosis and inhibiting IL-1β-related NLRP3 inflammasome activation. J. Clin. Med. 2018, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Chen, Y.Y.; Hsiao, C.M.; Pan, M.H.; Wang, B.J.; Chen, Y.C.; Ho, C.T.; Huang, K.C.; Chen, R.J. Induction of Autophagy by Pterostilbene Contributes to the Prevention of Renal Fibrosis via Attenuating NLRP3 Inflammasome Activation and Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2020, 8, 436. [Google Scholar] [CrossRef]
- Chen, L.; Lan, Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct. 2017, 8, 1785–1792. [Google Scholar] [CrossRef]
- Park, B.; Jo, K.; Lee, T.G.; Hyun, S.W.; Kim, J.S.; Kim, C.S. Polydatin inhibits NLRP3 inflammasome in dry eye disease by attenuating oxidative stress and inhibiting the nf-κb pathway. Nutrients 2019, 11, 2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Wang, C.; Wu, W.; Liu, T.; Ji, B.; Zhou, F. Cyanidin-3-glucoside alleviates 4-Hydroxyhexenal-induced NLRP3 inflammasome activation via JNK-c-Jun/AP-1 pathway in human retinal pigment epithelial cells. J. Immunol. Res. 2018, 2018, 5604610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wang, S.; Wan, T.; Huang, Y.; Pang, N.; Jiang, X.; Gu, Y.; Zhang, Z.; Luo, J.; Yang, L. Cyanidin-3-O-β-glucoside inactivates NLRP3 inflammasome and alleviates alcoholic steatohepatitis via SirT1/NF-κB signaling pathway. Free Radic. Biol. Med. 2020, 160, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.L.; Sun, H.Y.; Wu, X.B.; Cheng, L.; Ren, J.D. Epigallocatechin-3-gallate attenuates acute pancreatitis induced lung injury by targeting mitochondrial reactive oxygen species triggered NLRP3 inflammasome activation. Food Funct. 2021, 12, 5658–5667. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, M.; Yao, W.; Du, K.; He, M.; Jin, X.; Jiao, L.; Ma, G.; Wei, B.; Wei, M. Epigallocatechin-3-Gallate Attenuates Microglial Inflammation and Neurotoxicity by Suppressing the Activation of Canonical and Noncanonical Inflammasome via TLR4/NF-κB Pathway. Mol. Nutr. Food Res. 2019, 63, 1801230. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lee, Y.H.; Hsu, Y.H.; Chen, Y.J.; Lin, Y.F.; Cheng, F.Y.; Chiu, H.W. Resveratrol-loaded nanoparticles conjugated with kidney injury molecule-1 as a drug delivery system for potential use in chronic kidney disease. Nanomedicine 2017, 12, 2741–2756. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Loarca-Piña, G.; Gonzalez de Mejia, E. Gallic and butyric acids modulated NLRP3 inflammasome markers in a co-culture model of intestinal inflammation. Food Chem. Toxicol. 2020, 146, 111835. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.-Y.; Qian, F.; Wang, Y.; Granato, D. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Wang, T.; Cai, M.; Qian, F.; Sun, Y.; Wang, Y. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019, 10, 3898–3908. [Google Scholar] [CrossRef]
- Gao, X.; Fan, W.; Tan, L.; Shi, Y.; Ding, C.; Liu, S.; Miao, Y.; Luo, Y.; Shi, X.; DeSaeger, S.; et al. Soy isoflavones ameliorate experimental colitis by targeting ERα/NLRP3 inflammasome pathways. J. Nutr. Biochem. 2020, 83, 108438. [Google Scholar] [CrossRef]
- Fan, R.; You, M.; Toney, A.M.; Kim, J.; Giraud, D.; Xian, Y.; Ye, F.; Gu, L.; Ramer-Tait, A.E.; Chung, S. Red Raspberry Polyphenols Attenuate High-Fat Diet–Driven Activation of NLRP3 Inflammasome and its Paracrine Suppression of Adipogenesis via Histone Modifications. Mol. Nutr. Food Res. 2020, 64, e1900995. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.; Yang, C.; Li, L.; Ye, H.; Yan, H.; Wang, M.; Yuan, Y. Allicin alleviated acrylamide-induced NLRP3 inflammasome activation via oxidative stress and endoplasmic reticulum stress in Kupffer cells and SD rats liver. Food Chem. Toxicol. 2021, 148, 111937. