Lower Muscle and Blood Lactate Accumulation in Sickle Cell Trait Carriers in Response to Short High-Intensity Exercise
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental Design
2.2.1. Inclusion (V1)
2.2.2. Incremental Exercise Test to Exhaustion (V2)
2.2.3. Short High-Intensity Exercise Bout (V3)
2.3. Blood Lactate and Glucose Concentrations and Their Time-Courses during Recovery
2.4. Muscle Analyses
2.5. Statistical Analysis
3. Results
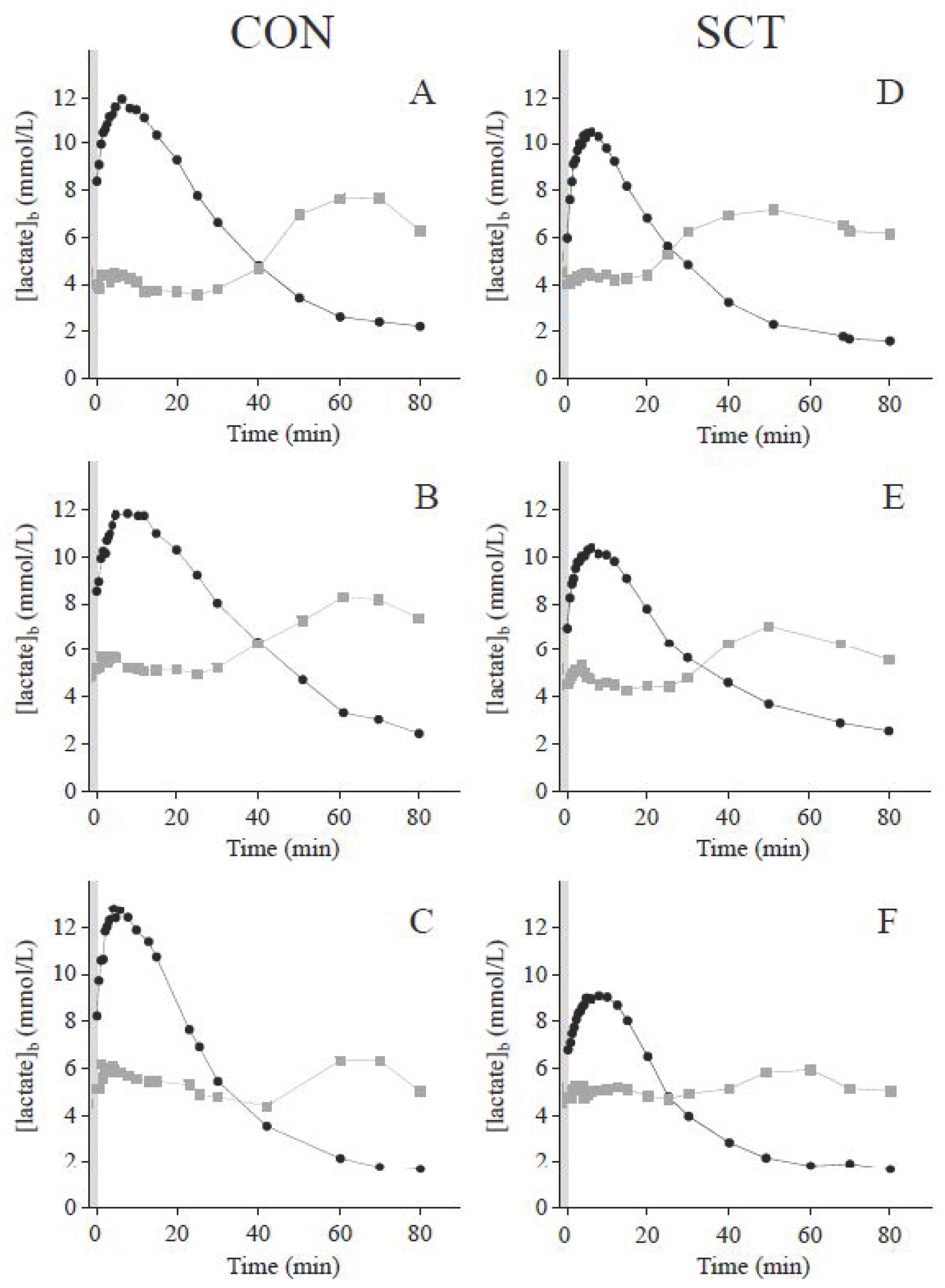
3.1. Blood Lactate Response to Short Supramaximal Exercise
3.2. Muscle pH Regulation, Lactate Transport, Metabolite Concentrations and Isoforms of Lactate Dehydrogenase
3.3. Blood Lactate and Glucose Kinetics during Recovery
4. Discussion
4.1. Metabolic Response to Short Supramaximal Exercise in SCT Carriers
4.2. Postexercise Blood Lactate Kinetics and Its Relation with Glycemia
4.3. Effects of α-Thalassemia
4.4. Clinical Relevance and Consequences on High-Intensity Exercise Performance
4.5. Nutritional and Metabolic Flexibility Perspectives/Hypotheses
4.6. Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binder, A.R.; Jones, S.R. Prevalence and awareness of sickle cell hemoglobin in a military population. Determination by a rapid screening method. JAMA 1970, 214, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.A.; Jones, S.R. Sickle-cell trait. N. Engl. J. Med. 1970, 282, 1158. [Google Scholar] [PubMed]
- Jones, S.R.; Binder, R.A.; Donowho, E.M.J. Sudden death in sickle-cell trait. N. Engl. J. Med. 1970, 282, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Hoiberg, A.; Ernst, J.E.; Uddin, D. Sickle cell trait and glucose-6-phosphate dehydrogenase deficiency. Effects on health and military performance in black Navy enlistees. Arch. Intern. Med. 1981, 141, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Kark, J.A.; Posey, D.M.; Schumacher, H.R.; Ruehle, C.J. Sickle-Cell Trait as a Risk Factor for Sudden Death in Physical Training. N. Engl. J. Med. 1987, 317, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.R. Sickle cell hemoglobin (Hb AS) in black football players. JAMA 1973, 225, 981–982. [Google Scholar] [CrossRef]
- Bergeron, M.F.; Cannon, J.G.; Hall, E.L.; Kutlar, A. Erythrocyte Sickling During Exercise and Thermal Stress. Clin. J. Sport Med. 2004, 14, 354–356. [Google Scholar] [CrossRef]
- Connes, P.; Sara, F.; Hardy-Dessources, M.-D.; Marlin, L.; Etienne, F.; Larifla, L.; Saint-Martin, C.; Hue, O. Effects of short supramaximal exercise on hemorheology in sickle cell trait carriers. Arbeitsphysiologie 2006, 97, 143–150. [Google Scholar] [CrossRef]
