Taste of Fat and Obesity: Different Hypotheses and Our Point of View
Abstract
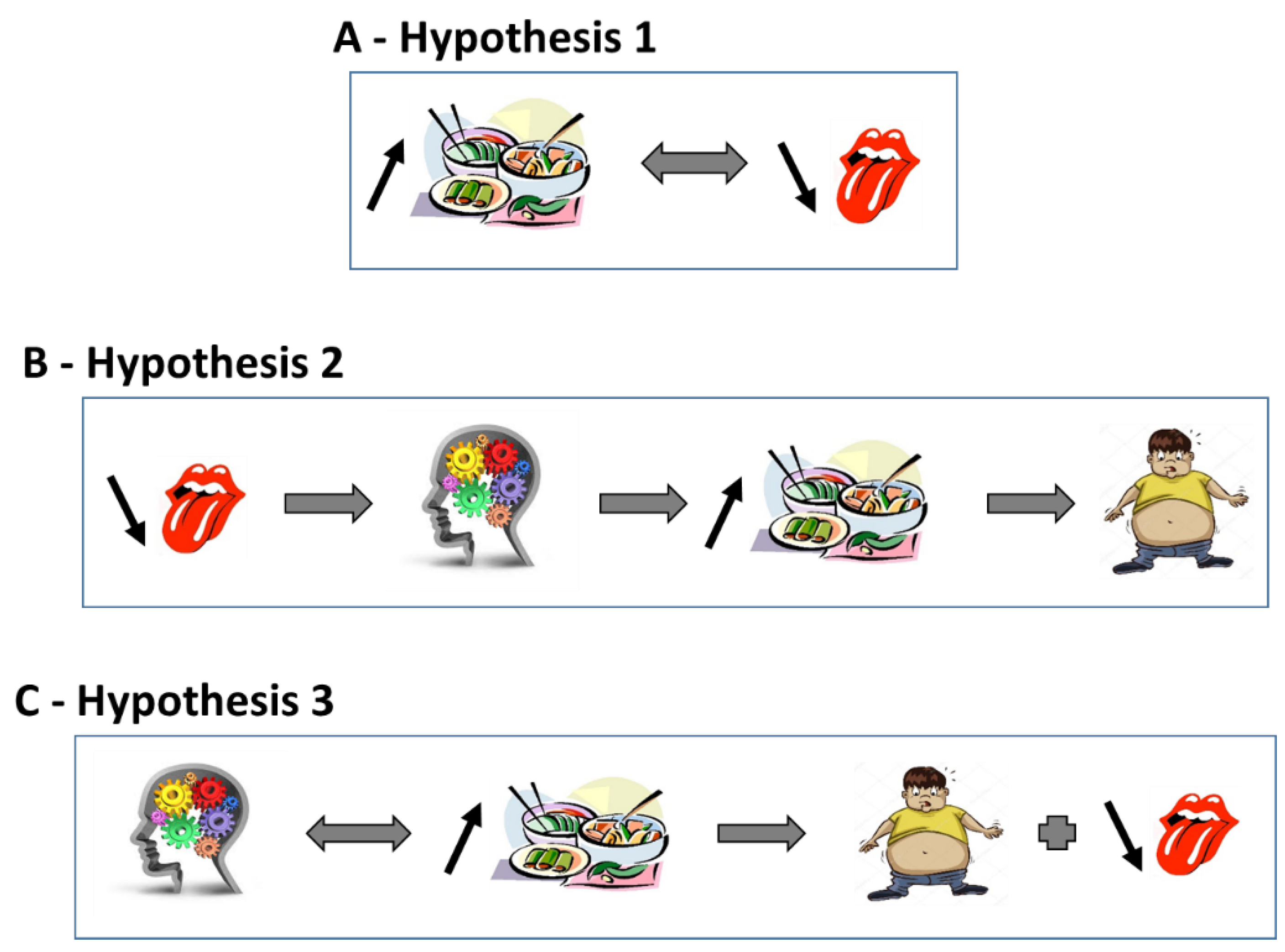
:1. Introduction
2. Teleonomy of the Taste for Fat
- Gustatory stimulation linked to fat is analysed by its qualitative and quantitative components thus indicating that the taste of fat/mouthfeel of fat exists [32].
- Fatty acids in the mouth induce gustatory evoked potentials, thus showing that fatty acids are well perceived at the cortical level, as we have observed [33].
- The presence of fatty acids in the mouth is perceived and processed by the central nervous system, thereby inducing adapted behaviour and anticipatory vegetative reactions [34].
3. High-Fat Diet and Fat Taste
4. Taste of Fat and Obesity
5. Fat Taste and Bariatric Surgery
6. Alteration of the Reward System, High-Fat Diet and Obesity
7. Taste/Mouthfeel of Fat and Obesity, Cause or Consequence?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cahill, G.F. Starvation in Man. N. Engl. J. Med. 1970, 282, 668–675. [Google Scholar] [CrossRef]
- Davis, C.M. Self Selection of Diet by Newly Weaned Infants: An Experimental Study. Nutr. Rev. 2009, 44, 114–116. [Google Scholar] [CrossRef]
- Davis, C.M. Results of the Self-Selection of Diets by Young Children. Can. Med. Assoc. J. 1939, 41, 257–261. [Google Scholar] [PubMed]
- Mayer, J. Regulation of Energy Intake and The Body Weight: The Glucostatic Theory and the Lipostatic Hypothesis. Ann. N. Y. Acad. Sci. 1955, 63, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Russek, M. Hepatic Receptors and the Neurophysiological Mechanisms Controlling Feeding Behavior. Neurosci. Res. 1971, 4, 213–282. [Google Scholar] [CrossRef] [PubMed]
- Flatt, J.P. The Difference in the Storage Capacities for Carbohydrate and for Fat, and Its Implications in the Regulation of Body Weighta. Ann. N. Y. Acad. Sci. 2006, 499, 104–123. [Google Scholar] [CrossRef]
- Kennedy, G.C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. Ser. B Boil. Sci. 1953, 140, 578–592. [Google Scholar] [CrossRef]
- Brondel, L.; Landais, L.; Romer, M.A.; Holley, A.; Pénicaud, L. Substrate oxidation influences liking, wanting, macronutrient selection, and consumption of food in humans. Am. J. Clin. Nutr. 2011, 94, 775–783. [Google Scholar] [CrossRef]
- Harnischfeger, F.; Dando, R. Obesity-induced taste dysfunction, and its implications for dietary intake. Int. J. Obes. 2021, 45, 1644–1655. [Google Scholar] [CrossRef]
- Boesveldt, S.; de Graaf, K. The Differential Role of Smell and Taste for Eating Behavior. Perception 2017, 46, 307–319. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Mattes, R.D. Nutrition and taste and smell dysfunction. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Loper, H.B.; La Sala, M.; Dotson, C.D.; I Steinle, N. Taste perception, associated hormonal modulation, and nutrient intake. Nutr. Rev. 2015, 73, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhagen, J.V.; Engelen, L. The neurocognitive bases of human multimodal food perception: Sensory integration. Neurosci. Biobehav. Rev. 2006, 30, 613–650. [Google Scholar] [CrossRef] [PubMed]
- Nicklaus, S. The role of food experiences during early childhood in food pleasure learning. Appetite 2016, 104, 3–9. [Google Scholar] [CrossRef]
- Pénicaud, L.; Valentin, D.; Brondel, L. Mechanisms involved in the control of feeding behavior in relation to food flavor. In Flavor: From Food to Behaviors, Wellbeing and Health; Etiévant, P., Guichard, E., Salles, C., Voilley, A., Eds.; Elsevier Ltd.: Cambridge, MA, USA, 2016; pp. 101–119. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste, olfactory and food texture reward processing in the brain and obesity. Int. J. Obes. 2010, 35, 550–561. [Google Scholar] [CrossRef] [Green Version]
- Cabanac, M. Sensory Pleasure. Q. Rev. Biol. 1979, 54, 1–29. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Zheng, H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.; Woods, S.C.; Seeley, R.J. Central Nervous System Mechanisms Linking the Consumption of Palatable High-Fat Diets to the Defense of Greater Adiposity. Cell Metab. 2012, 15, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Deglaire, A.; Méjean, C.; Castetbon, K.; Kesse-Guyot, E.; Hercberg, S.; Schlich, P. Associations between weight status and liking scores for sweet, salt and fat according to the gender in adults (The Nutrinet-Santé study). Eur. J. Clin. Nutr. 2014, 69, 40–46. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Lenard, N.R.; Shin, A.C. Food Reward, Hyperphagia, and Obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1266–R1277. [Google Scholar] [CrossRef] [Green Version]
- Cabanac, M. Physiological Role of Pleasure. Science 1971, 173, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2017, 27, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Leloup, C.; Brondel, L.; Mouillot, T.; Brindisi, M.; Pénicaud, L.; Jacquin-Piques, A. Contrôle de la prise alimentaire. In EMC Gastro-Entérologie; Elesevier Masson SAS: Paris, France, 2020. [Google Scholar] [CrossRef]
- Keast, R.; Costanzo, A.; Hartley, I. Macronutrient Sensing in the Oral Cavity and Gastrointestinal Tract: Alimentary Tastes. Nutrients 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Shanmugamprema, D.; Muthuswamy, K.; Subramanian, G.; Ponnusamy, V.; Krishnan, V.; Subramaniam, S. Fat taste signal transduction and its possible negative modulator components. Prog. Lipid Res. 2020, 79, 101035. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Schamarek, I.; Blüher, M. Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets? Diabetes Metab. J. 2020, 44, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Montmayeur, J.P.; le Coutre, J. (Eds.) Fat Detection: Taste, Texture, and Post Ingestive Effects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; 609p. [Google Scholar]
