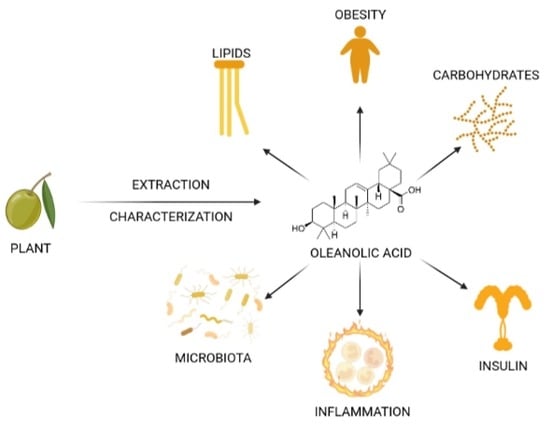
Oleanolic Acid: Extraction, Characterization and Biological Activity
Abstract
:1. Introduction
2. Oleanolic Acid Characterization
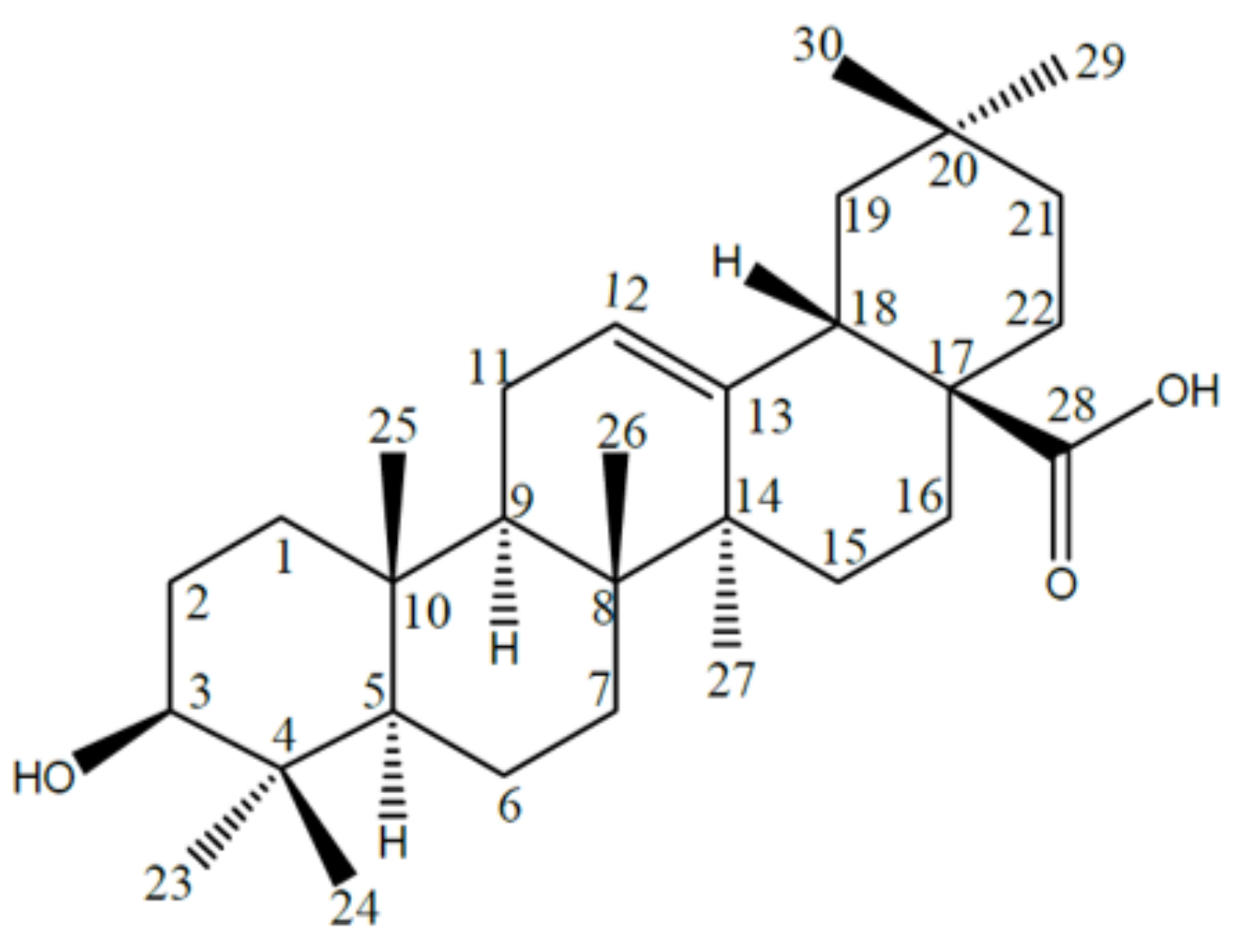
2.1. Names, Structure and Identifiers
2.2. Chemical and Physical Properties
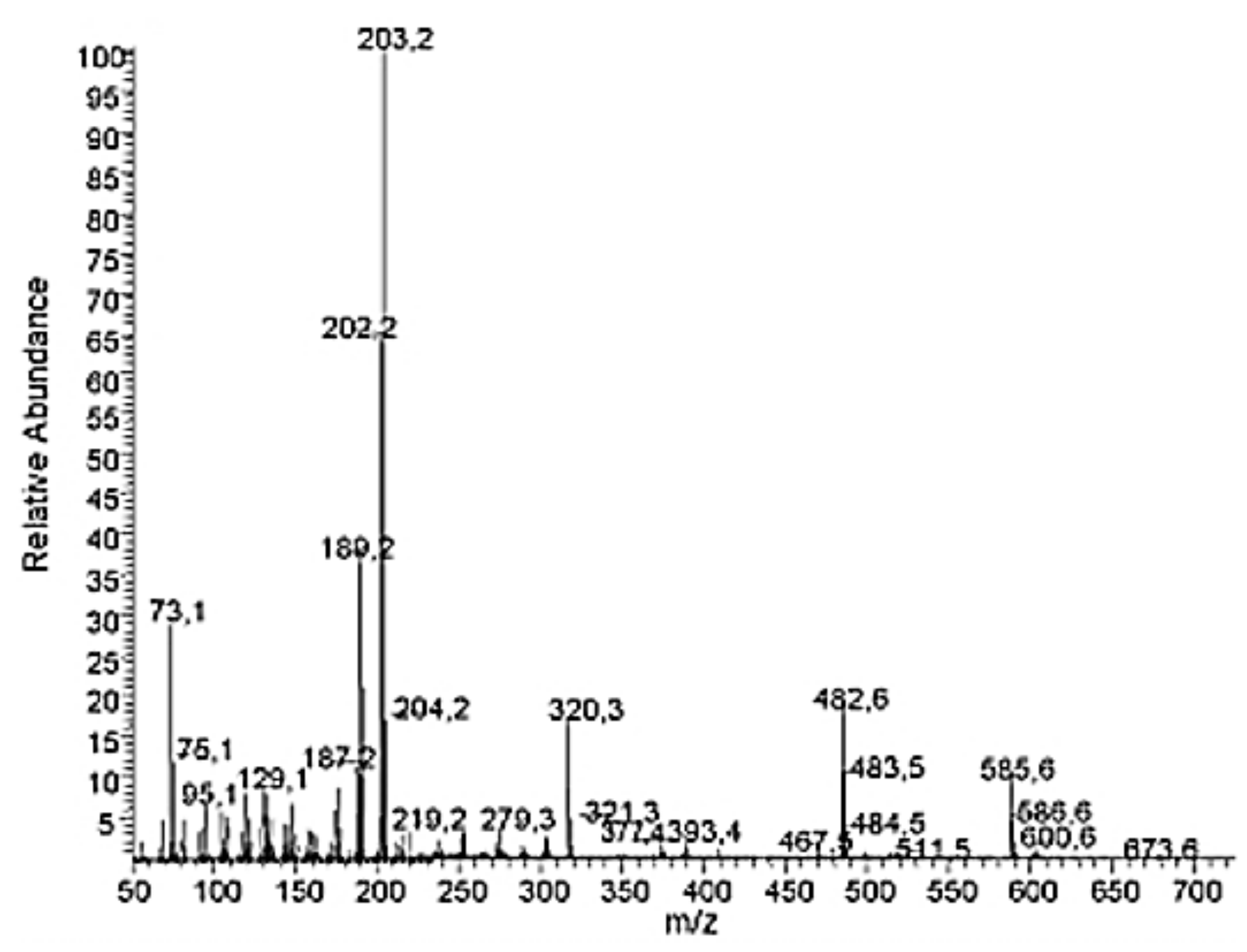
2.3. Spectral Information
3. Oleanolic Acid Extraction
3.1. Ultrasound-Assisted Extraction of OA
3.2. Microwave-Assisted Extraction of OA
3.3. Supercritical Carbon Dioxide Extraction of OA
4. Quantitative Analysis Techniques for OA
5. Oleanolic Acid Biosynthesis
6. Food Sources of OA
7. Biological Activity of OA
7.1. Absorption, Distribution, Metabolism and Excretion (ADME) of OA
7.2. OA Effects on Lipid Profile and Obesity
7.2.1. Animal Models
7.2.2. Transcriptional Gene Expression
7.2.3. Effects in Humans
7.3. OA Effects on Carbohydrate Metabolism
7.3.1. Activation of the β-Cell M3 Muscarinic Receptors
7.3.2. Agonist Action on the TGR5 Receptor
7.3.3. Enhancement of the Shp-2 Enzyme Activity
7.3.4. Promotion of β-Cell Survival and Proliferation
7.4. Improvement of the Insulin Signaling
7.4.1. Insulin-Mimetic Effect as IR Co-Activator
7.4.2. Inhibition of Protein-Tyrosine Phosphatases PTP1B and TCPTP
7.4.3. Activation of PI3K/Akt
7.4.4. Activation of LKB1/AMPK
7.4.5. Inhibition of Glycogen Synthase Kinase-3β
7.4.6. Effects on the Glycogen Pool
7.5. Effects on Metabolic Syndrome Components
7.5.1. Hypotensive Effect
7.5.2. Improvement of Hyperglycemia and Insulin Resistance
7.5.3. Inhibition of Polyol Pathway and AGEs
7.5.4. Alleviation of the Oxidative Stress-Induced Insulin Resistance
7.6. Oleanolic Acid and Gut Microbiota
7.7. Effects on Inflammatory Mediators
8. A Unifying Hypothesis of the Oleanolic Acid Pharmacological Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, H.; Wu, H.; Zheng, Y.; Xi, J.; Chow, A.H.; Chan, C.K. Physical characterization of oleanolic acid nonsolvate and solvates prepared by solvent recrystallization. Int. J. Pharm. 2008, 355, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Neil, M.J.; Smith, A.; Heckelman, P.E. The Merck Index, 13th ed.; Merck & Co., Inc.: Kenilworth, NJ, USA, 2001. [Google Scholar]
- Albi, T.; Guinda, A.; Lanzon, A. Obtaining procedure and determination of terpenic acids of olive leaf (Olea europaea). Grasas Aceites 2001, 52, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.C.; Jain, C.L.; Nigam, S.; Padhi, M.M. Rapid extraction, isolation, and quantification of oleanolic acid from Lantana camara L. Roots using microwave and HPLC-PDA techniques. Acta Chromatogr. 2013, 25, 181–199. [Google Scholar] [CrossRef]
- Guinda, Á.; Pérez-Camino, M.C.; Lanzón, A. Supplementation of oils with oleanolic acid from the olive leaf (Olea europaea). Eur. J. Lipid Sci. Technol. 2004, 106, 22–26. [Google Scholar] [CrossRef]
- Tostes, J.B.D.F.; Nakamura, M.J.; de Saboya, C.G.F.; Mazzei, J.L.; Siani, A.C. Efficient and selective method to separate triterpene acids by direct treatment of apple peels with alkaline ethanol. Sep. Sci. Technol. 2016, 51, 1986–1993. [Google Scholar] [CrossRef]
- Jin, I.J.; Ko, Y.I.; Kim, Y.M.; Han, S.K. Solubilization of oleanolic acid and ursolic acid by cosolvency. Arch. Pharmacal Res. 1997, 20, 269–274. [Google Scholar] [CrossRef]
- Jäger, S.; Winkler, K.; Pfüller, U.; Scheffler, A. Solubility Studies of Oleanolic Acid and Betulinic Acid in Aqueous Solutions and Plant Extracts of Viscum album L. Planta Med. 2007, 73, 157–162. [Google Scholar] [CrossRef]
- Schneider, P.; Hosseiny, S.; Szczotka, M.; Jordan, V.; Schlitter, K. Rapid solubility determination of the triterpenes oleanolic acid and ursolic acid by UV-spectroscopy in different solvents. Phytochem. Lett. 2009, 2, 85–87. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Yu, Y.-Y.; Xu, X.-R.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Ultrason. Sonochem. 2012, 19, 772–776. [Google Scholar] [CrossRef]
- Schneider, P.; Bischoff, F.; Müller, U.; Bart, H.-J.; Schlitter, K.; Jordan, V. Plant Extraction with Aqueous Two-Phase Systems. Chem. Eng. Technol. 2011, 34, 452–458. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Liang, Z.; Wang, W. Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason. Sonochem. 2010, 17, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-C.; Yang, Y.-C. Extraction characteristics and kinetic studies of oleanolic and ursolic acids from Hedyotis diffusa under ultrasound-assisted extraction conditions. Sep. Purif. Technol. 2014, 130, 182–192. [Google Scholar] [CrossRef]
- Guinda, Á.; Rada, M.; Delgado, T.; Adánez, M.P.G.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef] [PubMed]
- Guinda, A.; Rada, M.; Delgado, T.; Castellano, J.M. Pentacyclic triterpenic acids from Argania spinosa. Eur. J. Lipid Sci. Technol. 2010, 113, 231–237. [Google Scholar] [CrossRef]
- Geană, E.I.; Ionete, R.E.; Ciocarlan, A.; Aricu, A.; Fulga, A.; Ungur, N.; Podogova, M.; Nikolaeva, D. HPLC determination of oleanolic and ursolic acid in Apples and apple pomace. Prog. Cryog. Isot. Sep. 2014, 17, 53–62. [Google Scholar]