- Monchanin, G.; Connes, P.; Wouassi, D.; Francina, A.; Djoda, B.; Banga, P.E.; Owona, F.X.; Thiriet, P.; Massarelli, R.; Martin, C. Hemorheology, Sickle Cell Trait, and α-Thalassemia in Athletes: Effects of Exercise. Med. Sci. Sports Exerc. 2005, 37, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Monchanin, G.; Serpero, L.D.; Connes, P.; Tripette, J.; Wouassi, D.; Bezin, L.; Francina, A.; Ngongang, J.; De La Peña, M.; Massarelli, R.; et al. Effects of progressive and maximal exercise on plasma levels of adhesion molecules in athletes with sickle cell trait with or without α-thalassemia. J. Appl. Physiol. 2007, 102, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.W.; Weisman, I.M.; Zeballos, R.J.; Stephenson, S.R. Exercise and hypoxia increase sickling in venous blood from an exercising limb in individuals with sickle cell trait. Am. J. Med. 1989, 87, 48–56. [Google Scholar] [CrossRef]
- Bennett, M.A.; Heslop, R.W.; Meynell, M.J. Massive haematuria associated with sickle-cell trait. BMJ 1967, 1, 677–679. [Google Scholar] [CrossRef] [Green Version]
- Anzalone, M.L.; Green, V.S.; Buja, M.; Sanchez, L.A.; Harrykissoon, R.I.; Eichner, E.R. Sickle cell trait and fatal rhabdomyolysis in football training: A case study. Med. Sci. Sports Exerc. 2010, 42, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Koppes, G.; Daly, J.; Coltman, C.; Butkus, D. Exertion-Induced rhabdomyolysis with acute renal failure and disseminated intravascular coagulation in sickle cell trait. Am. J. Med. 1977, 63, 313–317. [Google Scholar] [CrossRef]
- Le Gallais, D.; Bile, A.; Mercier, J.; Paschel, M.A.; Tonellot, J.L.; Dauverchain, J.E. Exercise-induced death in sickle cell trait: Role of aging, training, and deconditioning. Med. Sci. Sports Exerc. 1996, 28, 541–544. [Google Scholar]
- Rosenthal, A.M.; Parker, D.J. Collapse of a young athlete. Ann. Emerg. Med. 1992, 21, 1493–1498. [Google Scholar] [CrossRef]
- Freund, H.; Lonsdorfer, J.; Oyono-Enguéllé, S.; Lonsdorfer, A.; Bogui, P. Lactate exchange and removal abilities in sickle cell patients and in untrained and trained healthy humans. J. Appl. Physiol. 1992, 73, 2580–2587. [Google Scholar] [CrossRef]
- Ueda, Y.; Nagel, R.L.; Bookchin, R.M. An increased Bohr effect in sickle cell anemia. Blood 1979, 53, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Juel, C.; Bangsbo, J.; Graham, T.; Saltin, B. Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol. Scand. 1990, 140, 147–159. [Google Scholar] [CrossRef]
- Freund, H.; Lonsdorfer, J.; Oyono-Enguéllé, S.; Lonsdorfe, A.; Dah, C.; Bogui, P. Lactate Exchange and Removal Abilities in Sickle Cell Trait Carriers During and After Incremental Exercise. Int. J. Sports Med. 1995, 16, 428–434. [Google Scholar] [CrossRef]