- E Hartley, I.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolhuis, D.; Newman, L.P.; Keast, R.S. Effects of Salt and Fat Combinations on Taste Preference and Perception. Chem. Senses 2015, 41, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalé-Rush, A.; Burgess, J.R.; Mattes, R.D. Evidence for Human Orosensory (Taste?) Sensitivity to Free Fatty Acids. Chem. Senses 2007, 32, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2016, 96, 151–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouillot, T.; Szleper, E.; Vagne, G.; Barthet, S.; Litime, D.; Brindisi, M.-C.; Leloup, C.; Penicaud, L.; Nicklaus, S.; Brondel, L.; et al. Cerebral gustatory activation in response to free fatty acids using gustatory evoked potentials in humans. J. Lipid Res. 2019, 60, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Chevrot, M.; Passilly-Degrace, P.; Ancel, D.; Bernard, A.; Enderli, G.; Gomes, M.; Robin, I.; Issanchou, S.; Vergès, B.; Nicklaus, S.; et al. Obesity interferes with the orosensory detection of long-chain fatty acids in humans. Am. J. Clin. Nutr. 2014, 99, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, D. ANNUAL LIPID CYCLES IN HIBERNATORS: Integration of Physiology and Behavior. Annu. Rev. Nutr. 2005, 25, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Greenwood, M. Cream and sugar: Human preferences for high-fat foods. Physiol. Behav. 1983, 30, 629–633. [Google Scholar] [CrossRef]
- Finlayson, G. Food addiction and obesity: Unnecessary medicalization of hedonic overeating. Nat. Rev. Endocrinol. 2017, 13, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.-D. Development, brain plasticity and reward: Early high-fat diet exposure confers vulnerability to obesity—View from the chair. Int. J. Obes. Suppl. 2012, 2, S3–S6. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; McPhee, L.; Birch, L. Conditioned preferences: Young children prefer flavors associated with high dietary fat. Physiol. Behav. 1991, 50, 1245–1251. [Google Scholar] [CrossRef]
- Drewnowski, A. Why do we Like Fat? J. Am. Diet. Assoc. 1997, 97, S58–S62. [Google Scholar] [CrossRef]
- Perszyk, E.; Hutelin, Z.; Trinh, J.; Kanyamibwa, A.; Fromm, S.; Davis, X.; Wall, K.; Flack, K.; DiFeliceantonio, A.; Small, D. Fat and Carbohydrate Interact to Potentiate Food Reward in Healthy Weight but Not in Overweight or Obesity. Nutrients 2021, 13, 1203. [Google Scholar] [CrossRef]
- Gutierrez, R.; Fonseca, E.; Simon, S.A. The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity. Cell. Mol. Life Sci. 2020, 77, 3469–3502. [Google Scholar] [CrossRef]
- Olszewski, P.K.; Wood, E.L.; Klockars, A.; Levine, A.S. Excessive Consumption of Sugar: An Insatiable Drive for Reward. Curr. Nutr. Rep. 2019, 8, 120–128. [Google Scholar] [CrossRef]
- Freeman, C.R.; Zehra, A.; Ramirez, V.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Impact of sugar on the body, brain, and behavior. Front. Biosci. 2018, 23, 2255–2266. [Google Scholar]
- Elfhag, K.; Erlanson-Albertsson, C. Sweet and fat taste preference in obesity have different associations with personality and eating behavior. Physiol. Behav. 2006, 88, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Salbe, A.D.; DelParigi, A.; E Pratley, R.; Drewnowski, A.; Tataranni, P.A. Taste preferences and body weight changes in an obesity-prone population. Am. J. Clin. Nutr. 2004, 79, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, R.; O’Neil, P.M.; A Hirsch, A.; Currey, H.S.; Moskowitz, G. Taste hedonics and thresholds in obesity. Int. J. Obes. 1980, 4, 203–212. [Google Scholar] [PubMed]
- Sobek, G.; Łuszczki, E.; Dąbrowski, M.; Dereń, K.; Baran, J.; Weres, A.; Mazur, A. Preferences for Sweet and Fatty Taste in Children and Their Mothers in Association with Weight Status. Int. J. Environ. Res. Public Health 2020, 17, 538. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.R.; Papantoni, A.; Veldhuizen, M.G.; Kamath, V.; Harris, C.; Moran, T.H.; Carnell, S.; Steele, K.E. Taste-related reward is associated with weight loss following bariatric surgery. J. Clin. Investig. 2020, 130, e137772. [Google Scholar] [CrossRef]
- Asano, M.; Hong, G.; Matsuyama, Y.; Wang, W.; Izumi, S.; Toda, T.; Kudo, T.-A. Association of Oral Fat Sensitivity with Body Mass Index, Taste Preference, and Eating Habits in Healthy Japanese Young Adults. Tohoku J. Exp. Med. 2016, 238, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Bolhuis, D.P.; Costanzo, A.; Newman, L.P.; Keast, R.S. Salt Promotes Passive Overconsumption of Dietary Fat in Humans. J. Nutr. 2015, 146, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Heinze, J.M.; Costanzo, A.; Baselier, I.; Fritsche, A.; Frank-Podlech, S.; Keast, R. Detection thresholds for four different fatty stimuli are associated with increased dietary intake of processed high-caloric food. Appetite 2018, 123, 7–13. [Google Scholar] [CrossRef]
- Liang, L.C.; Sakimura, J.; May, D.; Breen, C.; Driggin, E.; Tepper, B.J.; Chung, W.K.; Keller, K.L. Fat discrimination: A phenotype with potential implications for studying fat intake behaviors and obesity. Physiol. Behav. 2011, 105, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Keast, R.S.; Azzopardi, K.M.; Newman, L.P.; Haryono, R.Y. Impaired oral fatty acid chemoreception is associated with acute excess energy consumption. Appetite 2014, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Papantoni, A.; Shearrer, G.E.; Sadler, J.R.; Stice, E.; Burger, K.S. Longitudinal Associations between Taste Sensitivity, Taste Liking, Dietary Intake and BMI in Adolescents. Front. Psychol. 2021, 12, e597704. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S.J. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.E.; Newman, L.P.; Keast, R. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.; Šerý, O.; Plesnik, J.; Daoudi, H.; Rouabah, A.; Rouabah, L.; A Khan, N. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int. J. Obes. 2015, 39, 920–924. [Google Scholar] [CrossRef]
- E Stewart, J.; Seimon, R.V.; Otto, B.; Keast, R.S.; Clifton, P.M.; Feinle-Bisset, C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am. J. Clin. Nutr. 2011, 93, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.M.; Edlinger, C.; Craig, B.A.; Mattes, R.D. Associations between BMI and Fat Taste Sensitivity in Humans. Chem. Senses 2014, 39, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.M.; Nuessle, T.M.; Garneau, N.; Smutzer, G.; Mattes, R.D. No Difference in Perceived Intensity of Linoleic Acid in the Oral Cavity between Obese and Nonobese Individuals. Chem. Senses 2015, 40, 557–563. [Google Scholar] [CrossRef]
