Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women
Abstract
:1. Introduction
2. Absorption and Metabolism of Soy Isoflavones
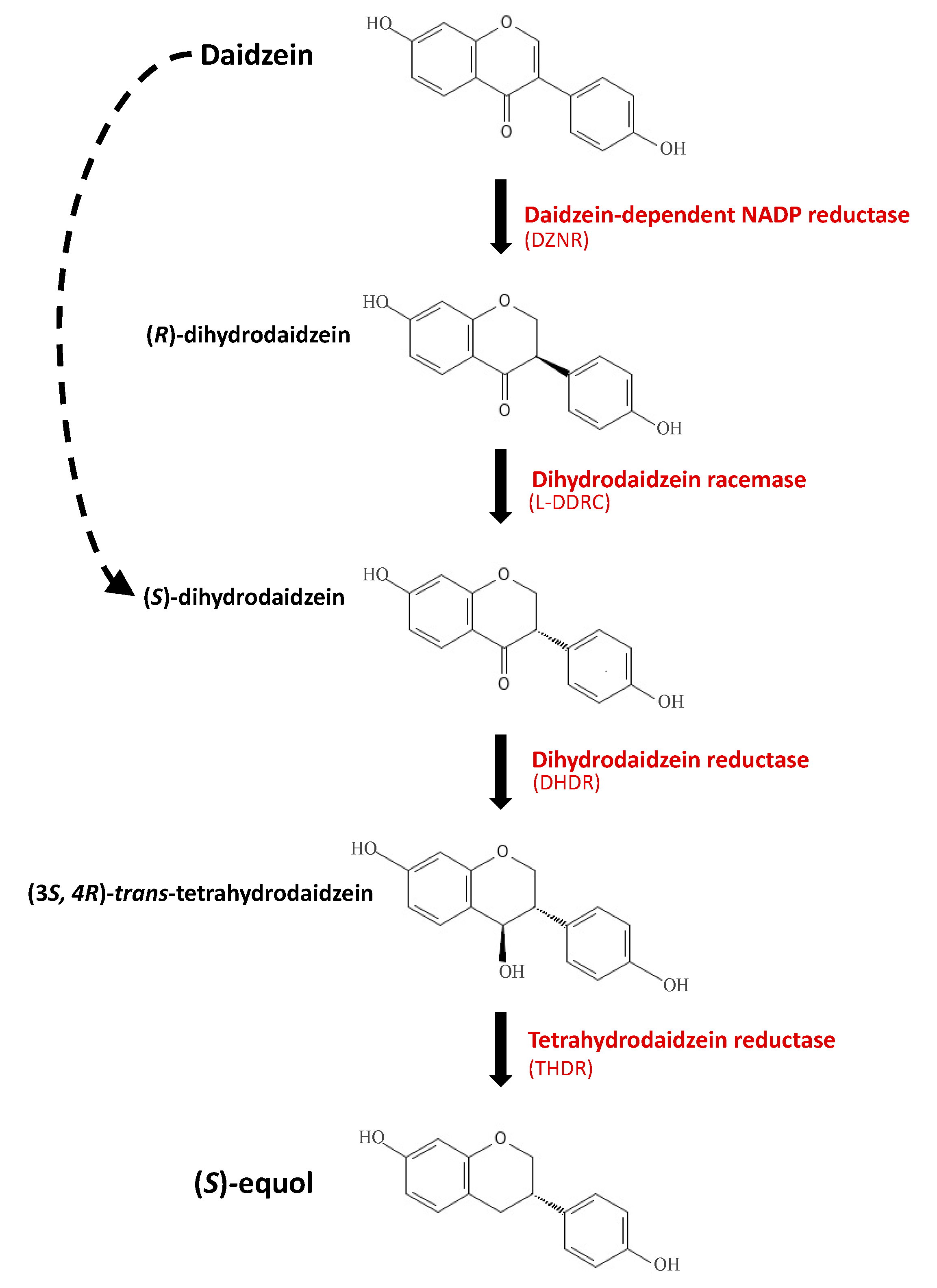
3. Bacterial Equol Production
4. Soy Isoflavones & Equol in Postmenopausal Women: Clinical Implications
4.1. Obesity and Diabetes
4.2. Cardiovascular Diseases
5. Current Limitations and Future Research of Soy Isoflavones and Equol: Developing Necessary Model Systems for Maximizing Estrogenic Potential of Daidzein
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPA | bisphenol A |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DZNR | daidzein-dependent NADP reductase |
| DDT | dichloro-diphenyl-trichloroethane |
| DDRC | dihydrodaidzein racemase |
| DHDR | dihydrodaidzein reductase |
| ERβ | estrogen receptor beta |
| GLUT4 | glucose transporter 4 |
| IL-6 | interleukin |
| LDL | low-density lipoprotein |
| MCP-1 | monocyte chemoattractant protein 1 |
| O-DMA | O-desmethylangolensin |
| PPARγ | proliferator-activated receptor γ |
| SERM | selective estrogen receptor modulators |
| THDR | tetrahydrodaidzein reductase |
| TNF-α | tumor necrosis factor-α |
| T2D | Type 2 diabetes |
| VCAM-1 | vascular cell adhesion molecule 1 |
References
- Rogers, N.H.; Perfield, J.W.; Strissel, K.J.; Obin, M.S.; Greenberg, A.S. Reduced Energy Expenditure and Increased Inflammation Are Early Events in the Development of Ovariectomy-Induced Obesity. Endocrinology 2009, 150, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Gracia, C.R. Obesity and reproductive hormone levels in the transition to menopause. Menopause 2010, 17, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschou, S.A.; Marina, L.V.; Spartalis, E.; Anagnostis, P.; Alexandrou, A.; Goulis, D.G.; Lambrinoudaki, I. Therapeutic strategies for type 2 diabetes mellitus in women after menopause. Maturitas 2019, 126, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Vitale, C.; Marazzi, G.; Volterrani, M. Menopause and cardiovascular disease: The evidence. Climacteric 2007, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surakasula, A.; Nagarjunapu, G.C.; Raghavaiah, K.V. A comparative study of pre- and post-menopausal breast cancer: Risk factors, presentation, characteristics and management. J. Res. Pharm. Pract. 2014, 3, 12–18. [Google Scholar] [CrossRef]
- Grodstein, F.; Manson, J.E.; Stampfer, M.J. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. A prospective, observational study. Ann. Intern. Med. 2001, 135, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Al-Safi, Z.A.; Santoro, N. Menopausal hormone therapy and menopausal symptoms. Fertil. Steril. 2014, 101, 905–915. [Google Scholar] [CrossRef]
- Dietel, M. Hormone replacement therapy (HRT), breast cancer and tumor pathology. Maturitas 2010, 65, 183–189. [Google Scholar] [CrossRef]
- Huntley, A.L.; Ernst, E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause 2003, 10, 465–476. [Google Scholar] [CrossRef]
- Posadzki, P.; Lee, M.; Moon, T.; Choi, T.; Park, T.; Ernst, E. Prevalence of complementary and alternative medicine (CAM) use by menopausal women: A systematic review of surveys. Maturitas 2013, 75, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Keinan-Boker, L.; Peeters, P.H.M.; A Mulligan, A.; Navarro, C.; Slimani, N.; Mattisson, I.; Lundin, E.; McTaggart, A.; E Allen, N.; Overvad, K.; et al. Soy product consumption in 10 European countries: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2002, 5, 1217–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, E.B.; Block, G.; Crawford, S.; Lachance, L.; Fitzgerald, G.; Miracle, H.; Sherman, S. Lifestyle and Demographic Factors in Relation to Vasomotor Symptoms: Baseline Results from the Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2004, 159, 1189–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Dupont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.C.; Morgan, M.R.A.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Lampe, J.W. Is equol the key to the efficacy of soy foods? Am. J. Clin. Nutr. 2009, 89, 1664S–1667S. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Qin, L.; Liu, A.; Uchiyama, S.; Ueno, T.; Li, X.; Wang, P. Prevalence of the Equol-Producer Phenotype and Its Relationship with Dietary Isoflavone and Serum Lipids in Healthy Chinese Adults. J. Epidemiol. 2010, 20, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.M.; Setchell, K.D.R. Animal Models Impacted by Phytoestrogens in Commercial Chow: Implications for Pathways Influenced by Hormones. Lab. Investig. 2001, 81, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Lamartiniere, C.A.; Wang, J.; Smith-Johnson, M.; Eltoum, I.-E. Daidzein: Bioavailability, Potential for Reproductive Toxicity, and Breast Cancer Chemoprevention in Female Rats. Toxicol. Sci. 2002, 65, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Kudou, S.; Fleury, Y.; Welti, D.; Magnolato, D.; Uchida, T.; Kitamura, K.; Okubo, K. Malonyl Isoflavone Glycosides in Soybean Seeds (Glycine max Merrill). Agric. Biol. Chem. 1991, 55, 2227–2233. [Google Scholar] [CrossRef]