- Vincent, L.; Féasson, L.; Oyono-Enguéllé, S.; Banimbek, V.; Denis, C.; Guarneri, C.; Aufradet, E.; Monchanin, G.; Martin, C.; Gozal, D.; et al. Remodeling of skeletal muscle microvasculature in sickle cell trait and α-thalassemia. Am. J. Physiol. Circ. Physiol. 2010, 298, H375–H384. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Thiriet, P.; Mbala, E.; Wouassi, D.; Gelas, H.; Geyssant, A.; Lacour, J.R. Effect of different modalities of exercise and recovery on exercise performance in subjects with sickle cell trait. Med. Sci. Sports Exerc. 1992, 24, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Thiriet, P.; Wouassi, D.; Bitanga, E.; Lacour, J.R.; Gozal, D. Hyperoxia during recovery from consecutive anaerobic exercises in the sickle cell trait. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bile, A.; Le Gallais, D.; Mercier, B.; Martinez, P.; Ahmaidi, S.; Prefaut, C.; Mercier, J. Blood lactate concentrations during incremental exercise in subjects with sickle cell trait. Med. Sci. Sports Exerc. 1998, 30, 649–654. [Google Scholar] [CrossRef]
- Sara, F.; Hardy-Dessources, M.-D.; Voltaire, B.; Etienne-Julan, M.; Hue, O. Lactic Response in Sickle Cell Trait Carriers in Comparison With Subjects With Normal Hemoglobin. Clin. J. Sport Med. 2003, 13, 96–101. [Google Scholar] [CrossRef]
- Marlin, L.; Sara, F.; Antoine-Jonville, S.; Connes, P.; Etienne-Julan, M.; Hue, O. Ventilatory and Lactic Thresholds in Subjects with Sickle Cell Trait. Int. J. Sports Med. 2007, 28, 916–920. [Google Scholar] [CrossRef]
- Bilé, A.; Le Gallais, D.; Mercier, B.; Martinez, P.; Ahmaidi, S.; Préfaut, C. Anaerobic Exercise Components During the Force-Velocity Test in Sickle Cell Trait. Int. J. Sports Med. 1996, 17, 254–258. [Google Scholar] [CrossRef]
- Connes, P.; Hardy-Dessources, M.-D.; Hue, O. Counterpoint: Sickle cell trait should not be considered asymptomatic and as a benign condition during physical activity. J. Appl. Physiol. 2007, 103, 2138–2140. [Google Scholar] [CrossRef]
- Le Gallais, D.; Lonsdorfer, J.; Bogui, P.; Fattoum, S. Point: Sickle cell trait should be considered asymptomatic and as a benign condition during physical activity. J. Appl. Physiol. 2007, 103, 2137–2138. [Google Scholar] [CrossRef] [Green Version]
- Marlin, L.; Connes, P.; Antoine-Jonville, S.; Tripette, J.; Montout-Hedreville, M.; Sanouiller, A.; Etienne-Julan, M.; Hue, O. Cardiorespiratory responses during three repeated incremental exercise tests in sickle cell trait carriers. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 102, 181–187. [Google Scholar] [CrossRef]