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Yen, C.C.; Kuo, L.L.; Lo, C.W.; Huang, C.S.; Chen, C.C.; Lii, C.K. Benzyl isothiocyanate ameliorates high-fat/cholesterol/cholic acid diet-induced nonalcoholic steatohepatitis through inhibiting cholesterol crystal-activated NLRP3 inflammasome in Kupffer cells. Toxicol. Appl. Pharmacol. 2020, 393, 114941. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shang, H.; Chen, Y.Q.; Pan, L.L.; Bhatia, M.; Sun, J. Sulforaphane Protects Pancreatic Acinar Cell Injury by Modulating Nrf2-Mediated Oxidative Stress and NLRP3 Inflammatory Pathway. Oxid. Med. Cell. Longev. 2016, 2016, 4916497. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, J.; Lee, M.J.; Kim, Y.J.; Cho, Y.W.; Lee, G.S. Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine 2015, 71, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Xia, Z.; Shao, N.; Li, B.; Xue, L.; Peng, Y.; Zhi, F.; Yang, Y. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci. Rep. 2017, 7, 12117. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Piscianz, E.; Zweyer, M.; Bortul, R.; Loganes, C.; Girardelli, M.; Baj, G.; Monasta, L.; Celeghini, C. Geranylgeraniol and neurological impairment: Involvement of apoptosis and mitochondrial morphology. Int. J. Mol. Sci. 2016, 17, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.M.; Panis, C.; Da Silva, S.S.; Macri, J.A.; Kawakami, N.Y.; Hayashida, T.H.; Madeira, T.B.; Acquaro, V.R.; Nixdorf, S.L.; Pizzatti, L.; et al. Kaurenoic acid possesses leishmanicidal activity by triggering a NLRP12/IL-1 β/cNOS/NO Pathway. Mediat. Inflamm. 2015, 2015, 392918. [Google Scholar] [CrossRef] [Green Version]
- Robblee, M.M.; Kim, C.C.; Abate, J.P.; Valdearcos, M.; Sandlund, K.L.M.; Shenoy, M.K.; Volmer, R.; Iwawaki, T.; Koliwad, S.K. Saturated Fatty Acids Engage an IRE1α-Dependent Pathway to Activate the NLRP3 Inflammasome in Myeloid Cells. Cell Rep. 2016, 14, 2611–2623. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, F.; Khazaei, M.; Komaki, A.; Amiri, I.; Jalili, C. Virgin coconut oil (VCO) by normalizing NLRP3 inflammasome showed potential neuroprotective effects in Amyloid-β induced toxicity and high-fat diet fed rat. Food Chem. Toxicol. 2018, 118, 68–83. [Google Scholar] [CrossRef]
- López-Tenorio, I.I.; Domínguez-López, A.; Miliar-García, Á.; Escalona-Cardoso, G.N.; Real-Sandoval, S.A.; Gómez-Alcalá, A.; Jaramillo-Flores, M.E. Modulation of the mRNA of the Nlrp3 inflammasome by Morin and PUFAs in an obesity model induced by a high-fat diet. Food Res. Int. 2020, 137, 109706. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, T.; Wang, F.; Yang, Y.; He, C.; Yang, W.; Zhang, J.; Zou, Z. High n-3 fatty acids counteract hyperglycemia-induced insulin resistance in fat-1 mice: Via pre-adipocyte NLRP3 inflammasome inhibition. Food Funct. 2021, 12, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Ma, T.; Geng, S.; Ning, D. Walnut oil alleviates DSS–induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microb. Pathog. 2021, 154, 104866. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, Y.; Chen, J.; Zong, Y.; Yang, X. DHA/AA alleviates LPS-induced Kupffer cells pyroptosis via GPR120 interaction with NLRP3 to inhibit inflammasome complexes assembly. Cell Death Dis. 2021, 12, 73. [Google Scholar] [CrossRef]
- Dang, R.; Zhou, X.; Tang, M.; Xu, P.; Gong, X.; Liu, Y.; Jiao, H.; Jiang, P. Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur. J. Nutr. 2018, 57, 893–906. [Google Scholar] [CrossRef]
- Liu, N.; Meng, B.; Zeng, L.; Yin, S.; Hu, Y.; Li, S.; Fu, Y.; Zhang, X.; Xie, C.; Shu, L.; et al. Discovery of a novel rice-derived peptide with significant anti-gout potency. Food Funct. 2020, 11, 10542–10553. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Y.; Zeng, L.; Yin, S.; Hu, Y.; Li, S.; Fu, Y.; Zhang, X.; Xie, C.; Shu, L.; et al. RDP3, A Novel Antigout Peptide Derived from Water Extract of Rice. J. Agric. Food Chem. 2020, 68, 7143–7151. [Google Scholar] [CrossRef]