- Costanzo, A.; Orellana, L.; Nowson, C.; Duesing, K.; Keast, R. Fat Taste Sensitivity Is Associated with Short-Term and Habitual Fat Intake. Nutrients 2017, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- E Stewart, J.; Keast, R. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2011, 36, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Newman, L.P.; Torres, S.J.; Bolhuis, D.P.; Keast, R.S. The influence of a high-fat meal on fat taste thresholds. Appetite 2016, 101, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Passilly-Degrace, P.; Gaillard, D.; Merlin, J.-F.; Chevrot, M.; Besnard, P. The Lipid-Sensor Candidates CD36 and GPR120 Are Differentially Regulated by Dietary Lipids in Mouse Taste Buds: Impact on Spontaneous Fat Preference. PLoS ONE 2011, 6, e24014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdener, M.H.; Subramaniam, S.; Sundaresan, S.; Sery, O.; Hashimoto, T.; Asakawa, Y.; Besnard, P.; Abumrad, N.A.; Khan, N.A. CD36- and GPR120-Mediated Ca2+ Signaling in Human Taste Bud Cells Mediates Differential Responses to Fatty Acids and Is Altered in Obese Mice. Gastroenterology 2014, 146, 995–1005.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbertson, T.; Liu, L.; York, D.A.; Bray, G.A. Dietary Fat Preferences Are Inversely Correlated with Peripheral Gustatory Fatty Acid Sensitivitya. Ann. N. Y. Acad. Sci. 1998, 855, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.P.; Bolhuis, D.P.; Torres, S.J.; Keast, R.S. Dietary fat restriction increases fat taste sensitivity in people with obesity. Obesity 2016, 24, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, A.; Liu, D.; Nowson, C.; Duesing, K.; Archer, N.; Bowe, S.; Keast, R. A low-fat diet up-regulates expression of fatty acid taste receptor gene FFAR4 in fungiform papillae in humans: A co-twin randomised controlled trial. Br. J. Nutr. 2019, 122, 1212–1220. [Google Scholar] [CrossRef]
- Gent, J.F.; McBurney, D.H. Time course of gustatory adaptation. Percept. Psychophys. 1978, 23, 171–175. [Google Scholar] [CrossRef]
- Kelley, A.E.; Will, M.J.; Steininger, T.L.; Zhang, M.; Haber, S.N. Restricted daily consumption of a highly palatable food (chocolate EnsureR) alters striatal enkephalin gene expression. Eur. J. Neurosci. 2003, 18, 2592–2598. [Google Scholar] [CrossRef]
- Liu, D.; Archer, N.; Duesing, K.; Hannan, G.; Keast, R. Mechanism of fat taste perception: Association with diet and obesity. Prog. Lipid Res. 2016, 63, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.A.; Dando, R.; Roper, S.D. Autocrine and Paracrine Roles for ATP and Serotonin in Mouse Taste Buds. J. Neurosci. 2009, 29, 13909–13918. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Love-Gregory, L.; Klein, S.; Abumrad, N.A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 2012, 53, 561–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vors, C.; Drai, J.; Gabert, L.; Pineau, G.; Laville, M.; Vidal, H.; Guichard, E.; Michalski, M.-C.; Feron, G. Salivary composition in obese vs. normal-weight subjects: Towards a role in postprandial lipid metabolism? Int. J. Obes. 2015, 39, 1425–1428. [Google Scholar] [CrossRef] [PubMed]
- E Abraham, J.; Maranian, M.J.; Spiteri, I.; Russell, R.; Ingle, S.; Luccarini, C.; Earl, H.M.; Pharoah, P.P.; Dunning, A.M.; Caldas, C. Saliva samples are a viable alternative to blood samples as a source of DNA for high throughput genotyping. BMC Med. Genom. 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, P.; Keast, R.S.J.; Roura, E. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br. J. Nutr. 2017, 118, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, C.; Riso, P.; Laureati, M.; Gargari, G.; Pagliarini, E. Exploring Associations between Interindividual Differences in Taste Perception, Oral Microbiota Composition, and Reported Food Intake. Nutrients 2019, 11, 1167. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, C.; Gargari, G.; Koirala, R.; Laureati, M.; Riso, P.; Guglielmetti, S.; Pagliarini, E. New insights into the relationship between taste perception and oral microbiota composition. Sci. Rep. 2019, 9, 3549. [Google Scholar] [CrossRef] [Green Version]
- Zeigler, C.C.; Persson, G.R.; Wondimu, B.; Marcus, C.; Sobko, T.; Modéer, T. Microbiota in the Oral Subgingival Biofilm Is Associated with Obesity in Adolescence. Obesity 2012, 20, 157–164. [Google Scholar] [CrossRef]
- Hankir, M.; Seyfried, F.; Hintschich, C.A.; Diep, T.A.; Kleberg, K.; Kranz, M.; Deuther-Conrad, W.; Tellez, L.A.; Rullmann, M.; Patt, M.; et al. Gastric Bypass Surgery Recruits a Gut PPAR-α-Striatal D1R Pathway to Reduce Fat Appetite in Obese Rats. Cell Metab. 2017, 25, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Bertino, M.; Beauchamp, G.K.; Engelman, K. Increasing dietary salt alters salt taste preference. Physiol. Behav. 1986, 38, 203–213. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X. Salt taste preference, sodium intake and gastric cancer in China. Asian Pacific journal of cancer pre-vention. Asian Pac. J. Cancer Prev. 2011, 12, 1207–1210. [Google Scholar] [PubMed]
- Sartor, F.; Donaldson, L.; Markland, D.A.; Loveday, H.; Jackson, M.; Kubis, H.-P. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite 2011, 57, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Skrandies, W.; Zschieschang, R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theunissen, M.; Polet, I.; Kroeze, J.; Schifferstein, H. Taste adaptation during the eating of sweetened yogurt. Appetite 2000, 34, 21–27. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Finkbeiner, S.; Beauchamp, G.K.; Mennella, J.A. Obese Women Have Lower Monosodium Glutamate Taste Sensitivity and Prefer Higher Concentrations Than Do Normal-weight Women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite 2016, 111, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Azinge, E.C.; Sofola, O.; O Silva, B. Relationship between salt intake, salt-taste threshold and blood pressure in Nigerians. West Afr. J. Med. 2011, 30, 373–376. [Google Scholar]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.-P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005, 115, 3177–3184. [Google Scholar] [CrossRef] [Green Version]