- Shinkaruk, S.; Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Comparative Effects of R- and S-equol and Implication of Transactivation Functions (AF-1 and AF-2) in Estrogen Receptor-Induced Transcriptional Activity. Nutrients 2010, 2, 340–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.D.R.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of Pure Isoflavones in Healthy Humans and Analysis of Commercial Soy Isoflavone Supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafii, F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; House, S.E.; Prior, R.L.; Fang, N.; Ronis, M.J.J.; Clarkson, T.B.; Wilson, M.E.; Badger, T.M. Metabolic Phenotype of Isoflavones Differ among Female Rats, Pigs, Monkeys, and Women. J. Nutr. 2006, 136, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.M.; Wood, D.M.; Bottomley, L.; Blagg, S.; Owen, K.; Hughes, P.J.; Waring, R.H.; Kirk, C.J. Phytoestrogens Are Potent Inhibitors of Estrogen Sulfation: Implications for Breast Cancer Risk and Treatment. J. Clin. Endocrinol. Metab. 2004, 89, 1779–1787. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol—A Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [Green Version]
- Magee, P.J. Is equol production beneficial to health? Proc. Nutr. Soc. 2011, 70, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Magee, P.J.; Allsopp, P.; Samaletdin, A.; Rowland, I.R. Daidzein, R-(+)equol and S-(−)equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2. Eur. J. Nutr. 2014, 53, 345–350. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; E Wolfe, B.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, C.; Frankenfeld, C.; Lampe, J.W. Gut Bacterial Metabolism of the Soy Isoflavone Daidzein: Exploring the Relevance to Human Health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Hur, H.-G.; Beger, R.D.; Heinze, T.M.; Lay, J.; Freeman, J.P.; Dore, J.; Rafii, F. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch. Microbiol. 2002, 178, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Schoefer, L.; Mohan, R.; Braune, A.; Birringer, M.; Blaut, M. Anaerobic C-ring cleavage of genistein and daidzein byEubacterium ramulus. FEMS Microbiol. Lett. 2002, 208, 197–202. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Atkinson, C.; Thomas, W.K.; Goode, E.L.; Gonzalez, A.; Jokela, T.; Wähälä, K.; Schwartz, S.M.; Li, S.S.; Lampe, J.W. Familial Correlations, Segregation Analysis, and Nongenetic Correlates of Soy Isoflavone–Metabolizing Phenotypes. Exp. Biol. Med. 2004, 229, 902–913. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Halm, B.M.; Pagano, I.; Kono, N.; Mack, W.J.; Hodis, H.N. Equol production changes over time in postmenopausal women. J. Nutr. Biochem. 2011, 23, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.A.; Lai, J.F.; Pagano, I.; Morimoto, Y.; Maskarinec, G. Equol production changes over time in pre-menopausal women. Br. J. Nutr. 2011, 107, 1201–1206. [Google Scholar] [CrossRef]
- Blair, R.M.; Appt, S.E.; Franke, A.A.; Clarkson, T.B. Treatment with Antibiotics Reduces Plasma Equol Concentration in Cynomolgus Monkeys (Macaca fascicularis). J. Nutr. 2003, 133, 2262–2267. [Google Scholar] [CrossRef] [Green Version]
- Halm, B.M.; Franke, A.A.; Ashburn, L.A.; Hebshi, S.M.; Wilkens, L.R. Oral Antibiotics Decrease Urinary Isoflavonoid Excretion in Children after Soy Consumption. Nutr. Cancer 2007, 60, 14–22. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T.; Iino, K.; Yokoyama, Y.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients 2019, 11, 433. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.R.; Wiseman, H.; Sanders, T.; Adlercreutz, H.; Bowey, E.A. Interindividual Variation in Metabolism of Soy Isoflavones and Lignans: Influence of Habitual Diet on Equol Production by the Gut Microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Jiang, H. High dietary fat intake lowers serum equol concentration and promotes prostate carcinogenesis in a transgenic mouse prostate model. Nutr. Metab. 2019, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.; Flórez, A.B.; Verbruggen, S.; Redruello, B.; Verhoeven, J.; Venema, K.; Mayo, B. Modulation of equol production via different dietary regimens in an artificial model of the human colon. J. Funct. Foods 2020, 66, 103819. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.; Ohta, H.; Ishigaki, Y. Inter-relationship between diet, lifestyle habits, gut microflora, and the equol-producer phenotype: Baseline findings from a placebo-controlled intervention trial. Menopause 2019, 26, 273–285. [Google Scholar] [CrossRef]