- Wei, M.-C.; Yang, Y.-C.; Hong, S.-J. Determination of Oleanolic and Ursolic Acids inHedyotis diffusaUsing Hyphenated Ultrasound-Assisted Supercritical Carbon Dioxide Extraction and Chromatography. Evid. Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gupta, R.; Dey, A.; Pandey, D.K. Simultaneous quantification of oleanolic acid, ursolic acid, betulinic acid and lupeol in different populations of five Swertia species by using HPTLC-densitometry: Comparison of different extraction methods and solvent selection. Ind. Crop. Prod. 2019, 130, 537–546. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; He, Q.; Zhang, B.; Zheng, X. Ionic Liquid Based Ultrasonic-Assisted Extraction of Oleanolic Acid from Grape Seeds. Open Access Libr. J. 2017, 4, e4148. [Google Scholar] [CrossRef]
- Anekpankul, T.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Extraction of anti-cancer damnacanthal from roots of Morinda citrifolia by subcritical water. Sep. Purif. Technol. 2007, 55, 343–349. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D. Supercritical CO2 extraction of grappa volatile compounds. Int. J. Food Sci. Technol. 2009, 44, 1927–1932. [Google Scholar] [CrossRef]
- Cai, J.; Liu, B.; Su, Q. Comparison of simultaneous distillation extraction and solid-phase microextraction for the determination of volatile flavor components. J. Chromatogr. A 2001, 930, 1–7. [Google Scholar] [CrossRef]
- Terigar, B.; Balasubramanian, S.; Boldor, D.; Xu, Z.; Lima, M.; Sabliov, C. Continuous microwave-assisted isoflavone extraction system: Design and performance evaluation. Bioresour. Technol. 2010, 101, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.C.; Yonker, C.R. Supercritical Fluid Chromatography, Pressurized Liquid Extraction, and Supercritical Fluid Extraction. Anal. Chem. 2006, 78, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zu, Y.-G.; Fu, Y.-J.; Luo, M.; Liu, W.; Li, J.; Efferth, T. Supercritical carbon dioxide extraction of seed oil from yellow horn (Xanthoceras sorbifolia Bunge.) and its anti-oxidant activity. Bioresour. Technol. 2010, 101, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Chen, F. Preparative isolation and purification of phillyrin from the medicinal plant Forsythia suspensa by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1083, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Wei, M.-C.; Huang, T.-C. Optimisation of an Ultrasound-assisted Extraction Followed by RP-HPLC Separation for the Simultaneous Determination of Oleanolic Acid, Ursolic Acid and Oridonin Content in Rabdosia rubescens. Phytochem. Anal. 2012, 23, 627–636. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.M.; Kentish, S.; Ashok Kumar, M. Use of Power Ultrasound to Improve Extraction and Modify Phase Transitions in Food Processing. Food Rev. Int. 2013, 29, 67–91. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Song, Y.; Ai, X.-X.; Guo, Y.-J.; Xu, X.-R.; Li, H.-B. A New High-Performance Liquid Chromatographic Method for the Determination and Distribution of Linalool in Michelia alba. Molecules 2010, 15, 4890–4897. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Wei, M.-C.; Lian, F.-Y.; Huang, T.-C. Simultaneous extraction and quantitation of oleanolic acid and ursolic acid Fromscutellaria barbata d. don by ultrasound-assisted extraction and high-performance liquid chromatography. Chem. Eng. Commun. 2013, 201, 482–500. [Google Scholar] [CrossRef]
- Wanigasekara, E.; Perera, S.; Crank, J.A.; Sidisky, L.; Shirey, R.; Berthod, A.; Armstrong, D.W. Bonded ionic liquid polymeric material for solid-phase microextraction GC analysis. Anal. Bioanal. Chem. 2009, 396, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Cláudio, A.F.; Freire, M.; Freire, C.; Silvestre, A.; Coutinho, J.A. Extraction of vanillin using ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2010, 75, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Vetal, M.D.; Chavan, R.S.; Rathod, V.K. Microwave assisted extraction of ursolic acid and oleanolic acid from Ocimum sanctum. Biotechnol. Bioprocess Eng. 2014, 19, 720–726. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J.; Yu, X.; Zhang, G.; Zhao, J. Optimization of microwave-assisted extraction followed by RP-HPLC for the simultaneous determination of oleanolic acid and ursolic acid in the fruits of Chaenomeles sinensis. J. Sep. Sci. 2010, 33, 1147–1155. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Sánchez-Avila, N.; Priego-Capote, F.; Jimenez, J.R.; Luquedecastro, M. Fast and selective determination of triterpenic compounds in olive leaves by liquid chromatography–tandem mass spectrometry with multiple reaction monitoring after microwave-assisted extraction. Talanta 2009, 78, 40–48. [Google Scholar] [CrossRef]
- Tian, Y.-Q.; Zhao, H.-T.; Zhang, X.-L.; Zhang, W.-T.; Liu, X.-C.; Gao, S.-H. Comparison of different extraction techniques and optimization of the microwave-assisted extraction of saponins from Aralia elata (Miq.) Seem fruits and rachises. Chem. Pap. 2020, 74, 3077–3087. [Google Scholar] [CrossRef]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects In Vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Wei, M.-C.; Huang, T.-C.; Lee, S.-Z. Extraction of protocatechuic acid from Scutellaria barbata D. Don using supercritical carbon dioxide. J. Supercrit. Fluids 2013, 81, 55–66. [Google Scholar] [CrossRef]