- Besnard, P. Lipids and obesity: Also a matter of taste? Rev. Endocr. Metab. Disord. 2016, 17, 159–170. [Google Scholar] [CrossRef]
- Khan, A.S.; Keast, R.; Khan, N.A. Preference for dietary fat: From detection to disease. Prog. Lipid Res. 2020, 78, 101032. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Townsend, R.L.; Patterson, L.M.; Berthoud, H.-R. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R1267–R1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevrot, M.; Bernard, A.; Ancel, D.; Buttet, M.; Martin, C.; Abdoul-Azize, S.; Merlin, J.-F.; Poirier, H.; Niot, I.; Khan, N.A.; et al. Obesity alters the gustatory perception of lipids in the mouse: Plausible involvement of lingual CD36. J. Lipid Res. 2013, 54, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scruggs, D.M.; Buffington, C.; Cowan, G.S.M., Jr. Taste Acuity of the Morbidly Obese before and after Gastric Bypass Surgery. Obes. Surg. 1994, 4, 24–28. [Google Scholar] [CrossRef]
- Mattes, R.D. Oral fatty acid signaling and intestinal lipid processing: Support and supposition. Physiol. Behav. 2011, 105, 27–35. [Google Scholar] [CrossRef]
- Tucker, R.M.; Laguna, L.; Quinn, R.; Mattes, R.D. The Effect of Short, Daily Oral Exposure on Non-esterified Fatty Acid Sensitivity. Chemosens. Percept. 2013, 6, 78–85. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Y.; Parker, J.K.; Kennedy, O.B.; Methven, L. Relative Effects of Sensory Modalities and Importance of Fatty Acid Sensitivity on Fat Perception in a Real Food Model. Chemosens. Percept. 2016, 9, 105–119. [Google Scholar] [CrossRef] [Green Version]
- Vignini, A.; Borroni, F.; Sabbatinelli, J.; Pugnaloni, S.; Alia, S.; Taus, M.; Ferrante, L.; Mazzanti, L.; Fabri, M. General Decrease of Taste Sensitivity Is Related to Increase of BMI: A Simple Method to Monitor Eating Behavior. Dis. Markers 2019, 2019, 2978026. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.M.; Kaiser, K.A.; Parman, M.A.; George, B.J.; Allison, D.B.; Mattes, R.D. Comparisons of Fatty Acid Taste Detection Thresholds in People Who Are Lean vs. Overweight or Obese: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169583. [Google Scholar] [CrossRef]
- Cox, D.N.; Hendrie, G.A.; Carty, D. Sensitivity, hedonics and preferences for basic tastes and fat amongst adults and children of differing weight status: A comprehensive review. Food Qual. Prefer. 2016, 48, 359–367. [Google Scholar] [CrossRef]
- Martínez-Ruiz, N.R.; López-Díaz, J.A.; Wall-Medrano, A.; Jiménez-Castro, J.A.; Angulo, O. Oral fat perception is related with body mass index, preference and consumption of high-fat foods. Physiol. Behav. 2014, 129, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kindleysides, S.; Beck, K.L.; Walsh, D.C.I.; Henderson, L.; Jayasinghe, S.N.; Golding, M.; Breier, B.H. Fat Sensation: Fatty Acid Taste and Olfaction Sensitivity and the Link with Disinhibited Eating Behaviour. Nutrients 2017, 9, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, A.; Kim, J.; Noel, C.; Dando, R. Taste loss with obesity in mice and men. Int. J. Obes. 2019, 44, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Liang, L.C.; Sakimura, J.; May, D.; van Belle, C.; Breen, C.; Driggin, E.; Tepper, B.J.; Lanzano, P.C.; Deng, L.; et al. Common Variants in the CD36 Gene Are Associated with Oral Fat Perception, Fat Preferences, and Obesity in African Americans. Obesity 2012, 20, 1066–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, T.; Kubow, S.; Nielsen, D.E. Common variants in the CD36 gene are associated with dietary fat intake, high-fat food consumption and serum triglycerides in a cohort of Quebec adults. Int. J. Obes. 2021, 45, 1193–1202. [Google Scholar] [CrossRef]
- Drewnowski, A.; Brunzell, J.D.; Sande, K.; Iverius, P.H.; Greenwood, M.R. Sweet tooth reconsidered: Taste responsiveness in human obesity. Physiol. Behav. 1985, 35, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Mela, D.J.; A Sacchetti, D. Sensory preferences for fats: Relationships with diet and body composition. Am. J. Clin. Nutr. 1991, 53, 908–915. [Google Scholar] [CrossRef]
- Ricketts, C. Fat preferences, dietary fat intake and body composition in children. Eur. J. Clin. Nutr. 1997, 51, 778–781. [Google Scholar] [CrossRef] [Green Version]
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998, 68, 1157–1173. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Bunn, D.; Brown, T.; Summerbell, C.D.; Skeaff, C.M. Effects of total fat intake on body weight. Cochrane Database Syst. Rev. 2015, CD011834. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Lenard, N.R.; Shin, A.C.; Berthoud, H.-R. Appetite control and energy balance regulation in the modern world: Reward-driven brain overrides repletion signals. Int. J. Obes. 2009, 33, S8–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.-J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Stice, E.; Spoor, S.; Bohon, C.; Veldhuizen, M.G.; Small, D.M. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008, 117, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuominen, L.; Tuulari, J.; Karlsson, H.; Hirvonen, J.; Helin, S.; Salminen, P.; Parkkola, R.; Hietala, J.; Nuutila, P.; Nummenmaa, L. Aberrant mesolimbic dopamine–opiate interaction in obesity. NeuroImage 2015, 122, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Roux, C.L. Bariatric surgery and taste: Novel mechanisms of weight loss. Curr. Opin. Gastroenterol. 2010, 26, 140–145. [Google Scholar] [CrossRef]
- Primeaux, S.D.; De Silva, T.; Tzeng, T.H.; Chiang, M.C.; Hsia, D.S. Recent advances in the modification of taste and food preferences following bariatric surgery. Rev. Endocr. Metab. Disord. 2016, 17, 195–207. [Google Scholar] [CrossRef]