- Sublette, M.; Cross, T.-W.; Korcarz, C.; Hansen, K.; Murga-Garrido, S.; Hazen, S.; Wang, Z.; Oguss, M.; Rey, F.; Stein, J. Effects of Smoking and Smoking Cessation on the Intestinal Microbiota. J. Clin. Med. 2020, 9, 2963. [Google Scholar] [CrossRef]
- Sepehr, E.; Cooke, G.; Robertson, P.; Gilani, G.S. Bioavailability of soy isoflavones in rats Part I: Application of accurate methodology for studying the effects of gender and source of isoflavones. Mol. Nutr. Food Res. 2007, 51, 799–812. [Google Scholar] [CrossRef]
- Lu, L.J.; Anderson, K.E. Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am. J. Clin. Nutr. 1998, 68, 1500S–1504S. [Google Scholar] [CrossRef] [Green Version]
- Kelly, G.E.; Nelson, C.; Waring, M.A.; Joannou, G.E.; Reeder, A.Y. Metabolites of dietary (soya) isoflavones in human urine. Clin. Chim. Acta 1993, 223, 9–22. [Google Scholar] [CrossRef]
- Faughnan, M.S.; Hawdon, A.; Ah-Singh, E.; Brown, J.; Millward, D.J.; Cassidy, A. Urinary isoflavone kinetics: The effect of age, gender, food matrix and chemical composition. Br. J. Nutr. 2004, 91, 567–574. [Google Scholar] [CrossRef]
- Lampe, J.W.; Karr, S.C.; Hutchins, A.M.; Slavin, J.L. Urinary Equol Excretion with a Soy Challenge: Influence of Habitual Diet. Exp. Biol. Med. 1998, 217, 335–339. [Google Scholar] [CrossRef]
- Cassidy, A.; Brown, J.E.; Hawdon, A.; Faughnan, M.S.; King, L.J.; Millward, J.; Zimmer-Nechemias, L.; Wolfe, B.; Setchell, K.D. Factors Affecting the Bioavailability of Soy Isoflavones in Humans after Ingestion of Physiologically Relevant Levels from Different Soy Foods. J. Nutr. 2006, 136, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Fujitani, M.; Mizushige, T.; Bhattarai, K.; Adhikari, S.; Ishikawa, J.; Kishida, T. Dietary daidzein induces accumulation of S-equol in enterohepatic circulation to far higher levels than that of daidzein in female rats with and without ovariectomy. Biomed. Res. 2019, 40, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Kim, M.J.; Yang, H.J.; Kang, S.; Park, S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Matthies, A.; Clavel, T.; Gütschow, M.; Engst, W.; Haller, D.; Blaut, M.; Braune, A. Conversion of Daidzein and Genistein by an Anaerobic Bacterium Newly Isolated from the Mouse Intestine. Appl. Environ. Microbiol. 2008, 74, 4847–4852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavel, T.; Lepage, P.; Charrier, C. The Family Coriobacteriaceae; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; pp. 201–238. [Google Scholar]
- Wang, X.-L.; Hur, H.-G.; Lee, J.H.; Kim, K.T.; Kim, S.-I. Enantioselective Synthesis of S -Equol from Dihydrodaidzein by a Newly Isolated Anaerobic Human Intestinal Bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Elghali, S.; Mustafa, S.; Amid, M.; Manap, M.Y.A.; Ismail, A.; Abas, F. Bioconversion of daidzein to equol by Bifidobacterium breve 15,700 and Bifidobacterium longum BB536. J. Funct. Foods 2012, 4, 736–745. [Google Scholar] [CrossRef]
- Yee, S.; Burdock, G.A.; Kurata, Y.; Enomoto, Y.; Narumi, K.; Hamada, S.; Itoh, T.; Shimomura, Y.; Ueno, T. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem. Toxicol. 2008, 46, 2713–2720. [Google Scholar] [CrossRef]
- Uchiyama, S.; Ueno, T.; Suzuki, T. Identification of a Newly Isolated Equol-Producing Lactic Acid Bacterium from the Human Feces. J. Intestig. Microbiol. 2007, 21, 217–220. [Google Scholar]
- Shimada, Y.; Yasuda, S.; Takahashi, M.; Hayashi, T.; Miyazawa, N.; Sato, I.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Cloning and Expression of a Novel NADP(H)-Dependent Daidzein Reductase, an Enzyme Involved in the Metabolism of Daidzein, from Equol-Producing Lactococcus Strain 20-92. Appl. Environ. Microbiol. 2010, 76, 5892–5901. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, S.-I.; Han, J.; Wang, X.-L.; Song, D.-G. Stereospecific Biotransformation of Dihydrodaidzein into (3S)-Equol by the Human Intestinal Bacterium Eggerthella Strain Julong 732. Appl. Environ. Microbiol. 2009, 75, 3062–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, C.; Matthies, A.; Engst, W.; Blaut, M.; Braune, A. Identification and Expression of Genes Involved in the Conversion of Daidzein and Genistein by the Equol-Forming Bacterium Slackia isoflavoniconvertens. Appl. Environ. Microbiol. 2013, 79, 3494–3502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, H.-G.; Lay, J.O., Jr.; Beger, R.D.; Freeman, J.P.; Rafii, F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 2000, 174, 422–428. [Google Scholar] [CrossRef]