- Vincent, L.; Féasson, L.; Oyono-Enguéllé, S.; Banimbek, V.; Monchanin, G.; Dohbobga, M.; Wouassi, D.; Martin, C.; Gozal, D.; Geyssant, A.; et al. Skeletal muscle structural and energetic characteristics in subjects with sickle cell trait, α-thalassemia, or dual hemoglobinopathy. J. Appl. Physiol. 2010, 109, 728–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, M.; Embury, S.H. Alpha-thalassemia in blacks: Genetic and clinical aspects and interactions with the sickle hemoglobin gene. Blood 1986, 68, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, M.H. Haemoglobin C/α Thalassaemia: Haematological and Biosynthetic Studies. Br. J. Haematol. 1975, 30, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Embury, S.H. Alpha thalassemia. A modifier of sickle cell disease. Ann. N. Y. Acad. Sci. 1989, 565, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Freund, H.; Oyono-Enguéllé, S.; Heitz, A.; Marbach, J.; Ott, C.; Zouloumian, P.; Lampert, E. Work rate-dependent lactate kinetics after exercise in humans. J. Appl. Physiol. 1986, 61, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Chatel, B.; Bret, C.; Edouard, P.; Oullion, R.; Freund, H.; Messonnier, L.A. Lactate recovery kinetics in response to high-intensity exercises. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 116, 1455–1465. [Google Scholar] [CrossRef]
- Messonnier, L.; Kristensen, M.; Juel, C.; Denis, C. Importance of pH regulation and lactate/H+transport capacity for work production during supramaximal exercise in humans. J. Appl. Physiol. 2007, 102, 1936–1944. [Google Scholar] [CrossRef] [Green Version]
- Messonnier, L.A.; Chatel, B.; Emhoff, C.-A.W.; Blervaque, L.; Oyono-Enguéllé, S. Delayed Rebound of Glycemia During Recovery Following Short-Duration High-Intensity Exercise: Are There Lactate and Glucose Metabolism Interactions? Front. Nutr. 2021, 8, 734152. [Google Scholar] [CrossRef]
- Tesch, P.A.; Wright, J.E. Recovery from short term intense exercise: Its relation to capillary supply and blood lactate concentration. Graefe’s Arch. Clin. Exp. Ophthalmol. 1983, 52, 98–103. [Google Scholar] [CrossRef]
- Juel, C. Lactate-proton cotransport in skeletal muscle. Physiol. Rev. 1997, 77, 321–358. [Google Scholar] [CrossRef]
- Chatel, B.; Bendahan, D.; Hourdé, C.; Pellerin, L.; Lengacher, S.; Magistretti, P.; Le Fur, Y.; Vilmen, C.; Bernard, M.; Messonnier, L.A. Role of MCT1 and CAII in skeletal muscle pH homeostasis, energetics, and function: In vivo insights from MCT1 haploinsufficient mice. FASEB J. 2017, 31, 2562–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning Fox, J.E.; Meredith, D.; Halestrap, A.P. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J. Physiol. 2000, 529, 285–293. [Google Scholar] [PubMed]
- Juel, C. Regulation of pH in human skeletal muscle: Adaptations to physical activity. Acta Physiol. 2008, 193, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.S.; Davies, A.J.; Halestrap, A.P. The Plasma Membrane Lactate Transporter MCT4, but Not MCT1, Is Up-regulated by Hypoxia through a HIF-1α-dependent Mechanism. J. Biol. Chem. 2006, 281, 9030–9037. [Google Scholar] [CrossRef] [Green Version]