- Coughlin, K.; Bell, R.M.; Bivins, B.A.; Wrobel, S.; Griffen, W.O. Preoperative and Postoperative Assessment of Nutrient Intakes in Patients Who Have Undergone Gastric Bypass Surgery. Arch. Surg. 1983, 118, 813–816. [Google Scholar] [CrossRef]
- Kruseman, M.; Leimgruber, A.; Zumbach, F.; Golay, A. Dietary, Weight, and Psychological Changes among Patients with Obesity, 8 Years after Gastric Bypass. J. Am. Diet. Assoc. 2010, 110, 527–534. [Google Scholar] [CrossRef]
- le Roux, C.W.; Bueter, M.; Theis, N.; Werling, M.; Ashrafian, H.; Löwenstein, C.; Athanasiou, T.; Bloom, S.R.; Spector, A.C.; Olbers, T.; et al. Gastric bypass reduces fat intake and preference. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R1057–R1066. [Google Scholar] [CrossRef] [Green Version]
- Olbers, T.; Björkman, S.; Lindroos, A.; Maleckas, A.; Lönn, L.L.; Sjöström, L.; Lönroth, H. Body Composition, Dietary Intake, and Energy Expenditure After Laparoscopic Roux-en-Y Gastric Bypass and Laparoscopic Vertical Banded Gastroplasty. Ann. Surg. 2006, 244, 715–722. [Google Scholar] [CrossRef]
- Bernard, A.; Bihan, J.L.B.-L.; Radoi, L.; Coupaye, M.; Sami, O.; Casanova, N.; Le May, C.; Collet, X.; Delaby, P.; Le Bourgot, C.; et al. Orosensory Perception of Fat/Sweet Stimuli and Appetite-Regulating Peptides before and after Sleeve Gastrectomy or Gastric Bypass in Adult Women with Obesity. Nutrients 2021, 13, 878. [Google Scholar] [CrossRef] [PubMed]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.-H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattes, R.D. Fat preference and adherence to a reduced-fat diet. Am. J. Clin. Nutr. 1993, 57, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ledikwe, J.H.; Ello-Martin, J.; Pelkman, C.L.; Birch, L.L.; Mannino, M.L.; Rolls, B.J. A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet. Appetite 2007, 49, 74–83. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Andersen, I.N.S.; Lange, B.; Ritz, C.; Le Roux, C.W.; Schmidt, J.B.; Sjödin, A.; Bredie, W.L. Bariatric Surgery Leads to Short-Term Effects on Sweet Taste Sensitivity and Hedonic Evaluation of Fatty Food Stimuli. Obesity 2019, 27, 1796–1804. [Google Scholar] [CrossRef]
- Behary, P.; Miras, A. Food preferences and underlying mechanisms after bariatric surgery. Proc. Nutr. Soc. 2015, 74, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Zakeri, R.; Batterham, R.L. Potential mechanisms underlying the effect of bariatric surgery on eating behaviour. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 3–11. [Google Scholar] [CrossRef]
- Shoar, S.; Naderan, M.; Shoar, N.; Modukuru, V.R.; Mahmoodzadeh, H. Alteration Pattern of Taste Perception After Bariatric Surgery: A Systematic Review of Four Taste Domains. Obes. Surg. 2019, 29, 1542–1550. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 2013, 22, E13–E20. [Google Scholar] [CrossRef]
- Nance, K.; Eagon, J.C.; Klein, S.; Pepino, M.Y. Effects of Sleeve Gastrectomy vs. Roux-en-Y Gastric Bypass on Eating Behavior and Sweet Taste Perception in Subjects with Obesity. Nutrients 2017, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- El Labban, S.; Safadi, B.; Olabi, A. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on taste acuity and sweetness acceptability in postsurgical subjects. Nutrition 2016, 32, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Bueter, M.; Miras, A.D.; Chichger, H.; Fenske, W.; Ghatei, M.A.; Bloom, S.R.; Unwin, R.J.; Lutz, T.; Spector, A.C.; le Roux, C.W. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 2011, 104, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.; Penney, N.; Darzi, A.; Purkayastha, S. Taste Changes after Bariatric Surgery: A Systematic Review. Obes. Surg. 2018, 28, 3321–3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melis, M.; Pintus, S.; Mastinu, M.; Fantola, G.; Moroni, R.; Pepino, M.Y.; Barbarossa, I.T. Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes. Nutrients 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Holinski, F.; Menenakos, C.; Haber, G.; Olze, H.; Ordemann, J. Olfactory and Gustatory Function After Bariatric Surgery. Obes. Surg. 2015, 25, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Altun, H.; Hanci, D.; Altun, H.; Batman, B.; Serin, R.K.; Karip, A.B.; Akyuz, U. Improved Gustatory Sensitivity in Morbidly Obese Patients After Laparoscopic Sleeve Gastrectomy. Ann. Otol. Rhinol. Laryngol. 2016, 125, 536–540. [Google Scholar] [CrossRef]
- Nance, K.; Acevedo, M.B.; Pepino, M.Y. Changes in taste function and ingestive behavior following bariatric surgery. Appetite 2020, 146, 104423. [Google Scholar] [CrossRef]
- A Kenler, H.; E Brolin, R.; Cody, R.P. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am. J. Clin. Nutr. 1990, 52, 87–92. [Google Scholar] [CrossRef]
- Burge, J.C.; Schaumburg, J.Z.; Choban, P.S.; DiSilvestro, R.A.; Flancbaum, L. Changes in Patients’ Taste Acuity after Roux-en-Y Gastric Bypass for Clinically Severe Obesity. J. Am. Diet. Assoc. 1995, 95, 666–670. [Google Scholar] [CrossRef]
- Mathes, C.M.; Spector, A.C. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: A direct-measures approach. Physiol. Behav. 2012, 107, 476–483. [Google Scholar] [CrossRef]
- Münzberg, H.; Laque, A.; Yu, S.; Rezai-Zadeh, K.; Berthoud, H.-R. Appetite and body weight regulation after bariatric surgery. Obes. Rev. 2015, 16, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vuuren, M.A.J.; Strodl, E.; White, K.; Lockie, P.D. Taste, Enjoyment, and Desire of Flavors Change After Sleeve Gastrectomy-Short Term Results. Obes. Surg. 2016, 27, 1466–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tichansky, D.S.; Boughter, J.D.; Madan, A.K. Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg. Obes. Relat. Dis. 2006, 2, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Zerrweck, C.; Zurita, L.; Álvarez, G.; Maydón, H.G.; Sepúlveda, E.M.; Campos, F.; Caviedes, A.; Guilbert, L. Taste and Olfactory Changes Following Laparoscopic Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2015, 26, 1296–1302. [Google Scholar] [CrossRef]