- Tamura, M.; Tsushida, T.; Shinohara, K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 2007, 13, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Kawada, Y.; Goshima, T.; Sawamura, R.; Yokoyama, S.-I.; Yanase, E.; Niwa, T.; Ebihara, A.; Inagaki, M.; Yamaguchi, K.; Kuwata, K.; et al. Daidzein reductase of Eggerthella sp. YY7918, its octameric subunit structure containing FMN/FAD/4Fe-4S, and its enantioselective production of R-dihydroisoflavones. J. Biosci. Bioeng. 2018, 126, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Kim, H.-J.; Kang, S.-I.; Kim, S.-I.; Hur, H.-G. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch. Microbiol. 2007, 187, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.-I.; Suzuki, T. Isolation and Characterization of a Novel Equol-Producing Bacterium from Human Feces. Biosci. Biotechnol. Biochem. 2008, 72, 2660–2666. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, L.; Flórez, A.B.; Redruello, B.; Mayo, B. Metabolism of Soy Isoflavones by Intestinal Bacteria: Genome Analysis of an Adlercreutzia Equolifaciens Strain That Does Not Produce Equol. Biomolecules 2020, 10, 950. [Google Scholar] [CrossRef]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Ohtani, T.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of Two Novel Reductases Involved in Equol Biosynthesis in Lactococcus Strain 20-92. J. Mol. Microbiol. Biotechnol. 2011, 21, 160–172. [Google Scholar] [CrossRef]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of a Novel Dihydrodaidzein Racemase Essential for Biosynthesis of Equol from Daidzein in Lactococcus sp. Strain 20-92. Appl. Environ. Microbiol. 2012, 78, 4902–4907. [Google Scholar] [CrossRef] [Green Version]
- Flórez, A.B.; Vázquez, L.; Rodríguez, J.; Redruello, B.; Mayo, B. Transcriptional Regulation of the Equol Biosynthesis Gene Cluster in Adlercreutzia equolifaciens DSM19450T. Nutrients 2019, 11, 993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-Y.; Kim, M.; Han, J. Stereospecific microbial production of isoflavanones from isoflavones and isoflavone glucosides. Appl. Microbiol. Biotechnol. 2011, 91, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.; Flórez, A.B.; Rodríguez, J.; Mayo, B. Heterologous expression of equol biosynthesis genes from Adlercreutzia equolifaciens. FEMS Microbiol. Lett. 2021, 368, fnab082. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-G.; Kim, J.; Kim, E.-J.; Jung, E.; Pandey, B.P.; Kim, B.-G. P212A Mutant of Dihydrodaidzein Reductase Enhances (S)-Equol Production and Enantioselectivity in a Recombinant Escherichia coli Whole-Cell Reaction System. Appl. Environ. Microbiol. 2016, 82, 1992–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, Y.; Yokoyama, S.-I.; Yanase, E.; Niwa, T.; Suzuki, T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci. Microbiota Food Health 2016, 35, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Bastida, A.R.; Peirotén, Á.; Langa, S.; Arqués, J.L.; Landete, J.M. Heterologous production of equol by lactic acid bacteria strains in culture medium and food. Int. J. Food Microbiol. 2021, 360, 109328. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Beydoun, M.A.; Liang, L.; Caballero, B.; Kumanyika, S.K. Will All Americans Become Overweight or Obese? Estimating the Progression and Cost of the US Obesity Epidemic. Obesity 2008, 16, 2323–2330. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [Green Version]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of Obesity and Trends in the Distribution of Body Mass Index among US Adults, 1999–2010. JAMA 2012, 307, 491. [Google Scholar] [CrossRef] [Green Version]
- Polotsky, H.N.; Polotsky, A.J. Metabolic Implications of Menopause. Semin. Reprod. Med. 2010, 28, 426–434. [Google Scholar] [CrossRef]