- Castola, V.; Marongiu, B.; Bighelli, A.; Floris, C.; Laï, A.; Casanova, J. Extractives of cork (Quercus suber L.): Chemical composition of dichloromethane and supercritical CO2 extracts. Ind. Crop. Prod. 2005, 21, 65–69. [Google Scholar] [CrossRef]
- Domingues, R.M.; de Melo, M.M.; Oliveira, E.L.; Neto, C.P.; Silvestre, A.J.; Silva, C.M. Optimization of the supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark using experimental design. J. Supercrit. Fluids 2013, 74, 105–114. [Google Scholar] [CrossRef]
- Pérez-Camino, M.C.; Cert, A. Quantitative Determination of Hydroxy Pentacyclic Triterpene Acids in Vegetable Oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef]
- Jemmali, Z.; Chartier, A.; Dufresne, C.; Elfakir, C. Optimization of the derivatization protocol of pentacyclic triterpenes prior to their gas chromatography–mass spectrometry analysis in plant extracts. Talanta 2016, 147, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.; Sousa, G.; Freire, C.; Silvestre, A.; Neto, C. Eucalyptus globulus biomass residues from pulping industry as a source of high value triterpenic compounds. Ind. Crop. Prod. 2010, 31, 65–70. [Google Scholar] [CrossRef]
- Leipold, D.; Wünsch, G.; Schmidt, M.; Bart, H.-J.; Bley, T.; Neuhaus, H.E.; Bergmann, H.; Richling, E.; Muffler, K.; Ulber, R. Biosynthesis of ursolic acid derivatives by microbial metabolism of ursolic acid with Nocardia sp. strains—Proposal of new biosynthetic pathways. Process Biochem. 2010, 45, 1043–1051. [Google Scholar] [CrossRef]
- Martelanc, M.; Vovk, I.; Simonovska, B. Separation and identification of some common isomeric plant triterpenoids by thin-layer chromatography and high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 6662–6670. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, T.; Zhou, H.; Feng, Y.; Fan, C.; Chen, W.; Crommen, J.; Jiang, Z. Fast separation of triterpenoid saponins using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2016, 121, 22–29. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Exarchou, V.; Troganis, A.; Gerothanassis, I.P. Rapid and novel discrimination and quantification of oleanolic and ursolic acids in complex plant extracts using two-dimensional nuclear magnetic resonance spectroscopy—Comparison with HPLC methods. Anal. Chim. Acta 2009, 635, 188–195. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Y.; Wang, D.; Yang, G.; Yu, A.; Zhang, H. MECC determination of oleanolic acid and ursolic acid isomers in Ligustrum lucidum Ait. J. Pharm. Biomed. Anal. 2003, 32, 479–485. [Google Scholar] [CrossRef]
- Yang, P.; Li, Y.; Liu, X.; Jiang, S. Determination of free isomeric oleanolic acid and ursolic acid in Pterocephalus hookeri by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2007, 43, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.C.H.; Vilegas, J.H.Y.; Lanças, F.M. Separation of underivatised triterpene acids by capillary supercritical fluid chromatography. Phytochem. Anal. 2001, 12, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Giménez, E.; Juan, M.E.; Calvo-Melià, S.; Barbosa, J.; Sanz-Nebot, V.; Planas, J.M. Pentacyclic triterpene in Olea europaea L: A simultaneous determination by high-performance liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2015, 1410, 68–75. [Google Scholar] [CrossRef]
- Wang, Z.; Zuo, G.; Hwang, S.H.; Kwon, S.H.; Kang, Y.-H.; Lee, J.-Y.; Lim, S.S. Affinity measurement of ligands in Perilla frutescens extract towards α-glucosidase using affinity-based ultrafiltration-high-performance liquid chromatography. J. Chromatogr. B 2019, 1125, 121725. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.B.; Nandy, A.K.; Roy, G. Triterpenoids. Phytochemistry 1992, 31, 2199–2249. [Google Scholar] [CrossRef]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2008, 25, 794–830. [Google Scholar] [CrossRef]
- Seo, S.; Yoshimura, Y.; Uomori, A.; Takeda, K.; Seto, H.; Ebizuka, Y.; Sankawa, U. Biosynthesis of triterpenes, ursolic acid and oleanolic acid in tissue cultures of Rabdosia japonica Hara fed [5-13C2H2]mevalonolactone and [2-13C2H3]acetate. J. Am. Chem. Soc. 1988, 110, 1740–1745. [Google Scholar] [CrossRef]
- Benveniste, P. Sterol Metabolism. In Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2002; p. e0004. [Google Scholar]
- Humphrey, A.J.; Beale, M.H. Terpenes. In Plant Secondary Metabolites; Blackwell Publishing: Singapore, 2006; pp. 47–101. [Google Scholar]
- Stiti, N.; Triki, S.; Hartmann, M.-A. Formation of Triterpenoids throughout Olea europaea Fruit Ontogeny. Lipids 2007, 42, 55–67. [Google Scholar] [CrossRef]
- Herrera, J.B.R.; Bartel, B.; Wilson, W.K.; Matsuda, S.P. Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochemistry 1998, 49, 1905–1911. [Google Scholar] [CrossRef]
- Ebizuka, Y.; Katsube, Y.; Tsutsumi, T.; Kushiro, T.; Shibuya, M. Functional genomics approach to the study of triterpene biosynthesis. Pure Appl. Chem. 2003, 75, 369–374. [Google Scholar] [CrossRef]