- Han, J.; Wang, X.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. Protective effects of tuna meat oligopeptides (TMOP) supplementation on hyperuricemia and associated renal inflammation mediated by gut microbiota. FASEB J. 2020, 34, 5061–5076. [Google Scholar] [CrossRef] [Green Version]
- Bitzer, Z.T.; Wopperer, A.L.; Chrisfield, B.J.; Tao, L.; Cooper, T.K.; Vanamala, J.; Elias, R.J.; Hayes, J.E.; Lambert, J.D. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo. J. Nutr. Biochem. 2017, 40, 201–208. [Google Scholar] [CrossRef]
- Gómez Castro, M.F.; Miculán, E.; Herrera, M.G.; Ruera, C.; Perez, F.; Prieto, E.D.; Barrera, E.; Pantano, S.; Carasi, P.; Chirdo, F.G. P31-43 gliadin peptide forms oligomers and induces NLRP3 inflammasome/caspase 1-dependent mucosal damage in small intestine. Front. Immunol. 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Xu, X.; Sho, T.; Luo, W.; Zhang, J.; Xu, W.; Yao, J.; Xu, J. Effects of n-acetyl-cysteine supplementation in late gestational diet on maternal-placental redox status, placental NLRP3 inflammasome, and fecal microbiota in sows. J. Anim. Sci. 2019, 97, 1757–1771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, X.; Jiang, D.; Chen, J.; Jia, H.; Wu, Z.; Kim, I.H.; Yang, Y. Glycine attenuates lipopolysaccharide-induced acute lung injury by regulating NLRP3 inflammasome and NRF2 signaling. Nutrients 2020, 12, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; Lu, X.Y.; Zhu, W.L.; Liu, X.Q.; Li, B.Y.; Song, L.; Liu, H.F.; Cai, W.W.; Deng, Y.X.; Xu, T.T.; et al. Carnosine ameliorates age-related dementia: Via improving mitochondrial dysfunction in SAMP8 mice. Food Funct. 2020, 11, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, T.; Yu, D.; Fang, X.; Fan, H.; Liu, Q.; Yi, G.; Yi, X.; Liu, Q. L-Homocarnosine attenuates inflammation in cerebral ischemia–reperfusion injury through inhibition of nod-like receptor protein 3 inflammasome. Int. J. Biol. Macromol. 2018, 118, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, X.; Chen, X.; Cai, Y.; Ma, Y.; Ma, J.; Zhang, Q.; Dai, L.; Fan, X.; Bai, Y. Choline Supplementation Ameliorates Behavioral Deficits and Alzheimer’s Disease-Like Pathology in Transgenic APP/PS1 Mice. Mol. Nutr. Food Res. 2019, 63, 1801407. [Google Scholar] [CrossRef]
- Han, X.; Song, J.; Lian, L.H.; Yao, Y.L.; Shao, D.Y.; Fan, Y.; Hou, L.S.; Wang, G.; Zheng, S.; Wu, Y.L.; et al. Ginsenoside 25-OCH3-PPD Promotes Activity of LXRs to Ameliorate P2X7R-Mediated NLRP3 Inflammasome in the Development of Hepatic Fibrosis. J. Agric. Food Chem. 2018, 66, 7023–7035. [Google Scholar] [CrossRef]
- Wang, F.; Park, J.S.; Ma, Y.; Ma, H.; Lee, Y.J.; Lee, G.R.; Yoo, H.S.; Roh, Y.S.; Hong, J.T. Ginseng saponin enriched in rh1 and rg2 ameliorates nonalcoholic fatty liver disease by inhibiting inflammasome activation. Nutrients 2021, 13, 856. [Google Scholar] [CrossRef]
- Li, C.W.; Deng, M.Z.; Gao, Z.J.; Dang, Y.Y.; Zheng, G.D.; Yang, X.J.; Chao, Y.X.; Cai, Y.F.; Wu, X.L. Effects of compound K, a metabolite of ginsenosides, on memory and cognitive dysfunction in db/db mice involve the inhibition of ER stress and the NLRP3 inflammasome pathway. Food Funct. 2020, 11, 4416–4427. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yang, Y.Z.; Zheng, Y.J.; Wang, S.C.; Gu, H.M.; Pan, Y.; Wang, S.J.; Xu, H.J.; Kong, L.D. Dataset on assessment of magnesium isoglycyrrhizinate injection for dairy diet and body weight in fructose-induced metabolic syndrome of rats. Data Br. 2018, 18, 69–75. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, N.N.; Hu, X.; Zheng, Y. Anti-colitic effects of Physalin B on dextran sodium sulfate-induced BALB/c mice by suppressing multiple inflammatory signaling pathways. J. Ethnopharmacol. 2020, 259, 112956. [Google Scholar] [CrossRef]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K.P. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fan, J.; Chen, H.W.; Liu, E.Q. Trametes orientalis polysaccharide alleviates PM2.5-induced lung injury in mice through its antioxidant and anti-inflammatory activities. Food Funct. 