- Vieira-Potter, V.J. Inflammation and macrophage modulation in adipose tissues. Cell. Microbiol. 2014, 16, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Walsh, J.M.E.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Sternfeld, B.; Wang, H.; Quesenberry, C.P.; Abrams, B.; Everson-Rose, S.; Greendale, G.A.; Matthews, K.A.; Torrens, J.I.; Sowers, M. Physical Activity and Changes in Weight and Waist Circumference in Midlife Women: Findings from the Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2004, 160, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hassager, C.; Ravn, P.; Wang, S.; Christiansen, C. Total and regional body-composition changes in early postmenopausal women: Age-related or menopause-related? Am. J. Clin. Nutr. 1994, 60, 843–848. [Google Scholar] [CrossRef]
- Trémollieres, F.A.; Pouilles, J.-M.; Ribot, C.A. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am. J. Obstet. Gynecol. 1996, 175, 1594–1600. [Google Scholar] [CrossRef]
- Homma, H.; Kurachi, H.; Nishio, Y.; Takeda, T.; Yamamoto, T.; Adachi, K.; Morishige, K.-I.; Ohmichi, M.; Matsuzawa, Y.; Murata, Y. Estrogen Suppresses Transcription of Lipoprotein Lipase Gene. J. Biol. Chem. 2000, 275, 11404–11411. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Igarashi, K.; Yu, C. Anti-obese and anti-diabetic effects of a mixture of daidzin and glycitin on C57BL/6J mice fed with a high-fat diet. Biosci. Biotechnol. Biochem. 2015, 79, 117–123. [Google Scholar] [CrossRef]
- Sakane, N.; Kotani, K.; Tsuzaki, K.; Takahashi, K.; Usui, T.; Uchiyama, S.; Fujiwara, S. Equol producers can have low leptin levels among prediabetic and diabetic females. Ann. D’endocrinologie 2014, 75, 25–28. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T.; Nogowski, L. Daidzein, coumestrol and zearalenone affect lipogenesis and lipolysis in rat adipocytes. Phytomedicine 2002, 9, 338–345. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Calleri, E.; Pochetti, G.; Dossou, K.S.S.; Laghezza, A.; Montanari, R.; Capelli, D.; Prada, E.; Loiodice, F.; Massolini, G.; Bernier, M.; et al. Resveratrol and Its Metabolites Bind to PPARs. ChemBioChem 2014, 15, 1154–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäckesjö, C.-M.; Li, Y.; Lindgren, U.; Haldosén, L.-A. Activation of Sirt1 Decreases Adipocyte Formation during Osteoblast Differentiation of Mesenchymal Stem Cells. J. Bone Miner. Res. 2006, 21, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.D.S.; Rohden, F.; Hammes, T.O.; Margis, R.; Bortolotto, J.W.; Padoin, A.V.; Mottin, C.C.; Guaragna, R.M. Resveratrol Upregulated SIRT1, FOXO1, and Adiponectin and Downregulated PPARγ1–3 mRNA Expression in Human Visceral Adipocytes. Obes. Surg. 2010, 21, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kanatsu, J.; Toh, M.; Naka, A.; Kondo, K.; Iida, K. The Dietary Isoflavone Daidzein Reduces Expression of Pro-Inflammatory Genes through PPARα/γ and JNK Pathways in Adipocyte and Macrophage Co-Cultures. PLoS ONE 2016, 11, e0149676. [Google Scholar] [CrossRef]
- Di Marzio, D. Peroxisome proliferator-activated receptor-γ agonists and diabetes: Current evidence and future perspectives. Vasc. Health Risk Manag. 2008, 4, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.W.; Lee, O.-H.; Banz, W.J.; Moustaid-Moussa, N.; Shay, N.F.; Kim, Y.-C. Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARγ transcriptional activity. J. Nutr. Biochem. 2010, 21, 841–847. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Naka, A.; Ohara, N.; Kondo, K.; Iida, K. Daidzein regulates proinflammatory adipokines thereby improving obesity-related inflammation through PPARγ. Mol. Nutr. Food Res. 2014, 58, 718–726. [Google Scholar] [CrossRef]
- Mezei, O.; Banz, W.J.; Steger, R.W.; Peluso, M.R.; Winters, T.A.; Shay, N. Soy Isoflavones Exert Antidiabetic and Hypolipidemic Effects through the PPAR Pathways in Obese Zucker Rats and Murine RAW 264.7 Cells. J. Nutr. 2003, 133, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Jayagopal, V.; Albertazzi, P.; Kilpatrick, E.S.; Howarth, E.M.; Jennings, P.E.; Hepburn, D.A.; Atkin, S.L. Beneficial Effects of Soy Phytoestrogen Intake in Postmenopausal Women with Type 2 Diabetes. Diabetes Care 2002, 25, 1709–1714. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-Cell Deficit and Increased beta-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, H.; Usami, A.; Shirai, R.; Harada, N.; Ikushiro, S.; Sakaki, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-Equol Activates cAMP Signaling at the Plasma Membrane of INS-1 Pancreatic β-Cells and Protects against Streptozotocin-Induced Hyperglycemia by Increasing β-Cell Function in Male Mice. J. Nutr. 2017, 147, 1631–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiuchi, H.; Harada, N.; Adachi, T.; Nakano, Y.; Inui, H.; Yamaji, R. S-Equol Enantioselectively Activates CAMP-Protein Kinase A Signaling and Reduces Alloxan-Induced Cell Death in INS-1 Pancreatic β-Cells. J. Nutr. Sci. Vitaminol. 