Motor Unit Fatigability following Chronic Carnosine Supplementation in Aged Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Study Design
2.4. Surgical and Electrophysiological Procedures
2.5. Testing Protocol and Data Analysis
2.6. Antioxidant Potential, Advanced Glycation End Products, and Enzyme Activity in MG Muscle
2.7. Muscle Content of Carnosine, Anserine, and Taurine
2.8. Statistical Analyses
3. Results
3.1. MUs Number and Contractile Properties
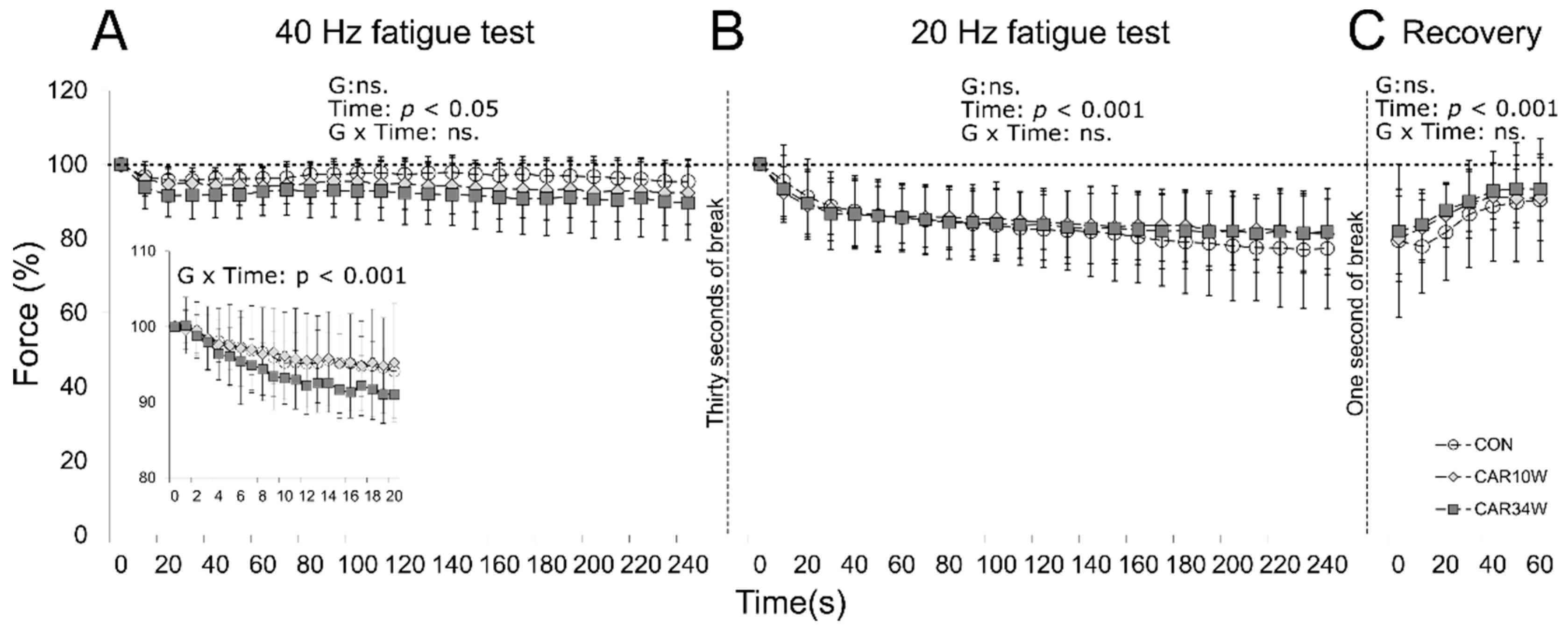
3.2. MUs Fatigability
3.2.1. FF Units
3.2.2. FR Units
3.2.3. Slow Units
3.3. Antioxidant Potential, Advanced Glycation End Products, and Enzyme Activity
3.4. Carnosine, Anserine, and Taurine Content in Muscle Samples
3.5. Body and MG Muscle Weight
3.6. Food, Water, and Carnosine Consumption
3.7. Animal Behaviour and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capel, F.; Rimbert, V.; Lioger, D.; Diot, A.; Rousset, P.; Mirand, P.P.; Boirie, Y.; Morio, B.; Mosoni, L. Due to Reverse Electron Transfer, Mitochondrial H2O2 Release Increases with Age in Human Vastus Lateralis Muscle Although Oxidative Capacity Is Preserved. Mech. Ageing Dev. 2005, 126, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Capel, F.; Buffière, C.; Patureau Mirand, P.; Mosoni, L. Differential Variation of Mitochondrial H2O2 Release during Aging in Oxidative and Glycolytic Muscles in Rats. Mech. Ageing Dev. 2004, 125, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Meli, A.C.; Umanskaya, A.; Xie, W.; Shiomi, T.; Zalk, R.; Lacampagne, A.; Marks, A.R. Ryanodine Receptor Oxidation Causes Intracellular Calcium Leak and Muscle Weakness in Aging. Cell Metab. 2011, 14, 196–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derbré, F.; Gratas-Delamarche, A.; Gómez-Cabrera, M.C.; Viña, J. Inactivity-Induced Oxidative Stress: A Central Role in Age-Related Sarcopenia? Eur. J. Sport Sci. 2014, 14 (Suppl. S1), S98–S108. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, B.; Larsson, L. Detection of an Aging-Related Increase in Advanced Glycation End Products in Fast- and Slow-Twitch Skeletal Muscles in the Rat. Biogerontology 2013, 14, 293–301. [Google Scholar] [CrossRef]
- Ramamurthy, B.; Höök, P.; Jones, A.D.; Larsson, L. Changes in Myosin Structure and Function in Response to Glycation. FASEB J. 2001, 15, 2415–2422. [Google Scholar] [CrossRef] [Green Version]
- Pastoris, O.; Boschi, F.; Verri, M.; Baiardi, P.; Felzani, G.; Vecchiet, J.; Dossena, M.; Catapano, M. The Effects of Aging on Enzyme Activities and Metabolite Concentrations in Skeletal Muscle from Sedentary Male and Female Subjects. Exp. Gerontol. 2000, 35, 95–104. [Google Scholar] [CrossRef]