- Kittrell, H.; Graber, W.; Mariani, E.; Czaja, K.; Hajnal, A.; Di Lorenzo, P.M. Taste and odor preferences following Roux-en-Y surgery in humans. PLoS ONE 2018, 13, e0199508. [Google Scholar] [CrossRef]
- Kapoor, N.; Al-Najim, W.; Le Roux, C.W.; Docherty, N.G. Shifts in Food Preferences After Bariatric Surgery: Observational Reports and Proposed Mechanisms. Curr. Obes. Rep. 2017, 6, 246–252. [Google Scholar] [CrossRef]
- Puputti, S.; Hoppu, U.; Sandell, M. Taste Sensitivity is Associated with Food Consumption Behavior but not with Recalled Pleasantness. Foods 2019, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Tepper, B.J. Nutritional Implications of Genetic Taste Variation: The Role of PROP Sensitivity and Other Taste Phenotypes. Annu. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Pecore, S.D. Taste perception of sodium chloride in relation to dietary intake of salt. Am. J. Clin. Nutr. 1982, 35, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, H.; Zheng, H.; Shin, A.C. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Ann. N. Y. Acad. Sci. 2012, 1264, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Fushiki, T. Why fat is so preferable: From oral fat detection to inducing reward in the brain. Biosci. Biotechnol. Biochem. 2014, 78, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Individual differences in the neurophysiology of reward and the obesity epidemic. Int. J. Obes. 2009, 33, S44–S48. [Google Scholar] [CrossRef] [Green Version]
- Berridge, K.C.; Ho, C.-Y.; Richard, J.; DiFeliceantonio, A. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010, 1350, 43–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weltens, N.; Zhao, D.; Van Oudenhove, L. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol. Motil. 2014, 26, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Berthoud, H.-R. Food reward functions as affected by obesity and bariatric surgery. Int. J. Obes. 2011, 35, S40–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, B.; Haburcak, M.; Avena, N.; Moyer, M.; Hoebel, B.; Pothos, E. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 2009, 159, 1193–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoshuk, L.M.; Duffy, V.B.; E Hayes, J.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Payne, T.; Kronenbuerger, M.; Wong, G. Gustatory Testing; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ribeiro, G.; Oliveira-Maia, A.J. Sweet taste and obesity. Eur. J. Intern. Med. 2021, 92, 3–10. [Google Scholar] [CrossRef]
- Heinze, J.M.; Preissl, H.; Fritsche, A.; Frank-Podlech, S. Controversies in fat perception. Physiol. Behav. 2015, 152, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Drewnowski, A.; Kurth, C.; Holden-Wiltse, J.; Saari, J. Food preferences in human obesity: Carbohydrates versus fats. Appetite 1992, 18, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, S.; Monteleone, E. Food Preferences and Obesity. Endocrinol. Metab. 2021, 36, 209–219. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Whisenhunt, B.L.; Williamson, D.A.; Greenway, F.L.; Netemeyer, R.G. Development and Validation of the Food-Craving Inventory. Obes. Res. 2002, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lanfer, A.; Knof, K.; Barba, G.; Veidebaum, T.; Papoutsou, S.; de Henauw, S.; Soós, T.; Moreno, L.A.; Ahrens, W.; Lissner, L. Taste preferences in association with dietary habits and weight status in European children: Results from the IDEFICS study. Int. J. Obes. 2012, 36, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leohr, J.; Kjellsson, M.C. Sweet/Fat Preference Taste in Subjects Who are Lean, Obese and Very Obese. Pharm. Res. 2020, 37, 244. [Google Scholar] [CrossRef]
- Lampuré, A.; Castetbon, K.; Deglaire, A.; Schlich, P.; Péneau, S.; Hercberg, S.; Méjean, C. Associations between liking for fat, sweet or salt and obesity risk in French adults: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.; Alonso-Alonso, M.; Audette, M.; Malbert, C.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Carnell, S.; Gibson, C.; Benson, L.; Ochner, C.N.; Geliebter, A. Neuroimaging and obesity: Current knowledge and future directions. Obes. Rev. 2012, 13, 43–56. [Google Scholar] [CrossRef]
- Ng, J.; Stice, E.; Yokum, S.; Bohon, C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 2011, 57, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.; Melhorn, S.J.; Smeraglio, A.; Tyagi, V.; Grabowski, T.; Schwartz, M.W.; A Schur, E. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am. J. Clin. Nutr. 2012, 96, 989–999. [Google Scholar] [CrossRef] [Green Version]
- Tryon, M.S.; Carter, C.S.; DeCant, R.; Laugero, K.D. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol. Behav. 2013, 120, 233–242. [Google Scholar] [CrossRef]
- Demos, K.E.; Heatherton, T.F.; Kelley, W.M. Individual Differences in Nucleus Accumbens Activity to Food and Sexual Images Predict Weight Gain and Sexual Behavior. J. Neurosci. 2012, 32, 5549–5552. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Hinton, E.C.; Parkinson, J.A.; Lawrence, A.D. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage 2012, 63, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Bullmore, E.; Keogh, J.; Gillard, J.; O’Rahilly, S.; Fletcher, P.C. Leptin Regulates Striatal Regions and Human Eating Behavior. Science 2007, 317, 1355. [Google Scholar] [CrossRef] [Green Version]