2014, 60, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Lang, H.; Wang, L.; Liu, K.; Zhou, Y.; Mi, M. S-Equol ameliorates insulin secretion failure through Chrebp/Txnip signaling via modulating PKA/PP2A activities. Nutr. Metab. 2020, 17, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalko, J.R.; Sandstead, H.H.; Johnson, L.K.; Inman, L.F.; Milne, D.B.; Warner, R.C.; A Haunz, E. Effect of consuming fiber from corn bran, soy hulls, or apple powder on glucose tolerance and plasma lipids in type II diabetes. Am. J. Clin. Nutr. 1984, 39, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Tsai, A.C.; I Vinik, A.; Lasichak, A.; Lo, G.S. Effects of soy polysaccharide on postprandial plasma glucose, insulin, glucagon, pancreatic polypeptide, somatostatin, and triglyceride in obese diabetic patients. Am. J. Clin. Nutr. 1987, 45, 596–601. [Google Scholar] [CrossRef]
- Hermansen, K.; Søndergaard, M.; Høie, L.; Carstensen, M.; Brock, B. Beneficial Effects of a Soy-Based Dietary Supplement on Lipid Levels and Cardiovascular Risk Markers in Type 2 Diabetic Subjects. Diabetes Care 2001, 24, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.-L.; Tang, X.-Y.; Deng, Y.; Zhong, Q.-W.; Wang, C.; Zhang, Z.-Q.; Chen, Y.-M. Urinary equol, but not daidzein and genistein, was inversely associated with the risk of type 2 diabetes in Chinese adults. Eur. J. Nutr. 2020, 59, 719–728. [Google Scholar] [CrossRef]
- Talaei, M.; Lee, B.L.; Ong, C.N.; van Dam, R.M.; Yuan, J.M.; Koh, W.P.; Pan, A. Urine phyto-oestrogen metabolites are not significantly associated with risk of type 2 diabetes: The Singapore Chinese health study. Br. J. Nutr. 2016, 115, 1607–1615. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Steinbrecher, A.; Kolonel, L.N.; Maskarinec, G. Soy consumption is not protective against diabetes in Hawaii: The Multiethnic Cohort. Eur. J. Clin. Nutr. 2010, 65, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Merz, C.N.B.; Andersen, H.; Sprague, E.; Burns, A.; Keida, M.; Walsh, M.N.; Greenberger, P.; Campbell, S.; Pollin, I.; McCullough, C.; et al. Knowledge, Attitudes, and Beliefs Regarding Cardiovascular Disease in Women. J. Am. Coll. Cardiol. 2017, 70, 123–132. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Nachvak, S.M.; Moradi, S.; Anjom-Shoae, J.; Rahmani, J.; Nasiri, M.; Maleki, V.; Sadeghi, O. Soy, Soy Isoflavones, and Protein Intake in Relation to Mortality from All Causes, Cancers, and Cardiovascular Diseases: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. J. Acad. Nutr. Diet. 2019, 119, 1483–1500.e17. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Iso, H.; Ishihara, J.; Okada, K.; Inoue, M.; Tsugane, S. Association of Dietary Intake of Soy, Beans, and Isoflavones With Risk of Cerebral and Myocardial Infarctions in Japanese Populations. Circulation 2007, 116, 2553–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakoshi, J.; Piskula, M.K.; Izumi, T.; Tobe, K.; Saito, M.; Kataoka, S.; Obata, A.; Kikuchi, M. Isoflavone Aglycone–Rich Extract without Soy Protein Attenuates Atherosclerosis Development in Cholesterol-Fed Rabbits. J. Nutr. 2000, 130, 1887–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, T.B.; Anthony, M.S.; Morgan, T.M. Inhibition of Postmenopausal Atherosclerosis Progression: A Comparison of the Effects of Conjugated Equine Estrogens and Soy Phytoestrogens1. J. Clin. Endocrinol. Metab. 2001, 86, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, G.; Evans, R.; Phillips, K.; Dahlgren, R.; Steinke, F. Effect of soy fiber and soy protein on cholesterol metabolism and atherosclerosis in rabbits. Atherosclerosis 1987, 64, 47–54. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Zhang, P.; Zhu, J.; Meng, G.; Xie, L.; Yu, Y.; Ji, Y.; Han, Y. Soy Isoflavone Protects Myocardial Ischemia/Reperfusion Injury through Increasing Endothelial Nitric Oxide Synthase and Decreasing Oxidative Stress in Ovariectomized Rats. Oxidative Med. Cell. Longev. 2016, 2016, 5057405. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Kono, N.; Azen, S.P.; Shoupe, D.; Hwang-Levine, J.; Petitti, D.; Whitfield-Maxwell, L.; Yan, M.; Franke, A.A.; et al. Isoflavone Soy Protein Supplementation and Atherosclerosis Progression in Healthy Postmenopausal Women. Stroke 2011, 42, 3168–3175. [Google Scholar] [CrossRef] [Green Version]