- Fueger, P.T.; Shearer, J.; Krueger, T.M.; Posey, K.A.; Bracy, D.P.; Heikkinen, S.; Laakso, M.; Rottman, J.N.; Wasserman, D.H. Hexokinase II Protein Content Is a Determinant of Exercise Endurance Capacity in the Mouse. J. Physiol. 2005, 566, 533–541. [Google Scholar] [CrossRef]
- Houmard, J.A.; Weidner, M.L.; Gavigan, K.E.; Tyndall, G.L.; Hickey, M.S.; Alshami, A. Fiber Type and Citrate Synthase Activity in the Human Gastrocnemius and Vastus Lateralis with Aging. J. Appl. Physiol. 1998, 85, 1337–1341. [Google Scholar] [CrossRef]
- Hunter, S.K.; Critchlow, A.; Enoka, R.M. Muscle Endurance Is Greater for Old Men Compared with Strength-Matched Young Men. J. Appl. Physiol. 2005, 99, 890–897. [Google Scholar] [CrossRef] [Green Version]
- Lindström, B.; Lexell, J.; Gerdle, B.; Downham, D. Skeletal Muscle Fatigue and Endurance in Young and Old Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, B59–B66. [Google Scholar] [CrossRef] [PubMed]
- Baudry, S.; Klass, M.; Pasquet, B.; Duchateau, J. Age-Related Fatigability of the Ankle Dorsiflexor Muscles during Concentric and Eccentric Contractions. Eur. J. Appl. Physiol. 2007, 100, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.A.d.P.; Nosek, T.M.; Kolbeck, R.C. Influence of Ageing on the Fatigability of Isolated Mouse Skeletal Muscles from Mature and Aged Mice. Exp. Physiol. 2002, 87, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łochyński, D.; Krutki, P.; Celichowski, J. Effect of Ageing on the Regulation of Motor Unit Force in Rat Medial Gastrocnemius Muscle. Exp. Gerontol. 2008, 43, 218–228. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Abe, H. Role of Histidine-Related Compounds as Intracellular Proton Buffering Constituents in Vertebrate Muscle. Biochemistry. Biokhimiia 2000, 65, 757–765. [Google Scholar]
- Dutka, T.L.; Lamboley, C.R.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. Effects of Carnosine on Contractile Apparatus Ca2+ Sensitivity and Sarcoplasmic Reticulum Ca2+ Release in Human Skeletal Muscle Fibers. J. Appl. Physiol. 2012, 112, 728–736. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, D.; \Lochyński, D.; Everaert, I.; Pawlak, M.; Derave, W.; Celichowski, J. Role of Histidyl Dipeptides in Contractile Function of Fast and Slow Motor Units in Rat Skeletal Muscle. J. Appl. Physiol. 2016, 121, 164–172. [Google Scholar] [CrossRef] [Green Version]
- McFarland, G.A.; Holliday, R. Retardation of the Senescence of Cultured Human Diploid Fibroblasts by Carnosine. Exp. Cell Res. 1994, 212, 167–175. [Google Scholar] [CrossRef]
- Pepper, E.D.; Farrell, M.J.; Nord, G.; Finkel, S.E. Antiglycation Effects of Carnosine and Other Compounds on the Long-Term Survival of Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 7925–7930. [Google Scholar] [CrossRef] [Green Version]