- Messonnier, L.A.; Emhoff, C.-A.W.; Fattor, J.A.; Horning, M.A.; Carlson, T.J.; Brooks, G.A. Lactate kinetics at the lactate threshold in trained and untrained men. J. Appl. Physiol. 2013, 114, 1593–1602. [Google Scholar] [CrossRef]
- Thomas, C.; Perrey, S.; Lambert, K.; Hugon, G.; Mornet, D.; Mercier, J. Monocarboxylate transporters, blood lactate removal after supramaximal exercise, and fatigue indexes in humans. J. Appl. Physiol. 2005, 98, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Sirvent, P.; Perrey, S.; Raynaud, E.; Mercier, J. Relationships between maximal muscle oxidative capacity and blood lactate removal after supramaximal exercise and fatigue indexes in humans. J. Appl. Physiol. 2004, 97, 2132–2138. [Google Scholar] [CrossRef]
- Chioléro, R.; Tappy, L.; Gillet, M.; Revelly, J.-P.; Roth, H.; Cayeux, C.; Schneiter, P.; Leverve, X. Effect of Major Hepatectomy on Glucose and Lactate Metabolism. Ann. Surg. 1999, 229, 505–513. [Google Scholar] [CrossRef]
- Nelson, D.A.; Deuster, P.A.; Carter, R., III; Hill, O.T.; Wolcott, V.L.; Kurina, L.M. Sickle Cell Trait, Rhabdomyolysis, and Mortality among U.S. Army Soldiers. N. Engl. J. Med. 2016, 375, 435–442. [Google Scholar] [CrossRef]
- Bilé, A.; Le Gallais, D.; Mercier, J.; Bogui, P.; Préfaut, C. Sickle Cell Trait in Ivory Coast Athletic Throw and Jump Champions, 1956–1995. Int. J. Sports Med. 1998, 19, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Marlin, L.; Etienne-Julan, M.; Le Gallais, D.; Hue, O. Sickle Cell Trait in French West Indian Elite Sprint Athletes. Int. J. Sports Med. 2005, 26, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Favero, T.G.; Zable, A.C.; Colter, D.; Abramson, J.J. Lactate inhibits Ca2+-activated Ca2+-channel activity from skeletal muscle sarcoplasmic reticulum. J. Appl. Physiol. 1997, 82, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Gladden, L.B.; Kurdak, S.S.; Poole, D.C. Increased [lactate] in working dog muscle reduces tension development independent of pH. Med. Sci. Sports Exerc. 1995, 27, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Albertsen, J.; Rentsch, M.; Juel, C. Lactate and force production in skeletal muscle. J. Physiol. 2005, 562, 521–526. [Google Scholar] [CrossRef]
- Fitts, R. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef]
- Fitts, R.H. The Role of Acidosis in Fatigue: Pro Perspective. Med. Sci. Sports Exerc. 2016, 48, 2335–2338. [Google Scholar] [CrossRef]
- Metzger, J.M.; Fitts, R.H. Role of intracellular pH in muscle fatigue. J. Appl. Physiol. 1987, 62, 1392–1397. [Google Scholar] [CrossRef]