- Burris, R.L.; Vick, S.C.; Popovic, B.; Fraungruber, P.E.; Nagarajan, S. Maternal exposure to soy diet reduces atheroma in hyperlipidemic F1 offspring mice by promoting macrophage and T cell anti-inflammatory responses. Atherosclerosis 2020, 313, 26–34. [Google Scholar] [CrossRef]
- Petersen, K.S. The Dilemma With the Soy Protein Health Claim. J. Am. Heart Assoc. 2019, 8, e013202. [Google Scholar] [CrossRef]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Mejia, S.B.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; A Khan, T.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Mejia, S.B.; Chiavaroli, L.; Viguiliouk, E.; Li, S.S.; Kendall, C.W.C.; Vuksan, V.; Sievenpiper, J.L. Cumulative Meta-Analysis of the Soy Effect over Time. J. Am. Heart Assoc. 2019, 8, e012458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.M.W.; Kendall, C.W.C.; Marchie, A.; Liu, Z.; Vidgen, E.; Holmes, C.; Jackson, C.-J.; Josse, R.G.; Pencharz, P.B.; Rao, A.V.; et al. Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. Am. J. Clin. Nutr. 2012, 95, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Daneshzad, E.; Azadbakht, L. The effects of isolated soy protein, isolated soy isoflavones and soy protein containing isoflavones on serum lipids in postmenopausal women: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 3414–3428. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Vafeiadou, K.; Hallund, J.; Bügel, S.; Koebnick, C.; Reimann, M.; Ferrari, M.; Branca, F.; Talbot, D.; Dadd, T.; et al. Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in postmenopausal women: Interactions with genotype and equol production. Am. J. Clin. Nutr. 2005, 82, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Kimiagar, M.; Mehrabi, Y.; Esmaillzadeh, A.; Hu, F.B.; Willett, W.C. Soy Consumption, Markers of Inflammation, and Endothelial Function. Diabetes Care 2007, 30, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, H. Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food Funct. 2017, 8, 2935–2944. [Google Scholar] [CrossRef]
- Roghani, F.; Vaez-Mahdavi, M.-R.; Nadoushan, M.J.; Baluchnejadmojarad, T.; Naderi, G.; Roghani-Dehkordi, F.; Joghataei, M.T.; Kord, M. Chronic Administration of Daidzein, a Soybean Isoflavone, Improves Endothelial Dysfunction and Attenuates Oxidative Stress in Streptozotocin-induced Diabetic Rats. Phytother. Res. 2013, 27, 112–117. [Google Scholar] [CrossRef]
- Kerry, N.; Abbey, M. The isoflavone genistein inhibits copper and peroxyl radical mediated low density lipoprotein oxidation in vitro. Atherosclerosis 1998, 140, 341–347. [Google Scholar] [CrossRef]
- Kapiotis, S.; Hermann, M.; Held, I.; Seelos, C.; Ehringer, H.; Gmeiner, B.M.K. Genistein, the Dietary-Derived Angiogenesis Inhibitor, Prevents LDL Oxidation and Protects Endothelial Cells from Damage by Atherogenic LDL. Arter. Thromb. Vasc. Biol. 1997, 17, 2868–2874. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.J.; Wähälä, K.; Ojala, S.; Vihma, V.; Adlercreutz, H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc. Natl. Acad. Sci. USA 1998, 95, 3106–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiseman, H.; O’Reilly, J.D.; Adlercreutz, H.; I Mallet, A.; A Bowey, E.; Rowland, I.R.; Sanders, T. Isoflavone phytoestrogens consumed in soy decrease F2-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 2000, 72, 395–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, Y.; Wang, L.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T.; Nakagawa, J.; Ashida, H. Black soybean improves the vascular function through an increase in nitric oxide and a decrease in oxidative stress in healthy women. Arch. Biochem. Biophys. 2020, 688, 108408. [Google Scholar] [CrossRef]
- Woodman, O.L.; Boujaoude, M. Chronic treatment of male rats with daidzein and 17β -oestradiol induces the contribution of EDHF to endothelium-dependent relaxation. J. Cereb. Blood Flow Metab. 2004, 141, 322–328. [Google Scholar] [CrossRef]
- Sobey, C.G.; Weiler, J.M.; Boujaoude, M.; Woodman, O.L. Effect of Short-Term Phytoestrogen Treatment in Male Rats on Nitric Oxide-Mediated Responses of Carotid and Cerebral Arteries: Comparison with 17β-Estradiol. J. Pharmacol. Exp. Ther. 2004, 310, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Gottstein, N.; Ewins, B.A.; Eccleston, C.; Hubbard, G.P.; Kavanagh, I.C.; Minihane, A.-M.; Weinberg, P.D.; Rimbach, G. Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Br. J. Nutr. 2003, 89, 607–615. [Google Scholar] [CrossRef]
- Moriyama, M.; Hashimoto, A.; Satoh, H.; Kawabe, K.; Ogawa, M.; Takano, K.; Nakamura, Y. S-Equol, a Major Isoflavone from Soybean, Inhibits Nitric Oxide Production in Lipopolysaccharide-Stimulated Rat Astrocytes Partially via the GPR30-Mediated Pathway. Int. J. Inflamm. 2018, 2018, 8496973. [Google Scholar] [CrossRef] [Green Version]