2019, 10, 8005–8015. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, S.; Chen, J.; Lin, J.; Xiong, Q.; Yang, Y.; Yuan, J.; Zhou, L.; He, L.; Hou, S.; et al. Therapeutic roles of polysaccharides from Dendrobium Officinaleon colitis and its underlying mechanisms. Carbohydr. Polym. 2018, 185, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xiao, N.; Zeng, L.; Xiao, J.; Huang, J.; Xu, Y.; Chen, Y.; Ren, Y.; Du, B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym. 2020, 250, 116958. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, Q.Z.; Wang, Z.J.; Hua, C. Anti-inflammatory and hepatoprotective effects of Ganoderma lucidum polysaccharides against carbon tetrachloride-induced liver injury in Kunming Mice. Pharmacology 2019, 103, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, Y.; Cai, J.; Ji, J.; Wang, Y.; Zhang, W.; Pan, W.; Chen, Y. Polysaccharides from: Armillariella tabescens mycelia ameliorate insulin resistance in type 2 diabetic mice. Food Funct. 2020, 11, 9675–9685. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Pan, L.L.; Niu, W.; Fang, X.; Liang, W.; Li, J.; Li, H.; Pan, X.; Chen, W.; Zhang, H.; et al. Modulation of Gut Microbiota by Low Methoxyl Pectin Attenuates Type 1 Diabetes in Non-obese Diabetic Mice. Front. Immunol. 2019, 10, 1733. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Pan, L.L.; Luo, Y.; Niu, W.; Fang, X.; Liang, W.; Li, J.; Li, H.; Pan, X.; Yang, G.; et al. Low Methoxyl Pectin Protects against Autoimmune Diabetes and Associated Caecal Dysfunction. Mol. Nutr. Food Res. 2019, 63, e1900307. [Google Scholar] [CrossRef]
- Castro-Alves, V.C.; Shiga, T.M.; Nascimento, J.R.O. do Polysaccharides from chayote enhance lipid efflux and regulate NLRP3 inflammasome priming in macrophage-like THP-1 cells exposed to cholesterol crystals. Int. J. Biol. Macromol. 2019, 127, 502–510. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Wang, Y.; Jin, W.; Guo, Y. The immunoenhancement effects of starfish: Asterias rollestoni polysaccharides in macrophages and cyclophosphamide-induced immunosuppression mouse models. Food Funct. 2020, 11, 10700–10708. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosazza, J.P.N.; Huang, Z.; Dostal, L.; Volm, T.; Rousseau, B. Review: Biocatalytic transformations of ferulic acid: An abundant aromatic natural product. J. Ind. Microbiol. 1995, 15, 457–471. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.C.; Chang, Y.H.; Chang, K.S. Structural moieties required for cinnamaldehyde-related compounds to inhibit canonical IL-1β secretion. Molecules 2018, 23, 3241. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Wang, J.; Wen, W.; Pan, T.; Chen, H.; Fu, Y.; Wang, F.; Huang, J.H.; Xu, S. Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int. Immunopharmacol. 2020, 84, 106570. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef]
- Netea-Maier, R.T.; Plantinga, T.S.; van de Veerdonk, F.L.; Smit, J.W.; Netea, M.G. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy 2016, 12, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur Compounds: A Review of Their Anti-inflammatory Effects in Human Health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Osipova, V.; Polovinkina, M.; Gracheva, Y.; Shpakovsky, D.; Osipova, A.; Berberova, N. Antioxidant activity of some organosulfur compounds in vitro. Arab. J. Chem. 2021, 14, 103068. [Google Scholar] [CrossRef]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Bardaweel, S.K.; Mubarak, M.S.; Koch, W.; Gaweł-Beben, K.; Antosiewicz, B.; Sharifi-Rad, J. Immunomodulatory Effects of Diterpenes and Their Derivatives Through NLRP3 Inflammasome Pathway: A Review. Front. Immunol. 2020, 11, 572136. [Google Scholar] [CrossRef]