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Mahato, S.B.; Sarkar, S.K.; Poddar, G. Triterpenoid saponins. Phytochemistry 1988, 27, 3037–3067. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T.; Nagao, T.; Kinjo, J.; Okabe, H.; Higo, H.; Akahane, H. Ursolic Acid as a Trypanocidal Constituent in Rosemary. Biol. Pharm. Bull. 2002, 25, 1485–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allouche, Y.; Jiménez, A.; Uceda, M.; Aguilera, M.P.; Gaforio, J.J.; Beltrán, G. Triterpenic Content and Chemometric Analysis of Virgin Olive Oils from Forty Olive Cultivars. J. Agric. Food Chem. 2009, 57, 3604–3610. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Noshita, T.; Kidachi, Y.; Umetsu, H.; Hayashi, M.; Komiyama, K.; Funayama, S.; Ryoyama, K. Isolation of Ursolic Acid from Apple Peels and Its Specific Efficacy as a Potent Antitumor Agent. J. Health Sci. 2008, 54, 654–660. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Liu, R.H. Triterpenoids Isolated from Apple Peels Have Potent Antiproliferative Activity and May Be Partially Responsible for Apple’s Anticancer Activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef]
- Tian, L.-T.; Ma, L.; Du, N.-S. Survey of pharmacology of oleanolic acid. China J. Chin. Mater. Med. 2002, 27, 884–901. [Google Scholar]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Saponins in Ipomoea batatas tubers: Isolation, characterization, quantification and antioxidant properties. Food Chem. 2009, 113, 411–419. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive Fruit Extracts Inhibit Proliferation and Induce Apoptosis in HT-29 Human Colon Cancer Cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, R.-Y.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep. 2009, 26, 1321–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamai, H.; Sawada, N.; Yoshida, T.; Seike, J.; Takizawa, H.; Kenzaki, K.; Miyoshi, T.; Kondo, K.; Bando, Y.; Ohnishi, Y.; et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis In Vivo. Int. J. Cancer 2009, 125, 952–960. [Google Scholar] [CrossRef]
- Eloy, J.O.; Saraiva, J.; de Albuquerque, S.; Marchetti, J.M. Preparation, characterization and evaluation of the In Vivo trypanocidal activity of ursolic acid-loaded solid dispersion with poloxamer 407 and sodium caprate. Braz. J. Pharm. Sci. 2015, 51, 101–109. [Google Scholar] [CrossRef]
- Jinhua, W. Ursolic acid: Pharmacokinetics process In Vitro and In Vivo, a mini review. Arch. Der Pharm. 2019, 352, e1800222. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Hang, T.-J.; Wang, Y.; Jiang, L.; Wu, X.-L.; Zhang, Z.; Shen, J.; Zhang, Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J. Pharm. Biomed. Anal. 2006, 40, 190–196. [Google Scholar] [CrossRef]
- Chen, R.-J.; Liu, X.; Li, P.-M.; Zhang, L.; Zhao, L.; Zhang, X.-L. Pharmacokinetic profiles of oleanolic acid formulations in healthy Chinese male volunteers. Chin. Pharm. J. 2010, 45, 621–626. [Google Scholar]
- Rada, M.; Castellano, J.M.; Perona, J.S.; Guinda, Á. GC-FID determination and pharmacokinetic studies of oleanolic acid in human serum. Biomed. Chromatogr. 2015, 29, 1687–1692. [Google Scholar] [CrossRef] [Green Version]
- De la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.-D.; Covas, M.-I.; Expósito, M.; Espejo, J.-A.; Sanchez-Rodriguez, E.; Díaz-Pellicer, P.; et al. Pharmacokinetics of maslinic and oleanolic acids from olive oil—Effects on endothelial function in healthy adults. A randomized, controlled, dose–response study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef]
- Rada, M.; Ruiz-Gutiérrez, V.; Guinda, Á. Determination of Triterpenic Acids in Human Serum by High-Performance Liquid Chromatography: Triterpenoid Interaction with Serum Protein. J. Agric. Food Chem. 2011, 59, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ding, F.; Jiang, Y.-T.; Peng, Y.-K. Bioavailability and Activity of Natural Food Additive Triterpenoids as Influenced by Protein. J. Agric. Food Chem. 2014, 62, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Dopierala, K.; Krajewska, M.M.; Weiss, M. Physicochemical Characterization of Oleanolic Acid—Human Serum Albumin Complexes for Pharmaceutical and Biosensing Applications. Langmuir 2020, 36, 3611–3623. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.A.; Grüne, T.; Curry, S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J. Mol. Biol. 2000, 303, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, R.; Gollapudi, A.; Bonigala, P.; Chinnaboina, M.; Amooru, D.G. Betulinic acid binding to human serum albumin: A study of protein conformation and binding affinity. J. Photochem. Photobiol. B Biol. 2009, 94, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Yunoki, K.; Sasaki, G.; Tokuji, Y.; Kinoshita, M.; Naito, A.; Aida, K.; Ohnishi, M. Effect of Dietary Wine Pomace Extract and Oleanolic Acid on Plasma Lipids in Rats Fed High-Fat Diet and Its DNA Microarray Analysis. J. Agric. Food Chem. 2008, 56, 12052–12058. [Google Scholar] [CrossRef] [PubMed]
- De Melo, C.L.; Queiroz, M.G.R.; Fonseca, S.G.; Bizerra, A.M.C.; Lemos, T.L.; Melo, T.S.; Santos, F.A.; Rao, V.S. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef]