- Franzosa, E.A.; Huang, K.; Meadow, J.F.; Gevers, D.; Lemon, K.P.; Bohannan, B.J.M.; Huttenhower, C. Identifying personal microbiomes using metagenomic codes. Proc. Natl. Acad. Sci. USA 2015, 112, E2930–E2938. [Google Scholar] [CrossRef] [Green Version]
- Axelson, M.; Setchell, K. The excretion of lignans in rats-evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981, 123, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Bowey, E.; Adlercreutz, H.; Rowland, I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem. Toxicol. 2003, 41, 631–636. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Orcutt, R.P.; Gianni, F.J.; Judge, R.J. Development of an “Altered Schaedler Flora” for NCI Gnotobiotic Rodents. Microecol. Ther. 1987, 17. [Google Scholar]
- Brugiroux, S.; Beutler, M.; Pfann, C.; Garzetti, D.; Ruscheweyh, H.-J.; Ring, D.; Diehl, M.; Herp, S.; Lötscher, Y.; Hussain, S.; et al. Genome-guided design of a defined mouse microbiota that confers colonization resistance against Salmonella enterica serovar Typhimurium. Nat. Microbiol. 2017, 2, 16215. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Kunath, J.; Loh, G.; Blaut, M. Human intestinal microbiota: Characterization of a simplified and stable gnotobiotic rat model. Gut Microbes 2011, 2, 25–33. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [Green Version]
- Eberl, C.; Ring, D.; Münch, P.C.; Beutler, M.; Basic, M.; Slack, E.C.; Schwarzer, M.; Srutkova, D.; Lange, A.; Frick, J.-S.; et al. Reproducible Colonization of Germ-Free Mice with the Oligo-Mouse-Microbiota in Different Animal Facilities. Front. Microbiol. 2020, 10, 2999. [Google Scholar] [CrossRef]
- Stehr, M.; Greweling, M.C.; Tischer, S.; Singh, M.; Blöcker, H.; A Monner, D.; Muller, W. Charles River altered Schaedler flora (CRASF®) remained stable for four years in a mouse colony housed in individually ventilated cages. Lab. Anim. 2009, 43, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of naturalS-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A Pilot Randomized, Placebo-Controlled Trial. Menopause 2011, 18, 563–574. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Zhao, X.; Shoaf, S.E.; Ragland, K. The Pharmacokinetics of S-(-)Equol Administered as SE5-OH Tablets to Healthy Postmenopausal Women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legette, L.L.; Prasain, J.; King, J.; Arabshahi, A.; Barnes, S.; Weaver, C.M. Pharmacokinetics of Equol, a Soy Isoflavone Metabolite, Changes with the Form of Equol (Dietary versus Intestinal Production) in Ovariectomized Rats. J. Agric. Food Chem. 2014, 62, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Hazim, S.; Curtis, P.; Schär, M.Y.; Ostertag, L.M.; Kay, C.; Minihane, A.-M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Bacterial Strain | Conversion | Source |
|---|---|---|
| Adlercreutzia equolifaciens strain DSM 19450 | Daidzein → Equol | [53] |
| Lactobacillus intestinalis | Daidzein → Equol | [54] |
| Coriobacteriaceae strain Mt1B8 | Daidzein → Equol | [55] |
| Eggerthella sp. strain Julong 732 | Dihydrodaidzein → Equol | [57,62] |
| Bifidobacterium breve strain 15700 | Daidzein → Equol | [58] |
| Bifidobacterium longum strain BB536 | Daidzein → Equol | [58] |
| Lactococcus garvieae strain 20–92 | Daidzein → Equol | [60] |
| Slackia Isoflavoniconvertens strain DSM 22006 | Daidzein → Equol | [63] |
| Clostridium sp. strain HGH6 | Daidzein → Dihydrodaidzein | [64] |
| Coprobacillus strain TM-40 | Daidzein → Dihydrodaidzein | [65] |
| Lactobacillus sp. Niu-O16 | Daidzein → Dihydrodaidzein | [66,67] |
| Eggerthella sp. YY7918 | Daidzein → Equol | [68] |
| Adlercreutzia equolifaciens strain W18.34a | No conversion of daidzein | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonard, L.M.; Choi, M.S.; Cross, T.-W.L. Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients 2022, 14, 553. https://doi.org/10.3390/nu14030553
Leonard LM, Choi MS, Cross T-WL. Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients. 2022; 14(3):553. https://doi.org/10.3390/nu14030553
Chicago/Turabian StyleLeonard, Lindsay M., Mun Sun Choi, and Tzu-Wen L. Cross. 2022. "Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women" Nutrients 14, no. 3: 553. https://doi.org/10.3390/nu14030553
APA StyleLeonard, L. M., Choi, M. S., & Cross, T.-W. L. (2022). Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients, 14(3), 553. https://doi.org/10.3390/nu14030553