- Zhao, X.; Pu, D.; Zhao, Z.; Zhu, H.; Li, H.; Shen, Y.; Zhang, X.; Zhang, R.; Shen, J.; Xiao, W.; et al. Teuvincenone F suppresses LPS-induced inflammation and NLRP3 inflammasome activation by attenuating NEMO ubiquitination. Front. Pharmacol. 2017, 8, 565. [Google Scholar] [CrossRef] [Green Version]
- Aachoui, Y.; Chowdhury, R.R.; Fitch, R.W.; Ghosh, S.K. Molecular signatures of phytol-derived immunostimulants in the context of chemokine-cytokine microenvironment and enhanced immune response. Cell. Immunol. 2011, 271, 227–238. [Google Scholar] [CrossRef]
- Liu, D.; Qin, H.; Yang, B.; Du, B.; Yun, X. Oridonin ameliorates carbon tetrachloride-induced liver fibrosis in mice through inhibition of the NLRP3 inflammasome. Drug Dev. Res. 2020, 81, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Duan, M.X.; Yuan, Y.; Liu, C.; Cai, Z.; Xie, Q.; Hu, T.; Tang, Q.; Wu, Q.Q. Indigo fruits ingredient, aucubin, protects against LPS-induced cardiac dysfunction in mice. J. Pharmacol. Exp. Ther. 2019, 371, 348–359. [Google Scholar] [CrossRef]
- Zhao, C.C.; Xu, J.; Xie, Q.M.; Zhang, H.Y.; Fei, G.H.; Wu, H.M. Abscisic acid suppresses the activation of NLRP3 inflammasome and oxidative stress in murine allergic airway inflammation. Phyther. Res. 2021, 35, 3298–3309. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Azimullah, S.; Laham, F.; Tariq, S.; Goyal, S.N.; Adeghate, E.; Ojha, S. α-Bisabolol protects against β-adrenergic agonist-induced myocardial infarction in rats by attenuating inflammation, lysosomal dysfunction, NLRP3 inflammasome activation and modulating autophagic flux. Food Funct. 2020, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, F.; Li, L.; Lynch, E.C.; Xie, L.; Zhao, Y.; Fang, K.; Li, J.; Luo, J.; Xu, L.; et al. Celastrol alleviates metabolic disturbance in high-fat diet-induced obese mice through increasing energy expenditure by ameliorating metabolic inflammation. Phyther. Res. 2021, 35, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Wang, X.; Zhong, X.; Ji, L.; Guo, Z.; Liu, Y.; Shang, X. Sesquiterpenoids and their anti-inflammatory activity: Evaluation of Ainsliaea yunnanensis. Molecules 2019, 24, 1701. [Google Scholar] [CrossRef] [Green Version]
- Gianfrancesco, M.A.; Dehairs, J.; L’homme, L.; Herinckx, G.; Esser, N.; Jansen, O.; Habraken, Y.; Lassence, C.; Swinnen, J.V.; Rider, M.H.; et al. Saturated fatty acids induce NLRP3 activation in human macrophages through K + efflux resulting from phospholipid saturation and Na, K-ATPase disruption. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 1017–1030. [Google Scholar] [CrossRef]
- Witcher, K.J.; Novick, R.P.; Schlievert, P.M. Modulation of immune cell proliferation by glycerol monolaurate. Clin. Diagn. Lab. Immunol. 1996, 3, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Yang, Y.; Ou, T.; Key, C.C.C.; Tong, S.H.; Sequeira, R.C.; Nelson, J.M.; Nie, Y.; Wang, Z.; Boudyguina, E.; et al. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J. Lipid Res. 2017, 58, 1808–1821. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Lv, Y.; Liu, Y.; Li, J.; Wang, X.; Zhou, Z.; Tipoe, G.L.; Ouyang, S.; Guo, Y.; Zhang, J.; et al. Wolfberry-Derived Zeaxanthin Dipalmitate Attenuates Ethanol-Induced Hepatic Damage. Mol. Nutr. Food Res. 2019, 63, e1801339. [Google Scholar] [CrossRef]
- Wu, L.; Lyu, Y.; Srinivasagan, R.; Wu, J.; Ojo, B.; Tang, M.; El-Rassi, G.D.; Metzinger, K.; Smith, B.J.; Lucas, E.A.; et al. Astaxanthin-Shifted gut microbiota is associated with inflammation and metabolic homeostasis in mice. J. Nutr. 2020, 150, 2687–2698. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Dolinay, T.; Kim, Y.S.; Howrylak, J.; Hunninghake, G.M.; An, C.H.; Fredenburgh, L.; Massaro, A.F.; Rogers, A.; Gazourian, L.; Nakahira, K.; et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K. Carnosine and homocarnosine, the forgotten, enigmatic peptides of the brain. Neurochem. Res. 2005, 30, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Lin, C.Y.; Huang, S.F.; Lin, H.C.; Chang, W.L.; Chang, T.C. Effect and mechanism of ginsenosides CK and Rg1 on stimulation of glucose uptake in 3T3-L1 adipocytes. J. Agric. Food Chem. 2010, 58, 6039–6047. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.N.; Yan, M.H.; Xing, J.J.; Liu, W.C.; Li, W. Caspase-Mediated Anti-Apoptotic Effect of Ginsenoside Rg5, a Main Rare Ginsenoside, on Acetaminophen-Induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017, 65, 9226–9236. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Wan, Y.; Jin, X.J.; Ouyang, B.Q.; Bai, T.; Zhao, Y.Q.; Nan, J.X. 25-OCH3-PPD induces the apoptosis of activated t-HSC/Cl-6 cells via c-FLIP-mediated NF-κB activation. Chem. Biol. Interact. 2011, 194, 106–112. [Google Scholar] [CrossRef]
- Han, G.C.; Ko, S.K.; Sung, J.H.; Chung, S.H. Compound K enhances insulin secretion with beneficial metabolic effects in db/db mice. J. Agric. Food Chem. 2007, 55, 10641–10648. [Google Scholar] [CrossRef]
- Song, W.; Wei, L.; Du, Y.; Wang, Y.; Jiang, S. Protective effect of ginsenoside metabolite compound K against diabetic nephropathy by inhibiting NLRP3 inflammasome activation and NF-κB/p38 signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Int. Immunopharmacol. 2018, 63, 227–238. [Google Scholar] [CrossRef]
- Mao, Y.M.; Zeng, M.D.; Chen, Y.; Chen, C.W.; Fu, Q.C.; Cai, X.; Wu, S.M.; Chen, Y.G.; Sun, Y.; Li, J.; et al. Magnesium isoglycyrrhizinate in the treatment of chronic liver diseases: A randomized, double-blind, multi-doses, active drug controlled, multi-center study. Chin. J. Hepatol. 2009, 17, 847–851. [Google Scholar]
- Zhao, X.J.; Yang, Y.Z.; Zheng, Y.J.; Wang, S.C.; Gu, H.M.; Pan, Y.; Wang, S.J.; Xu, H.J.; Kong, L.D. Magnesium isoglycyrrhizinate blocks fructose-induced hepatic NF-κB/NLRP3 inflammasome activation and lipid metabolism disorder. Eur. J. Pharmacol. 2017, 809, 141–150. [Google Scholar] [CrossRef]
- Vieira, A.T.; Pinho, V.; Lepsch, L.B.; Scavone, C.; Ribeiro, I.M.; Tomassini, T.; Ribeiro-Dos-Santos, R.; Soares, M.B.P.; Teixeira, M.M.; Souza, D.G. Mechanisms of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. Br. J. Pharmacol. 2005, 146, 244–251. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Q.; Li, Z.; Gao, Y.; Pang, Z.; Wu, Y.; Li, G.; Lu, D.; Zhang, L.; Li, D. Astragalus Polysaccharides Attenuate Ovalbumin-Induced Allergic Rhinitis in Rats by Inhibiting NLRP3 Inflammasome Activation and NOD2-Mediated NF-?B Activation. J. Med. Food 2021, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Ye, Z.; Zhang, X.; Chen, H.; Ye, M. Protective effect of: Lachnum polysaccharide on dextran sulfate sodium-induced colitis in mice. Food Funct. 2020, 11, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, S.; Zhang, Z.; Zhang, W.; Yang, J.; Wan, Z.; Qin, L. Cereal Fiber Ameliorates High-Fat/Cholesterol-Diet-Induced Atherosclerosis by Modulating the NLRP3 Inflammasome Pathway in ApoE-/- Mice. J. Agric. Food Chem. 2018, 66, 4827–4834. [Google Scholar] [CrossRef]
- Wallert, M.; Börmel, L.; Lorkowski, S. Inflammatory Diseases and Vitamin E—What Do We Know and Where Do We Go? Mol. Nutr. Food Res. 2021, 65, 2000097. [Google Scholar] [CrossRef] [PubMed]
- Tapia, G.; Silva, D.; Romero, N.; Pettinelli, P.; Dossi, C.G.; de Miguel, M.; González-Mañán, D. Role of dietary α- and γ-tocopherol from Rosa mosqueta oil in the prevention of alterations induced by high-fat diet in a murine model. Nutrition 2018, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, Y. Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice. Nutr. Res. Pract. 2019, 13, 377–383. [Google Scholar] [CrossRef]
- Mullin, G.E.; Limektkai, B.; Wang, L.; Hanaway, P.; Marks, L.; Giovannucci, E. Dietary Supplements for COVID-19. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2021; Volume 1318, pp. 499–515. [Google Scholar]