- Stvolinsky, S.; Antipin, M.; Meguro, K.; Sato, T.; Abe, H.; Boldyrev, A. Effect of Carnosine and Its Trolox-Modified Derivatives on Life Span of Drosophila Melanogaster. Rejuvenation Res. 2010, 13, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Yuneva, A.O.; Kramarenko, G.G.; Vetreshchak, T.V.; Gallant, S.; Boldyrev, A.A. Effect of Carnosine on Drosophila Melanogaster Lifespan. Bull. Exp. Biol. Med. 2002, 133, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Yuneva, M.O.; Bulygina, E.R.; Gallant, S.C.; Kramarenko, G.G.; Stvolinsky, S.L.; Semyonova, M.L.; Boldyrev, A.A. Effect of Carnosine on Age-Induced Changes in Senescence-Accelerated Mice. J. Anti-Aging Med. 1999, 2, 337–342. [Google Scholar] [CrossRef]
- Aydín, A.F.; Küskü-Kiraz, Z.; Doğru-Abbasoğlu, S.; Uysal, M. Effect of Carnosine Treatment on Oxidative Stress in Serum, ApoB-Containing Lipoproteins Fraction and Erythrocytes of Aged Rats. Pharmacol. Rep. 2010, 62, 733–739. [Google Scholar] [CrossRef]
- Freemont, A.; Hoyland, J. Morphology, Mechanisms and Pathology of Musculoskeletal Ageing. J. Pathol. 2007, 211, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A. Muscle Carnosine Metabolism and β-Alanine Supplementation in Relation to Exercise and Training. Sports Med. 2010, 40, 247–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Favero, S.; Roschel, H.; Solis, M.Y.; Hayashi, A.P.; Artioli, G.G.; Otaduy, M.C.; Benatti, F.B.; Harris, R.C.; Wise, J.A.; Leite, C.C.; et al. Beta-Alanine (CarnosynTM) Supplementation in Elderly Subjects (60–80 Years): Effects on Muscle Carnosine Content and Physical Capacity. Amino Acids 2012, 43, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Łochyński, D.; Kaczmarek, D.; Krutki, P.; Celichowski, J. Effect of Ageing on the Force Development in Tetanic Contractions of Motor Units in Rat Medial Gastrocnemius Muscle. Mech. Ageing Dev. 2010, 131, 545–553. [Google Scholar] [CrossRef]
- Celichowski, J.; Grottel, K. The Dependence of the Twitch Course of Medial Gastrocnemius Muscle of the Rat and Its Motor Units on Stretching of the Muscle. Arch. Ital. De Biol. 1992, 130, 315–324. [Google Scholar]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E. Physiological Types and Histochemical Profiles in Motor Units of the Cat Gastrocnemius. J. Physiol. 1973, 234, 723–748. [Google Scholar] [CrossRef]
- Grottel, K.; Celichowski, J. Division of Motor Units in Medial Gastrocnemius Muscle of the Rat in the Light of Variability of Their Principal Properties. Acta Neurobiol. Exp. 1990, 50, 571–587. [Google Scholar]