- Vollmert, C.; Grosshans, M.; Vollstädt-Klein, S.; Tost, H.; Leber, S.; Bach, P.; Bühler, M.; Von Der Goltz, C.; Mutschler, J.; Loeber, S.; et al. Association of Leptin with Food Cue–Induced Activation in Human Reward Pathways. Arch. Gen. Psychiatry 2012, 69, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batterham, R.L.; Ffytche, D.H.; Rosenthal, J.M.; Zelaya, F.O.; Barker, G.J.; Withers, D.J.; Williams, S.C.R. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007, 450, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Simmons, W.K.; Herscovitch, P.; Martin, A.; Hall, K.D. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol. Psychiatry 2014, 19, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.P.; Kessler, R.M.; Feurer, I.D.; Volkow, N.D.; Patterson, B.W.; Ansari, M.S.; Li, R.; Marks-Shulman, P.; Abumrad, N.N. Relationship of Dopamine Type 2 Receptor Binding Potential with Fasting Neuroendocrine Hormones and Insulin Sensitivity in Human Obesity. Diabetes Care 2012, 35, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Eisenstein, S.A.; Antenor-Dorsey, J.A.V.; Gredysa, D.M.; Koller, J.M.; Bihun, E.C.; Ranck, S.A.; Arbeláez, A.M.; Klein, S.; Perlmutter, J.S.; Moerlein, S.; et al. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[11C]methyl)benperidol. Synapse 2013, 67, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Pak, K.; Kim, S.-J.; Kim, I.J. Obesity and Brain Positron Emission Tomography. Nucl. Med. Mol. Imaging 2017, 52, 16–23. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2012, 14, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, P.J. Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron 2011, 69, 664–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.C.; Dixon, J.B. Food for Thought: Reward Mechanisms and Hedonic Overeating in Obesity. Curr. Obes. Rep. 2017, 6, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.-J.; Morris, M.J. The role of reward circuitry and food addiction in the obesity epidemic: An update. Biol. Psychol. 2018, 131, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.T.; Jakobsen, T.A.; Nielsen, M.S.; Sjödin, A.; Le Roux, C.W.; Schmidt, J.B. Hedonic Changes in Food Choices Following Roux-en-Y Gastric Bypass. Obes. Surg. 2016, 26, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, S.; Miras, A.; Chhina, N.; Prechtl, C.G.; Sleeth, M.L.; Daud, N.M.; Ismail, N.A.; Durighel, G.; Ahmed, A.R.; Olbers, T.; et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2013, 63, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Tellez, L.A.; Niu, J.; Medina, S.; Ferreira, T.; Zhang, X.; Su, J.; Tong, J.; Schwartz, G.J.; Pol, A.V.D.; et al. Striatal Dopamine Links Gastrointestinal Rerouting to Altered Sweet Appetite. Cell Metab. 2015, 23, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Frank, G.K. Neuroimaging and eating disorders. Curr. Opin. Psychiatry 2019, 32, 478–483. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Reynolds, J.R.; E Shott, M.; Jappe, L.; Yang, T.T.; Tregellas, J.R.; O’Reilly, R. Anorexia Nervosa and Obesity are Associated with Opposite Brain Reward Response. Neuropsychopharmacology 2012, 37, 2031–2046. [Google Scholar] [CrossRef]
- Olivo, G.; Gaudio, S.; Schiöth, H.B. Brain and Cognitive Development in Adolescents with Anorexia Nervosa: A Systematic Review of fMRI Studies. Nutrients 2019, 11, 1907. [Google Scholar] [CrossRef] [Green Version]
- Södersten, P.; Bergh, C.; Leon, M.; Zandian, M. Dopamine and anorexia nervosa. Neurosci. Biobehav. Rev. 2016, 60, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.K.; Joseph, P.V.; Feldman, D.E.; Kroll, D.S.; Burns, J.A.; Manza, P.; Volkow, N.D.; Wang, G.-J. Brain Imaging of Taste Perception in Obesity: A Review. Curr. Nutr. Rep. 2019, 8, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.Y.; Zeffiro, T.A. Hunger and BMI modulate neural responses to sweet stimuli: FMRI meta-analysis. Int. J. Obes. 2020, 44, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Brondel, L.; Jacquin, A.; Meillon, S.; Pénicaud, L. Le goût: Physiologie, rôles et dysfonctionnements. Nutrition Clinique et Métabolisme 2013, 27, 123–133. [Google Scholar] [CrossRef]
- Mattes-Kulig, D.A.; Henkin, R.I. Energy and nutrient consumption of patients with dysgeusia. J. Am. Diet. Assoc. 1985, 85, 822–826. [Google Scholar] [CrossRef]
- Davis, C.; Strachan, S.; Berkson, M. Sensitivity to reward: Implications for overeating and overweight. Appetite 2004, 42, 131–138. [Google Scholar] [CrossRef]
- Han, P.; Bagenna, B.; Fu, M. The sweet taste signalling pathways in the oral cavity and the gastrointestinal tract affect human appetite and food intake: A review. Int. J. Food Sci. Nutr. 2018, 70, 125–135. [Google Scholar] [CrossRef]
- Schiff, S.; Amodio, P.; Testa, G.; Nardi, M.; Montagnese, S.; Caregaro, L.; di Pellegrino, G.; Sellitto, M. Impulsivity toward food reward is related to BMI: Evidence from intertemporal choice in obese and normal-weight individuals. Brain Cogn. 2016, 110, 112–119. [Google Scholar] [CrossRef]
- Berg, L.V.D.; Pieterse, K.; Malik, J.; Luman, M.; Van Dijk, K.W.; Oosterlaan, J.; Waal, H.A.D.-V.D. Association between impulsivity, reward responsiveness and body mass index in children. Int. J. Obes. 2011, 35, 1301–1307. [Google Scholar] [CrossRef] [Green Version]
- Neseliler, S.; Han, J.-E.; Dagher, A. The Use of Functional Magnetic Resonance Imaging in the Study of Appetite and Obesity. In Appetite and Food Intake, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 117–134. [Google Scholar] [CrossRef]
- López, A.L.O.; Johnson, L. Associations between Restrained Eating and the Size and Frequency of Overall Intake, Meal, Snack and Drink Occasions in the UK Adult National Diet and Nutrition Survey. PLoS ONE 2016, 11, e0156320. [Google Scholar] [CrossRef] [Green Version]
- Elfhag, K.; Morey, L. Personality traits and eating behavior in the obese: Poor self-control in emotional and external eating but personality assets in restrained eating. Eat. Behav. 2008, 9, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.C.; Steele, T.J.; Gwinner, M.; Rosenkranz, S.K.; Kirkpatrick, K. The relationship between dietary fat intake, impulsive choice, and metabolic health. Appetite 2021, 165, 105292. [Google Scholar] [CrossRef] [PubMed]
- Gero, D.; Steinert, R.E.; Le Roux, C.W.; Bueter, M. Do Food Preferences Change After Bariatric Surgery? Curr. Atheroscler. Rep. 2017, 19, 38. [Google Scholar] [CrossRef]
- Woods, S.C.; Langhans, W. Inconsistencies in the assessment of food intake. Am. J. Physiol. Metab. 2012, 303, E1408–E1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, C.; Siegrist, M. Does personality influence eating styles and food choices? Direct and indirect effects. Appetite 2015, 84, 128–138. [Google Scholar] [CrossRef]