- Sheng, K.; He, S.; Sun, M.; Zhang, G.; Kong, X.; Wang, J.; Wang, Y. Synbiotic supplementation containing: Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis. Food Funct. 2020, 11, 3964–3974. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamazaki, T.; Ohshio, K.; Sugamata, M.; Yoshikawa, M.; Kanauchi, O.; Morita, Y. A Specific Strain of Lactic Acid Bacteria, Lactobacillus paracasei, Inhibits Inflammasome Activation In Vitro and Prevents Inflammation-Related Disorders. J. Immunol. 2020, 205, 811–821. [Google Scholar] [CrossRef]
- Pan, X.; Fang, X.; Wang, F.; Li, H.; Niu, W.; Liang, W.; Wu, C.; Li, J.; Tu, X.; Pan, L.L.; et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br. J. Pharmacol. 2019, 176, 4446–4461. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.C.; Ouyang, C.N.; Yuan, S.N.; Lin, H.C.; Huang, K.Y.; Wu, P.S.; Liu, C.Y.; Tsai, K.J.; Loi, L.K.; Chen, Y.J.; et al. Pretreatment with a heat-killed probiotic modulates the NLRP3 inflammasome and attenuates colitis-associated colorectal cancer in mice. Nutrients 2019, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Loss, H.; Aschenbach, J.R.; Ebner, F.; Tedin, K.; Lodemann, U. Effects of a pathogenic ETEC strain and a probiotic Enterococcus faecium strain on the inflammasome response in porcine dendritic cells. Vet. Immunol. Immunopathol. 2018, 203, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.J.; Xu, J.J.; Wang, X.; Zhu, Y.H.; Wu, Q.; Wang, J.F. Lactobacillus johnsonii l531 ameliorates Escherichia coli-induced cell damage via inhibiting NLRP3 inflammasome activity and promoting ATG5/ATG16L1-mediated autophagy in porcine mammary epithelial cells. Vet. Sci. 2020, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Y.; Chu, B.; Yuan, L.; Liu, N.; Zhu, Y.; Wang, J. Lactobacillus johnsonii l531 alleviates the damage caused by Salmonella typhimurium via inhibiting tlr4, nf-κb, and nlrp3 inflammasome signaling pathways. Microorganisms 2021, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J. Lactobacillus rhamnosus GR-1 Prevents Escherichia coli-Induced Apoptosis Through PINK1/Parkin-Mediated Mitophagy in Bovine Mastitis. Front. Immunol. 2021, 12, 3743. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Bioactive Substances of Animal Origin. In Handbook of Food Chemistry; Springer: Berlin, Germany, 2015; pp. 1009–1033. ISBN 9783642366055. [Google Scholar]
- Zhao, Y.; Wu, Y.Z.; Wang, M. Bioactive Substances of Plant Origin. In Handbook of Food Chemistry; Springer: Berlin, Germany, 2015; pp. 967–1008. ISBN 9783642366055. [Google Scholar]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Ditu, L.M.; Grigore, M.E.; Camen-Comanescu, P.; Holban, A.M. Introduction in Nutraceutical and Medicinal Foods. In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–12. ISBN 9780128146262. [Google Scholar]
- Hung, W.L.; Ho, C.T.; Pan, M.H. Targeting the NLRP3 Inflammasome in Neuroinflammation: Health Promoting Effects of Dietary Phytochemicals in Neurological Disorders. Mol. Nutr. Food Res. 2020, 64, 1900550. [Google Scholar] [CrossRef]
- Bagherniya, M.; Khedmatgozar, H.; Fakheran, O.; Xu, S.; Johnston, T.P.; Sahebkar, A. Medicinal plants and bioactive natural products as inhibitors of NLRP3 inflammasome. Phyther. Res. 2021, 35, 4804–4833. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.Q.; Pan, R.; Tang, Y.; Zhou, X.G.; Wu, J.M.; Yu, L.; Law, B.Y.K.; Ai, W.; Yu, C.L.; Qin, D.L.; et al. Lychee seed polyphenol inhibits Aβ-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction. Biomed. Pharmacother. 2020, 130, 110575. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth-Walter, F.; Moskovskich, A.; Gomez-Casado, C.; Diaz-Perales, A.; Oida, K.; Singer, J.; Kinaciyan, T.; Fuchs, H.C.; Jensen-Jarolim, E. Immune suppressive effect of cinnamaldehyde due to inhibition of proliferation and induction of apoptosis in immune cells: Implications in cancer. PLoS ONE 2014, 9, e108402. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Williamson, G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H. Drug-Like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128010761. [Google Scholar]