- Kernell, D.; Eerbeek, O.; Verhey, B.A. Relation between Isometric Force and Stimulus Rate in Cat’s Hindlimb Motor Units of Different Twitch Contraction Time. Exp. Brain Res. 1983, 50, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janaszewska, A.; Bartosz, G. Assay of Total Antioxidant Capacity: Comparison of Four Methods as Applied to Human Blood Plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Blancquaert, L.; Everaert, I.; Missinne, M.; Baguet, A.; Stegen, S.; Volkaert, A.; Petrovic, M.; Vervaet, C.; Achten, E.; DE Maeyer, M.; et al. Effects of Histidine and β-Alanine Supplementation on Human Muscle Carnosine Storage. Med. Sci. Sports Exerc. 2017, 49, 602–609. [Google Scholar] [CrossRef]
- Masoro, E.J. Mortality and Growth Characteristics of Rat Strains Commonly Used in Aging Research. Exp. Aging Res. 1980, 6, 219–233. [Google Scholar] [CrossRef]
- Derave, W.; Jones, G.; Hespel, P.; Harris, R.C. Creatine Supplementation Augments Skeletal Muscle Carnosine Content in Senescence-Accelerated Mice (SAMP8). Rejuvenation Res. 2008, 11, 641–647. [Google Scholar] [CrossRef]
- Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B.; Hobson, R.M.; Harris, R.C. Carnosine: From Exercise Performance to Health. Amino Acids 2013, 44, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, A. Pathways of Glycolysis. Physiol. Rev. 1943, 23, 124–138. [Google Scholar] [CrossRef]
- Tesch, P.A.; Komi, P.V.; Häkkinen, K. Enzymatic Adaptations Consequent to Long-Term Strength Training. Int. J. Sports Med. 1987, 8 (Suppl. S1), 66–69. [Google Scholar] [CrossRef] [PubMed]
- Artioli, G.G.; Sale, C.; Jones, R.L. Carnosine in Health and Disease. Eur. J. Sport Sci. 2019, 19, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kilis-Pstrusinska, K. Carnosine and Kidney Diseases: What We Currently Know? Curr. Med. Chem. 2019, 27, 1764–1781. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Fantauzzi, C.B.; Pugliese, G. L-Carnosine and Its Derivatives as New Therapeutic Agents for the Prevention and Treatment of Vascular Complications of Diabetes. Curr. Med. Chem. 2019, 27, 1744–1763. [Google Scholar] [CrossRef]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; de Courten, B. The Potential of Carnosine in Brain-Related Disorders: A Comprehensive Review of Current Evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.J.; Artioli, G.G.; Turner, M.D.; Sale, C. The Physiological Roles of Carnosine and β-Alanine in Exercising Human Skeletal Muscle. Med. Sci. Sports Exerc. 2019, 51, 2098–2108. [Google Scholar] [CrossRef]
- Cararo, J.H.; Streck, E.L.; Schuck, P.F.; da C Ferreira, G. Carnosine and Related Peptides: Therapeutic Potential in Age-Related Disorders. Aging Dis. 2015, 6, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Hipkiss, A.R.; Baye, E.; de Courten, B. Carnosine and the Processes of Ageing. Maturitas 2016, 93, 28–33. [Google Scholar] [CrossRef]