- Levin, B.E.; Routh, V.H. Role of the brain in energy balance and obesity. Am. J. Physiol. Integr. Comp. Physiol. 1996, 271, R491–R500. [Google Scholar] [CrossRef]
- Wang, G.-J.; Volkow, N.D.; Fowler, J.S. The role of dopamine in motivation for food in humans: Implications for obesity. Expert Opin. Ther. Targets 2002, 6, 601–609. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Hebebrand, J. The concept of “food addiction” helps inform the understanding of overeating and obesity: Debate Consensus. Am. J. Clin. Nutr. 2021, 113, 274–276. [Google Scholar] [CrossRef] [PubMed]
- López, L.M.; Contreras-Rodriguez, O.; Soriano-Mas, C.; Stamatakis, E.A.; Verdejo-Garcia, A. Disrupted functional connectivity in adolescent obesity. NeuroImage Clin. 2016, 12, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.M.; Tse, N.Y.; Chen, Y.; Henning, E.; Hodges, J.R.; Kiernan, M.C.; Irish, M.; Farooqi, I.S.; Piguet, O. Neural correlates of fat preference in frontotemporal dementia: Translating insights from the obesity literature. Ann. Clin. Transl. Neurol. 2021, 8, 1318–1329. [Google Scholar] [CrossRef]
- Jiang, T.; Soussignan, R.; Schaal, B.; Royet, J.-P. Reward for food odors: An fMRI study of liking and wanting as a function of metabolic state and BMI. Soc. Cogn. Affect. Neurosci. 2014, 10, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, A.; Green, E.; Haase, L.; Szajer, J.; Murphy, C. Differential Effects of BMI on Brain Response to Odor in Olfactory, Reward and Memory Regions: Evidence from fMRI. Nutrients 2019, 11, 926. [Google Scholar] [CrossRef] [Green Version]
- Roger, C.; Lasbleiz, A.; Guye, M.; Dutour, A.; Gaborit, B.; Ranjeva, J.-P. The Role of the Human Hypothalamus in Food Intake Networks: An MRI Perspective. Front. Nutr. 2022, 8, e760914. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.; Shott, M.E.; DeGuzman, M.C. The Neurobiology of Eating Disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schebendach, J.E.; Klein, D.A.; Mayer, L.E.; Devlin, M.J.; Attia, E.; Walsh, B.T. Assessment of fat taste in individuals with and without anorexia nervosa. Int. J. Eat. Disord. 2013, 47, 215–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, A.M.; Roy, A.; Franks, A.T.; Joseph, P.V. A Systematic Review of Taste Differences Among People with Eating Disorders. Biol. Res. Nurs. 2019, 22, 82–91. [Google Scholar] [CrossRef]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef]
- Davis, C.; Levitan, R.D.; Kaplan, A.S.; Carter, J.; Reid, C.; Curtis, C.; Patte, K.; Hwang, R.; Kennedy, J.L. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 32, 620–628. [Google Scholar] [CrossRef]
- Little, T.J. Oral and gastrointestinal sensing of dietary fat and appetite regulation in humans: Modification by diet and obesity. Front. Neurosci. 2010, 1, 178. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Colquitt, L.; Wise, P.; Breslin, P.A.S.; E Rawson, N.; Genovese, F.; Maina, I.; Joseph, P.; Fomuso, L.; Slade, L.; et al. Studies of Human Twins Reveal Genetic Variation That Affects Dietary Fat Perception. Chem. Senses 2020, 45, 467–481. [Google Scholar] [CrossRef]
- Berland, C.; Montalban, E.; Perrin, E.; Di Miceli, M.; Nakamura, Y.; Martinat, M.; Sullivan, M.; Davis, X.S.; Shenasa, M.A.; Martin, C.; et al. Circulating Triglycerides Gate Dopamine-Associated Behaviors through DRD2-Expressing Neurons. Cell Metab. 2020, 31, 773–790.e11. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.W.; Fordahl, S.C. Obesity and dietary fat influence dopamine neurotransmission: Exploring the convergence of metabolic state, physiological stress, and inflammation on dopaminergic control of food intake. Nutr. Res. Rev. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Miyagawa, K.; Mizuo, K.; Yoshida, T.; Suzuki, T. Changes in central dopaminergic systems and morphine reward by prenatal and neonatal exposure to bisphenol-A in mice: Evidence for the importance of exposure period. Addict. Biol. 2007, 12, 167–172. [Google Scholar] [CrossRef] [PubMed]
- DelParigi, A.; Chen, K.; Salbe, A.D.; O Hill, J.; Wing, R.R.; Reiman, E.M.; A Tataranni, P. Persistence of abnormal neural responses to a meal in postobese individuals. Int. J. Obes. 2003, 28, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Speakman, J.R.; Levitsky, D.; Allison, D.; Bray, M.S.; de Castro, J.M.; Clegg, D.J.; Clapham, J.C.; Dulloo, A.; Gruer, L.; Haw, S.; et al. Set points, settling points and some alternative models: Theoretical options to understand how genes and environments combine to regulate body adiposity. Dis. Model. Mech. 2011, 4, 733–745. [Google Scholar] [CrossRef] [Green Version]
- Running, C.A.; Mattes, R.D.; Tucker, R.M. Fat taste in humans: Sources of within- and between-subject variability. Prog. Lipid Res. 2013, 52, 438–445. [Google Scholar] [CrossRef]
- Drewnowski, A.; Kurth, C.L.; E Rahaim, J. Taste preferences in human obesity: Environmental and familial factors. Am. J. Clin. Nutr. 1991, 54, 635–641. [Google Scholar] [CrossRef]
- Frijters, J.E.R.; Rasmussen-Conrad, E.L. Sensory Discrimination, Intensity Perception, and Affective Judgment of Sucrose-Sweetness in the Overweight. J. Gen. Psychol. 1982, 107, 233–247. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brondel, L.; Quilliot, D.; Mouillot, T.; Khan, N.A.; Bastable, P.; Boggio, V.; Leloup, C.; Pénicaud, L. Taste of Fat and Obesity: Different Hypotheses and Our Point of View. Nutrients 2022, 14, 555. https://doi.org/10.3390/nu14030555
Brondel L, Quilliot D, Mouillot T, Khan NA, Bastable P, Boggio V, Leloup C, Pénicaud L. Taste of Fat and Obesity: Different Hypotheses and Our Point of View. Nutrients. 2022; 14(3):555. https://doi.org/10.3390/nu14030555
Chicago/Turabian StyleBrondel, Laurent, Didier Quilliot, Thomas Mouillot, Naim Akhtar Khan, Philip Bastable, Vincent Boggio, Corinne Leloup, and Luc Pénicaud. 2022. "Taste of Fat and Obesity: Different Hypotheses and Our Point of View" Nutrients 14, no. 3: 555. https://doi.org/10.3390/nu14030555