- Giada, M.D.L.R. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ruiz-Rodriguez, A.; Marín, F.R.; Ocaña, A.; Soler-Rivas, C. Effect of domestic processing on bioactive compounds. Phytochem. Rev. 2008, 7, 345–384. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Medina-Remón, A.; Pérez-Jiménez, J.; Neveu, V.; Knaze, V.; Slimani, N.; Scalbert, A. Effects of food processing on polyphenol contents: A systematic analysis using Phenol-Explorer data. Mol. Nutr. Food Res. 2015, 59, 160–170. [Google Scholar] [CrossRef]
- Al-juhaimi, F.; Ghafoor, K.; Özcan, M.M.; Jahurul, M.H.A.; Babiker, E.E.; Jinap, S.; Sahena, F.; Sharifudin, M.S.; Zaidul, I.S.M. Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. J. Food Sci. Technol. 2018, 55, 3872–3880. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, D.; von Brugger, J.; Bialow, S. Functional food science: Differences and similarities with food science. Funct. Foods Health Dis. 2021, 11, 408–430. [Google Scholar] [CrossRef]
- Vettorazzi, A.; de Cerain, A.L.; Sanz-Serrano, J.; Gil, A.G.; Azqueta, A. European regulatory framework and safety assessment of food-related bioactive compounds. Nutrients 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.W.; Li, C.I.; Huang, K.C.; Liu, C.S.; Chen, H.L.; Lee, C.C.; Chiou, Y.Y.; Chen, R.J. 3-MCPD and glycidol coexposure induces systemic toxicity and synergistic nephrotoxicity via NLRP3 inflammasome activation, necroptosis, and autophagic cell death. J. Hazard. Mater. 2021, 405, 124241. [Google Scholar] [CrossRef]
- Jia, X.; Qiu, T.; Yao, X.; Jiang, L.; Wang, N.; Wei, S.; Tao, Y.; Pei, P.; Wang, Z.; Zhang, J.; et al. Arsenic induces hepatic insulin resistance via mtROS-NLRP3 inflammasome pathway. J. Hazard. Mater. 2020, 399, 123034. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Cai, D.; Li, P.; Jin, J.; Jiang, X.; Li, Z.; Tian, L.; Chen, G.; Sun, J.; et al. Chronic oral exposure to cadmium causes liver inflammation by NLRP3 inflammasome activation in pubertal mice. Food Chem. Toxicol. 2021, 148, 111944. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Yan, D.; Wang, Y.; Wang, N.; Liu, Y.; Tan, A.; Chen, X.; Yan, H. Chronic acrylamide exposure induced glia cell activation, NLRP3 inflammasome upregulation and cognitive impairment. Toxicol. Appl. Pharmacol. 2020, 393, 114949. [Google Scholar] [CrossRef]
- Bo, N.; Yilin, H.; Chaoyue, Y.; Lu, L.; Yuan, Y. Acrylamide induces NLRP3 inflammasome activation via oxidative stress- and endoplasmic reticulum stress-mediated MAPK pathway in HepG2 cells. Food Chem. Toxicol. 2020, 145, 111679. [Google Scholar] [CrossRef]
- Lee, P.Y.; Liu, C.C.; Wang, S.C.; Chen, K.Y.; Lin, T.C.; Liu, P.L.; Chiu, C.C.; Chen, I.C.; Lai, Y.H.; Cheng, W.C.; et al. Mycotoxin zearalenone attenuates innate immune responses and suppresses nlrp3 inflammasome activation in lps-activated macrophages. Toxins 2021, 13, 593. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Shi, L.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; Zhang, H.; et al. Zearalenone induces NLRP3-dependent pyroptosis via activation of NF-κB modulated by autophagy in INS-1 cells. Toxicology 2019, 428, 152304. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Wang, S.; Jiang, L.; Jiao, Y.; Sun, X.; Li, J.; Yang, L.; Hou, Y.; Wang, N.; Yao, X.; et al. Patulin induces pyroptosis through the autophagic-inflammasomal pathway in liver. Food Chem. Toxicol. 2021, 147, 111867. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Lee, S.; Jayasooriya, R.G.P.T.; Jin, C.Y.; Choi, Y.H.; Kim, G.Y. Deoxynivalenol enhances IL-1ß expression in bv 2. Microglial cells through activation of the NF-ΚB pathway and the ASC/NLRP 3. inflammasome. EXCLI J. 2019, 18, 356–369. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).