- Bingül, İ.; Yılmaz, Z.; Aydın, A.F.; Çoban, J.; Doğru-Abbasoğlu, S.; Uysal, M. Antiglycation and Anti-Oxidant Efficiency of Carnosine in the Plasma and Liver of Aged Rats. Geriatr. Gerontol. Int. 2017, 17, 2610–2614. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.; Tur, J.A. Antioxidant Supplementation and Adaptive Response to Training: A Systematic Review. Curr. Pharm. Des. 2019, 25, 1889–1912. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Orioli, M.; Carini, M.; Maffei Facino, R. Profiling Histidine-Containing Dipeptides in Rat Tissues by Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. J. Mass Spectrom. 2004, 39, 1417–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, N.; Tsunemori, F.; Wakabayashi, M.; Hama, T. Effect of Histidine-Free and -Excess Diets on Anserine and Carnosine Contents in Rat Gastrocnemius Muscle. J. Nutr. Sci. Vitaminol. 1977, 23, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Stuerenburg, H.J.; Kunze, K. Concentrations of Free Carnosine (a Putative Membrane-Protective Antioxidant) in Human Muscle Biopsies and Rat Muscles. Arch. Gerontol. Geriatr. 1999, 29, 107–113. [Google Scholar] [CrossRef]
- Johnson, P.; Hammer, J.L. Histidine Dipeptide Levels in Ageing and Hypertensive Rat Skeletal and Cardiac Muscles. Comp. Biochem. Physiol. B 1992, 103, 981–984. [Google Scholar] [CrossRef]
- Derave, W.; Eijnde, B.O.; Hespel, P. Creatine Supplementation in Health and Disease: What Is the Evidence for Long-Term Efficacy? Mol. Cell. Biochem. 2003, 244, 49–55. [Google Scholar] [CrossRef]
- Guerrero-Ontiveros, M.L.; Wallimann, T. Creatine Supplementation in Health and Disease. Effects of Chronic Creatine Ingestion in Vivo: Down-Regulation of the Expression of Creatine Transporter Isoforms in Skeletal Muscle. Mol. Cell. Biochem. 1998, 184, 427–437. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional Recommendations for the Management of Sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Saunders, B.; DE Salles Painelli, V.; DE Oliveira, L.F.; DA Eira Silva, V.; DA Silva, R.P.; Riani, L.; Franchi, M.; Gonçalves, L.D.S.; Harris, R.C.; Roschel, H.; et al. Twenty-Four Weeks of β-Alanine Supplementation on Carnosine Content, Related Genes, and Exercise. Med. Sci. Sports Exerc. 2017, 49, 896–906. [Google Scholar] [CrossRef] [Green Version]
- Stuerenburg, H.J. The Roles of Carnosine in Aging of Skeletal Muscle and in Neuromuscular Diseases. Biochemistry 2000, 65, 862–865. [Google Scholar] [PubMed]




| Motor Units Properties | Control (n = 94) | CAR10W (n = 95) | CAR34W (n = 97) | p |
|---|---|---|---|---|
| FF | ||||
| TwF (mN) | 72.3 ± 41.6 | 61.1 ± 39.6 | 74.0 ± 37.1 | 0.36 |
| TetF (mN) | 236.8 ± 138.3 | 214.3 ± 126.0 | 248.9 ± 125.4 | 0.52 |
| CT (ms) | 13.7 ± 1.8 | 13.0 ± 2.4 | 14.2 ± 2.9 | 0.18 |
| HRT (ms) | 20.4 ± 2.4 | 19.5 ± 2.4 | 20.9 ± 2.4 | 0.10 |
| FR | ||||
| TwF (mN) | 32.7 ± 27.9 | 34.2 ± 21.2 | 40.0 ± 33.2 | 0.36 |
| TetF (mN) | 116.8 ± 87.2 | 144.8 ± 100.8 | 176.4 ± 163.6 | 0.25 |