- Nelson, C.R.; DeBold, E.P.; Fitts, R.H. Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am. J. Physiol. Physiol. 2014, 307, C939–C950. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, C.W.; Hunter, S.K.; Trappe, S.W.; Smith, C.S.; Fitts, R.H. Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: Implications for muscle fatigue in humans. J. Physiol. 2018, 596, 3993–4015. [Google Scholar] [CrossRef] [Green Version]
- Westerblad, H. Acidosis Is Not a Significant Cause of Skeletal Muscle Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2339–2342. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.B.; de Paoli, F.V.; Overgaard, K. Protective effects of lactic acid on force production in rat skeletal muscle. J. Physiol. 2001, 536, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Emhoff, C.-A.W.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Direct and indirect lactate oxidation in trained and untrained men. J. Appl. Physiol. 2013, 115, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, J.L., Jr.; Tietz, E.; Two-Feathers, T.; Paull, J.; Chapman, K. Lactate, Fructose and Glucose Oxidation Profiles in Sports Drinks and the Effect on Exercise Performance. PLoS ONE 2007, 2, e927. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in contemporary biology: A phoenix risen. J. Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
| CON (n = 15) | SCT (n = 15) | p Value | |
|---|---|---|---|
| Anthropometric and physiological characteristics | |||
| Age (year) | 24 (2) | 23 (2) | 0.052 |
| Body mass (kg) | 66 (5) | 69 (6) | 0.151 |
| Pmax (W) | 210 (170–241) | 210 (140–245) | 0.475 |
| Pmax (W·kg−1) | 3.02 (0.36) | 3.01 (0.40) | 0.920 |
| DEE (kJ·day−1) | 10,868 (1474) | 11,664 (1321) | 0.131 |
| Hemoglobin and hematological data | |||
| HbS (%) | not present | 34.3 (3.6) | na |
| Hct (%) | 43.0 (2.7) | 43.2 (2.8) | 0.826 |
| MCV (fL) | 84.71 (5.37) | 80.00 (3.87) | 0.010 |
| MCH (pg) | 27.29 (2.08) | 25.93 (1.39) | 0.044 |
| MCHC (g·dL−1) | 32.17 (0.67) | 32.33 (0.56) | 0.483 |
| RBC (M·µL−1) | 5.09 (0.42) | 5.42 (0.45) | 0.053 |
| CON (n = 15) | SCT (n = 15) | p Value | |
|---|---|---|---|
| Blood lactate concentrations | |||
| [lactate]b(r) (mmol·L−1) | 1.356 (0.336) | 1.301 (0.323) | 0.648 |
| [lactate]b(0) (mmol·L−1) | 8.59 (1.40) [14] | 7.08 (1.57) | 0.011 |
| Bicarbonate-dependent muscle pH regulation mechanisms | |||
| CAII (a.u.) | 1.28 (0.40) [13] | 1.43 (0.36) [14] | 0.317 |
| CAIII (a.u.) | 1.03 (0.34) [13] | 1.05 (0.24) [13] | 0.857 |
| NBC (a.u.) | 3.98 (0.67) [14] | 3.95(1.06) [13] | 0.928 |
| Sarcolemmal H+ transport | |||
| MCT1 (a.u.) | 2.14 (0.53) [14] | 2.33 (0.61) [13] | 0.392 |
| MCT4 (a.u.) | 1.18 (1.75–4.81) [14] | 2.70 (1.34–5.78) [14] | 0.006 |
| Muscle metabolite concentrations | |||