- Kalaiarasi, P.; Kaviarasan, K.; Pugalendi, K.V. Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2009, 612, 93–97. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, D.; Han, Y.; Han, Z.; Zhong, W.; Wang, C. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int. J. Biochem. Cell Biol. 2015, 69, 142–152. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, F.; Song, Z.; Huang, H.; Chen, Y.; Shen, Y.; Jia, Y.; Chen, J. Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1–7) upregulation. Biomed. Pharmacother. 2018, 97, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules 2019, 24, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molepo, M.; Ayeleso, A.; Nyakudya, T.; Erlwanger, K.; Mukwevho, E. A Study on Neonatal Intake of Oleanolic Acid and Metformin in Rats (Rattus norvegicus) with Metabolic Dysfunction: Implications on Lipid Metabolism and Glucose Transport. Molecules 2018, 23, 2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, K.; Maeda, N.; Oiso, S.; Kariyazono, H. Decreased Plasma Octanoylated Ghrelin Levels in Mice by Oleanolic Acid. J. Oleo Sci. 2019, 68, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, Y.; Wang, X.; Tian, Z.; Qi, D.; Li, Y.; Jiang, H. Antihypertensive activity of oleanolic acid is mediated via downregulation of secretory phospholipase A2 and fatty acid synthase in spontaneously hypertensive rats. Int. J. Mol. Med. 2020, 46, 2019–2034. [Google Scholar] [CrossRef]
- Chen, S.; Wen, X.; Zhang, W.; Wang, C.; Liu, J.; Liu, C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1β axis in high-fat diet-induced hyperlipidemic mice. FASEB J. 2016, 31, 1085–1096. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Liu, J.; Ouyang, Q.; Xuan, C.; Wang, L.; Li, T.; Liu, J. The effects of oleanolic acid on atherosclerosis in different animal models. Acta Biochim. Biophys. Sin. 2017, 49, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.H.W.; Yang, Q.; Harada, M.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Pomegranate Flower Extract Diminishes Cardiac Fibrosis in Zucker Diabetic Fatty Rats—Modulation of cardiac endothelin-1 and nuclear factor-kappa B pathways. J. Cardiovasc. Pharmacol. 2005, 46, 856–862. [Google Scholar] [CrossRef]
- Lee, W.S.; Im, K.-R.; Park, Y.-D.; Sung, N.-D.; Jeong, T.-S. Human ACAT-1 and ACAT-2 Inhibitory Activities of Pentacyclic Triterpenes from the Leaves of Lycopus lucidus TURCZ. Biol. Pharm. Bull. 2006, 29, 382–384. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Mimaki, Y.; Ohtomo, T.; Yamada, J.; Nishiyama, T.; Mae, T.; Kishida, H.; Kawada, T. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. J. Nat. Med. 2012, 66, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-Y.; Kang, S.-W.; Kim, J.-L.; Li, J.; Lee, E.-S.; Gong, J.-H.; Han, S.J.; Kang, Y.-H. Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr. Res. 2010, 30, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.T.; Van Minh, C.; Van Kiem, P.; Thao, N.P.; Tai, B.H.; Nhiem, N.X.; Song, S.B.; Kim, Y.H. Effect of triterpenes and triterpene saponins from the stem bark of Kalopanax pictus on the transactivational activities of three PPAR subtypes. Carbohydr. Res. 2011, 346, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-Q.; Shen, J.; Chen, C.-P.; Ma, X.; Lin, C.; Ouyang, Q.; Xuan, C.-X.; Liu, J.; Sun, H.-B.; Liu, J. Lipid-lowering effects of oleanolic acid in hyperlipidemic patients. Chin. J. Nat. Med. 2018, 16, 339–346. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.; Jiménez, D.N.S.C.G.; Castillo-España, P.; Ramírez-Ávila, G.; Villalobos-Molina, R.; Estrada-Soto, S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. J. Ethnopharmacol. 2007, 109, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Komaki, E.; Yamaguchi, S.; Maru, I.; Kinoshita, M.; Kakehi, K.; Ohta, Y.; Tsukada, Y. Identification of Anti-.ALPHA.-Amylase Components from Olive Leaf Extracts. Food Sci. Technol. Res. 2003, 9, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.-H.; Wu, Y.-C.; Liu, I.-M.; Cheng, J.-T. Release of acetylcholine to raise insulin secretion in Wistar rats by oleanolic acid, one of the active principles contained in Cornus officinalis. Neurosci. Lett. 2006, 404, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Gilon, P. Mechanisms and Physiological Significance of the Cholinergic Control of Pancreatic-Cell Function. Endocr. Rev. 2001, 22, 565–604. [Google Scholar] [CrossRef] [PubMed]
- Whalley, N.M.; Pritchard, L.E.; Smith, D.M.; White, A. Processing of proglucagon to GLP-1 in pancreatic α-cells: Is this a paracrine mechanism enabling GLP-1 to act on β-cells? J. Endocrinol. 2011, 211, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Genet, C.; Strehle, A.; Schmidt, C.; Boudjelal, G.; Lobstein, A.; Schoonjans, K.; Souchet, M.; Auwerx, J.; Saladin, R.; Wagner, A. Structure−Activity Relationship Study of Betulinic Acid, A Novel and Selective TGR5 Agonist, and Its Synthetic Derivatives: Potential Impact in Diabetes. J. Med. Chem. 2010, 53, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Maczewsky, J.; Kaiser, J.; Gresch, A.; Gerst, F.; Düfer, M.; Krippeit-Drews, P.; Drews, G. TGR5 Activation Promotes Stimulus-Secretion Coupling of Pancreatic β-Cells via a PKA-Dependent Pathway. Diabetes 2018, 68, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lu, Y.-F.; Wu, Q.; Xu, S.-F.; Shi, F.-G.; Klaassen, C.D. Oleanolic acid reprograms the liver to protect against hepatotoxicants, but is hepatotoxic at high doses. Liver Int. 2019, 39, 427–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, Y.; Shi, T.; Meng, M.; Kong, G.; Tian, Y.; Chen, Q.; Yao, X.; Feng, G.; Cheng, H.; Lu, Z. A novel screening model for the molecular drug for diabetes and obesity based on tyrosine phosphatase Shp2. Bioorg. Med. Chem. Lett. 2011, 21, 874–878. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic acid enhances insulin secretion in pancreatic β-cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef] [Green Version]