| CT (ms) | 15.3 ± 3.2 | 15.9 ± 6.8 | 15.9 ± 4.1 | 0.61 |
| HRT (ms) | 21.5 ± 3.3 | 22.2 ± 6.0 | 22.2 ± 4.0 | 0.87 |
| S | ||||
| TwF (mN) | 8.8 ± 3.4 | 11.4 ± 10.8 | 13.7 ± 6.3 * | 0.00 |
| TetF (mN) | 63.3 ± 31.1 | 54.4 ± 44.6 | 66.1 ± 34.0 | 0.05 |
| CT (ms) | 25.6 ± 6.5 | 27.5 ± 7.5 | 27.1 ± 6.0 | 0.51 |
| HRT (ms) | 32.8 ± 6.8 | 34.5 ± 7.3 | 34.3 ± 5.2 | 0.54 |
| Control (n = 9) | CAR10W (n = 8) | CAR34W (n = 9) | F | |
|---|---|---|---|---|
| AGEs (AU/mg of supernatant) | 395.6 ± 28.90 | 386.2 ± 48.25 | 372.4 ± 31.36 | 0.91 |
| Hexokinase (mU/mL) | 9.19 ± 2.03 | 4.95 ± 3.22 # | 6.10 ± 2.04 # | 6.89 ** |
| Pyruvate Kinase (mU/mL) | 2.83 ± 1.02 | 3.01 ± 1.77 | 2.06 ± 1.15 | 1.25 |
| Citrate synthase (U/mL) | 30.1 ± 0.81 | 30.0 ± 1.35 | 29.0 ± 0.72 | 3.02 |
| Group | Red Gastrocnemius | Calculated Statistics | White Gastrocnemius | Calculated Statistics | |
|---|---|---|---|---|---|
| Carnosine | |||||
| CON | 2.74 ± 0.46 | 2.13 ± 0.57 | |||
| CAR10W | 2.52 ± 1.00 | 2.15 ± 0.81 | |||
| CAR34W | 1.94 ± 0.45 # | H = 6.34 * | 2.04 ± 0.58 | F = 0.06 | |
| Anserine | |||||
| CON | 6.20 ± 1.27 | 10.78 ± 2.20 | |||
| CAR10W | 5.04 ± 1.17 | 9.23 ± 3.21 | |||
| CAR34W | 4.96 ± 0.85 | F = 3.70 * | 8.60 ± 1.89 | F = 2.00 | |
| Histidyl dipeptides | |||||
| CON | 8.94 ± 1.44 | 12.91 ± 2.69 | |||
| CAR10W | 7.57 ± 2.12 | 11.38 ± 3.99 | |||
| CAR34W | 6.90 ± 1.10 # | F = 4.16 * | 10.64 ± 2.18 | F = 1.43 | |
| Taurine | |||||
| CON | 20.10 ± 4.41 | 32.65 ± 4.61 | |||
| CAR10W | 20.95 ± 6.23 | 33.29 ± 6.25 | |||
| CAR34W | 19.71 ± 3.04 | F = 0.16 | 32.82 ± 2.90 | F = 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łochyński, D.; Pawlak, M.; Everaert, I.; Podgórski, T.; Gartych, M.; Borucka, A.-M.; Celichowski, J.; Derave, W.; Kaczmarek, D. Motor Unit Fatigability following Chronic Carnosine Supplementation in Aged Rats. Nutrients 2022, 14, 514. https://doi.org/10.3390/nu14030514
Łochyński D, Pawlak M, Everaert I, Podgórski T, Gartych M, Borucka A-M, Celichowski J, Derave W, Kaczmarek D. Motor Unit Fatigability following Chronic Carnosine Supplementation in Aged Rats. Nutrients. 2022; 14(3):514. https://doi.org/10.3390/nu14030514
Chicago/Turabian StyleŁochyński, Dawid, Maciej Pawlak, Inge Everaert, Tomasz Podgórski, Magdalena Gartych, Anna-Maria Borucka, Jan Celichowski, Wim Derave, and Dominik Kaczmarek. 2022. "Motor Unit Fatigability following Chronic Carnosine Supplementation in Aged Rats" Nutrients 14, no. 3: 514. https://doi.org/10.3390/nu14030514
APA StyleŁochyński, D., Pawlak, M., Everaert, I., Podgórski, T., Gartych, M., Borucka, A.-M., Celichowski, J., Derave, W., & Kaczmarek, D. (2022). Motor Unit Fatigability following Chronic Carnosine Supplementation in Aged Rats. Nutrients, 14(3), 514. https://doi.org/10.3390/nu14030514