| [lactate]m(0) (mmol·kg−1 d.m.) | 132 (95–201) [13] | 113 (83–130) | 0.022 |
| [pyruvate]m(0) (mmol·kg−1 d.m.) | 1.85 (0.63–5.76) [13] | 2.07 (1.13–3.59) | 0.914 |
| [lactate]m(0)/[pyruvate]m ratio | 60.7 (26.3–225.5) [13] | 55.2 (31.5–99.3) | 0.440 |
| [ATP]m(0) (mmol·kg−1 d.m.) | 14.4 (9.0–18.3) [13] | 12.6 (9.6–21.9) | 0.908 |
| [ADP]m(0) (mmol·kg−1 d.m.) | 6.0 (2.60–9.10) [13] | 6.70 (3.2–10.3) | 0.903 |
| [ATP]m/[ADP]m(0) | 2.73 (1.22–4.38) [13] | 1.96 (1.30–4.56) | 0.339 |
| LDH isoform proportions | |||
| M-LDH (%) | 0.81 (0.71–0.84) [13] | 0.81 (0.63–0.88) | 0.610 |
| H-LDH (%) | 0.19 (0.16–0.29) [13] | 0.19 (0.12–0.37) | 0.610 |
| β2-adrenergic receptors | |||
| β2AR (a.u.) | 0.87 (0.13–1.60) [14] | 0.25 (0.08–1.53) [11] | 0.021 |
| CON (n = 15) | SCT (n = 15) | p Value | |
|---|---|---|---|
| Blood lactate kinetics parameters | |||
| γ1 (min−1) | 0.207 (0.086) [14] | 0.227 (0.104) | 0.586 |
| γ2 (min−1) | 0.045 (0.011) [14] | 0.061 (0.022) | 0.020 |
| [lactate]bpeak (mmol·L−1) | 12.1 (1.7) [14] | 10.4 (1.6) | 0.009 |
| Cross-over point of blood glucose and lactate concentrations | |||
| Concentration (mmol·L−1) | 5.21 (0.64) [14] | 4.98 (0.34) | 0.242 |
| Time into recovery (min) | 36.5 (26.3–66.9) [14] | 30.8 (22.0–36.1) | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messonnier, L.A.; Oyono-Enguéllé, S.; Vincent, L.; Dubouchaud, H.; Chatel, B.; Sanchez, H.; Malgoyre, A.; Martin, C.; Galactéros, F.; Bartolucci, P.; et al. Lower Muscle and Blood Lactate Accumulation in Sickle Cell Trait Carriers in Response to Short High-Intensity Exercise. Nutrients 2022, 14, 501. https://doi.org/10.3390/nu14030501
Messonnier LA, Oyono-Enguéllé S, Vincent L, Dubouchaud H, Chatel B, Sanchez H, Malgoyre A, Martin C, Galactéros F, Bartolucci P, et al. Lower Muscle and Blood Lactate Accumulation in Sickle Cell Trait Carriers in Response to Short High-Intensity Exercise. Nutrients. 2022; 14(3):501. https://doi.org/10.3390/nu14030501
Chicago/Turabian StyleMessonnier, Laurent A., Samuel Oyono-Enguéllé, Lucile Vincent, Hervé Dubouchaud, Benjamin Chatel, Hervé Sanchez, Alexandra Malgoyre, Cyril Martin, Frédéric Galactéros, Pablo Bartolucci, and et al. 2022. "Lower Muscle and Blood Lactate Accumulation in Sickle Cell Trait Carriers in Response to Short High-Intensity Exercise" Nutrients 14, no. 3: 501. https://doi.org/10.3390/nu14030501
APA StyleMessonnier, L. A., Oyono-Enguéllé, S., Vincent, L., Dubouchaud, H., Chatel, B., Sanchez, H., Malgoyre, A., Martin, C., Galactéros, F., Bartolucci, P., Thiriet, P., & Féasson, L. (2022). Lower Muscle and Blood Lactate Accumulation in Sickle Cell Trait Carriers in Response to Short High-Intensity Exercise. Nutrients, 14(3), 501. https://doi.org/10.3390/nu14030501