- Zito, C.I.; Kontaridis, M.I.; Fornaro, M.; Feng, G.-S.; Bennett, A.M. SHP-2 regulates the phosphatidylinositide 3?-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J. Cell. Physiol. 2004, 199, 227–236. [Google Scholar] [CrossRef]
- White, S.A.; James, R.F.L.; Swift, S.M.; Kimber, R.M.; Nicholson, M.L. Human islet cell transplantation—Future prospects. Diabet. Med. 2001, 18, 78–103. [Google Scholar] [CrossRef]
- Nataraju, A.; Saini, D.; Ramachandran, S.; Benshoff, N.; Liu, W.; Chapman, W.; Mohanakumar, T. Oleanolic Acid, a Plant Triterpenoid, Significantly Improves Survival and Function of Islet Allograft. Transplantation 2009, 88, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.H.; Ha, Y.J.; Shim, E.K.; Choi, S.Y.; Jin, J.L.; Yun-Choi, H.S.; Lee, J.R. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem. J. 2007, 403, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Galic, S.; Hauser, C.; Kahn, B.B.; Haj, F.G.; Neel, B.G.; Tonks, N.K.; Tiganis, T. Coordinated Regulation of Insulin Signaling by the Protein Tyrosine Phosphatases PTP1B and TCPTP. Mol. Cell. Biol. 2005, 25, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Paoli, P.; Camici, G.; Navarrete-Vázquez, G.; Ortiz-Andrade, R.; Estrada-Soto, S. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP–1B: In Vitro, In Silico, and In Vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.-W.; Shen, Q.; Yang, F.; Wang, B.; Zou, H.; Li, J.-Y.; Li, J.; Tang, J. Synthesis and biological evaluation of heterocyclic ring-substituted maslinic acid derivatives as novel inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2009, 19, 6618–6622. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, P.; Chen, X.; He, G. PI3K and ERK/Nrf2 pathways are involved in oleanolic acid-induced heme oxygenase-1 expression in rat vascular smooth muscle cells. J. Cell. Biochem. 2011, 112, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Yang, H.; Long, C. Oleanolic Acid Improves the Symptom of Renal Ischemia Reperfusion Injury via the PI3K/AKT Pathway. Urol. Int. 2020, 105, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Lee, M.; An, H.-J. Oleanolic acid protects against mast cell-mediated allergic responses by suppressing Akt/NF-κB and STAT1 activation. Phytomedicine 2020, 80, 153340. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, X.; Wu, X. Effect of Oleanolic Acid on Apoptosis and Autophagy of SMMC-7721 Hepatoma Cells. Med. Sci. Monit. 2020, 26, e921606-1–e921606-12. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Y.-P.; Cantley, J.; Iseli, T.J.; Molero, J.C.; Hegarty, B.D.; Kraegen, E.W.; Ye, Y.; Ye, J.-M. Oleanolic Acid Reduces Hyperglycemia beyond Treatment Period with Akt/FoxO1-Induced Suppression of Hepatic Gluconeogenesis in Type-2 Diabetic Mice. PLoS ONE 2012, 7, e42115. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.T.; Tuan, D.T.; Thu, N.B.; Nhiem, N.X.; Ngoc, T.M.; Yim, N.; Bae, K. Palbinone and triterpenes from Moutan Cortex (Paeonia suffruticosa, Paeoniaceae) stimulate glucose uptake and glycogen synthesis via activation of AMPK in insulin-resistant human HepG2 Cells. Bioorg. Med. Chem. Lett. 2009, 19, 5556–5559. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Duan, W.; Mu, D.; Zhang, L. Maslinic Acid Reduces Blood Glucose in KK-Ay Mice. Biol. Pharm. Bull. 2007, 30, 2075–2078. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.K.; Reddy, V.T.; Konopleva, M.; Andreeff, M.; Chan, L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J. Biol. Chem. 2010, 285, 40581–40592. [Google Scholar] [CrossRef] [Green Version]
- Matumba, M.G.; Ayeleso, A.O.; Nyakudya, T.; Erlwanger, K.; Chegou, N.N.; Mukwevho, E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients 2019, 11, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangeetha, K.N.; Sujatha, S.; Muthusamy, V.S.; Anand, S.; Nithya, N.; Velmurugan, D.; Balakrishnan, A.; Lakshmi, B.S. 3β-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochim. Biophys. Acta BBA Gen. Subj. 2010, 1800, 359–366. [Google Scholar] [CrossRef]
- Ahamed, K.B.M.; Gowdru, H.B.; Rajashekarappa, S.; Malleshappa, K.S.H.; Krishna, V. Molecular docking of glycogen synthase kinase3-β inhibitor oleanolic acid and its wound-healing activity in rats. Med. Chem. Res. 2012, 22, 156–164. [Google Scholar] [CrossRef]
- Jang, S.-M.; Kim, M.-J.; Choi, M.-S.; Kwon, E.-Y.; Lee, M.-K. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism 2010, 59, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Luo, D.; Sun, H.-B.; Shang, J.; Zhang, L.-Y. Anti-proliferative effect of pentacyclic triterpenes associated with glycogen accumulation in A549 cells. Chin. J. New Drugs 2011, 20, 2350–2353. [Google Scholar]
- Wen, X.; Sun, H.; Liu, J.; Wu, G.; Zhang, L.; Wu, X.; Ni, P. Pentacyclic triterpenes. Part 1: The first examples of naturally occurring pentacyclic triterpenes as a new class of inhibitors of glycogen phosphorylases. Bioorg. Med. Chem. Lett. 2005, 15, 4944–4948. [Google Scholar] [CrossRef]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E.; et al. Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure—Activity Relationships, and X-ray Crystallographic Studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
- Neto, J.C.G.L.; Xavier, M.D.A.; Borges, J.W.P.; de Araújo, M.F.M.; Damasceno, M.M.C.; Freitas, R. Prevalence of Metabolic Syndrome in individuals with Type 2 Diabetes Mellitus. Rev. Bras. Enferm. 2017, 70, 265–270. [Google Scholar] [CrossRef]
- Mc Cullough, A.J. Epidemiology of the metabolic syndrome in the USA. J. Dig. Dis. 2011, 12, 333–340. [Google Scholar] [CrossRef]
- Fernández-Aparicio, Á.; Schmidt-Rio Valle, J.; Perona, J.S.; Correa-Rodríguez, M.; Castellano, J.M.; González-Jiménez, E.; Aparicio, F.; Valle, S.-R.; Rodríguez, C.; Jiménez, G. Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review. J. Clin. Med. 2019, 8, 1294. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.M.; Choi, Y.H.; Yoon, J.J.; Lee, Y.J.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic acid modulates the renin-angiotensin system and cardiac natriuretic hormone concomitantly with volume and pressure balance in rats. Eur. J. Pharmacol. 2017, 809, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.D.; Bachhav, S.S.; Bhutada, M.S.; Patil, S.P.; Sharma, K.S. Oleanolic acid prevents increase in blood pressure and nephrotoxicity in nitric oxide dependent type of hypertension in rats. Pharmacogn. Res. 2015, 7, 385–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madlala, H.P.; Van Heerden, F.; Mubagwa, K.; Musabayane, C.T. Changes in Renal Function and Oxidative Status Associated with the Hypotensive Effects of Oleanolic Acid and Related Synthetic Derivatives in Experimental Animals. PLoS ONE 2015, 10, e0128192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, J.; Gu, T.; Yamahara, J.; Li, Y. Oleanolic acid supplement attenuates liquid fructose-induced adipose tissue insulin resistance through the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in rats. Toxicol. Appl. Pharmacol. 2014, 277, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Kim, H.M.; Kang, J.S.; Yadav, D.; Kwon, M.-H.; Kim, Y.M.; Kim, H.S.; Chung, C. Oleanolic acid and N-acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol. Dial. Transplant. 2015, 31, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, Y.; Abdelkader, D.; Hassan, W.; Sun, H.; Liu, J. Combination Therapy with Oleanolic Acid and Metformin as a Synergistic Treatment for Diabetes. J. Diabetes Res. 2015, 2015, 973287. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The Effects of Plant-Derived Oleanolic Acid on Selected Parameters of Glucose Homeostasis in a Diet-Induced Pre-Diabetic Rat Model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef] [Green Version]
- Djeziri, F.Z.; Belarbi, M.; Murtaza, B.; Hichami, A.; Benammar, C.; Khan, N.A. Oleanolic acid improves diet-induced obesity by modulating fat preference and inflammation in mice. Biochimie 2018, 152, 110–120. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Quintela, J.C.; Pérez-Montero, M.; Miñano, J.; De Sotomayor, M.A.; Herrera, M.D.; Rodríguez-Rodríguez, A.R. Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice. Nutrients 2020, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-derived oleanolic acid ameliorates markers of subclinical inflammation and innate immunity activation in diet-induced pre-diabetic rats. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820935771. [Google Scholar] [CrossRef]
- Su, S.; Wu, G.; Cheng, X.; Fan, J.; Peng, J.; Su, H.; Xu, Z.; Cao, M.; Long, Z.; Hao, Y.; et al. Oleanolic acid attenuates PCBs-induced adiposity and insulin resistance via HNF1b-mediated regulation of redox and PPARγ signaling. Free Radic. Biol. Med. 2018, 124, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-C.; Chan, K.-C. Nonenzymatic Antioxidative and Antiglycative Effects of Oleanolic Acid and Ursolic Acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, Q.; Li, Y.; Liu, Z.; Fan, Y.; Liu, Z.; Zhao, H.; Li, J.; Han, Z. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother. Res. 2009, 23, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial Dysfunction and Type 2 Diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allouche, Y.; Warleta, F.; Campos, M.; Sánchez-Quesada, C.; Uceda, M.; Beltrán, G.; Gaforio, J.J. Antioxidant, Antiproliferative, and Pro-apoptotic Capacities of Pentacyclic Triterpenes Found in the Skin of Olives on MCF-7 Human Breast Cancer Cells and Their Effects on DNA Damage. J. Agric. Food Chem. 2010, 59, 121–130. [Google Scholar] [CrossRef]
- Gutierrez, B.; Gallardo, I.; Ruiz, L.; Alvarez, Y.; Cachofeiro, V.; Margolles, A.; Hernandez, M.; Nieto, M.L. Oleanolic acid ameliorates intestinal alterations associated with EAE. J. Neuroinflamm. 2020, 17, 363. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Sun, H.; Xie, J. Synthesis of Oleanolic Acid Dimers as Inhibitors of Glycogen Phosphorylase. Chem. Biodivers. 2010, 7, 690–697. [Google Scholar] [CrossRef]
- Dharmappa, K.K.; Kumar, R.V.; Nataraju, A.; Mohamed, R.; Shivaprasad, H.V.; Vishwanath, B.S. Anti-Inflammatory Activity of Oleanolic Acid by Inhibition of Secretory Phospholipase A2. Planta Med. 2009, 75, 211–215. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Yin, M.-C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef]
- Du, Y.; Ko, K.M. Oleanolic Acid Protects against Myocardial Ischemia-Reperfusion Injury by Enhancing Mitochondrial Antioxidant Mechanism Mediated by Glutathione and α-Tocopherol in Rats. Planta Med. 2006, 72, 222–227. [Google Scholar] [CrossRef]
- Soobrattee, M.; Neergheen, V.; Luximon-Ramma, A.; Aruoma, O.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Bonner-Weir, S. Five Stages of Evolving Beta-Cell Dysfunction During Progression to Diabetes. Diabetes 2004, 53, S16–S21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkanen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome—The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef] [Green Version]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Jáuregui, O.; Amézqueta, S.; Dasilva, G.; Medina, I.; Nogués, M.R.; Romeu, M.; et al. Mechanistically different effects of fat and sugar on insulin resistance, hypertension, and gut microbiota in rats. Am. J. Physiol. Metab. 2018, 314, E552–E563. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.W.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Janssen, A.W.F.; Kersten, S. Potential mediators linking gut bacteria to metabolic health: A critical view. J. Physiol. 2016, 595, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Zhao, X.; Biswas, D. Polyphenols and tri-terpenoids from Olea europaea L. in alleviation of enteric pathogen infections through limiting bacterial virulence and attenuating inflammation. J. Funct. Foods 2017, 36, 132–143. [Google Scholar] [CrossRef]
- Callejo, M.N.; Gallardo, I.; Gutierrez, B.; Cabero, M.; Ruiz, L.; Alvarez, Y.; Simon, I.; Calvo, H.; Munoz, J.; Margolles, A.; et al. Oleanolic acid protection against experimental autoimmune myocarditis modulates the microbiota and the intestinal barrier integrity. Eur. Hear. J. 2020, 41, ehaa946.3716. [Google Scholar] [CrossRef]
- Dinh, C.H.L.; Yu, Y.; Szabo, A.; Zhang, Q.; Zhang, P.; Huang, X.-F. Bardoxolone Methyl Prevents High-Fat Diet-Induced Colon Inflammation in Mice. J. Histochem. Cytochem. 2016, 64, 237–255. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, Y.; Ma, W.; Wang, Z. Effects of Ligustrum lucidum on egg production, egg quality, and caecal microbiota of hens during the late laying period. Ital. J. Anim. Sci. 2020, 19, 687–696. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, C.; Zhou, J.; Wu, F.; Li, J.; Li, T.; Yin, Y. Disturbance of the intestinal microbial community by ursolic acid contributes to its function as a regulator of fat deposition. J. Funct. Foods 2015, 14, 456–468. [Google Scholar] [CrossRef]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Kwek, E.; He, Z.; Zhu, H.; Liu, J.; Zhao, Y.; Ma, K.Y.; He, W.-S.; Chen, Z.-Y. Ursolic acid alleviates hypercholesterolemia and modulates the gut microbiota in hamsters. Food Funct. 2020, 11, 6091–6103. [Google Scholar] [CrossRef]
- Staats, S.; Wagner, A.E.; Lüersen, K.; Künstner, A.; Meyer, T.; Kahns, A.K.; Derer, S.; Graspeuntner, S.; Rupp, J.; Busch, H.; et al. Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. BioFactors 2019, 45, 169–186. [Google Scholar] [CrossRef]
- Wan, S.; Huang, C.; Wang, A.; Zhu, X. Ursolic acid improves the bacterial community mapping of the intestinal tract in liver fibrosis mice. PeerJ 2020, 8, e9050. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.-Z.; Liu, C.; Huang, C.-K.; Luo, F.-Y.; Zhu, X. Ursolic Acid Improves Intestinal Damage and Bacterial Dysbiosis in Liver Fibrosis Mice. Front. Pharmacol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, D.; Jian, J.; Huang, C.; Luo, F.; Wan, S.; Jiang, M.; Wan, Y.; Wang, A.; Li, B.; et al. Protective Effect of Ursolic Acid on the Intestinal Mucosal Barrier in a Rat Model of Liver Fibrosis. Front. Physiol. 2019, 10, 956. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Luo, F.; Huang, C.; Liu, C.; Luo, Q.; Zhu, X. Ursolic acid reverses liver fibrosis by inhibiting interactive NOX4/ROS and RhoA/ROCK1 signalling pathways. Aging 2020, 12, 10614–10632. [Google Scholar] [CrossRef]
- Bala, V.; Rajagopal, S.; Kumar, D.P.; Nalli, A.D.; Mahavadi, S.; Sanyal, A.J.; Grider, J.R.; Murthy, K.S. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-ε pathway and modulated by endogenous H2S. Front. Physiol. 2014, 5, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.K.; Sharma, A.; Arora, S.; Blomenkamp, K.; Jun, I.C.; Luong, R.; Westrich, D.J.; Mittal, A.; Buchanan, P.M.; Guzman, M.A.; et al. Preserved Gut Microbial Diversity Accompanies Upregulation of TGR5 and Hepatobiliary Transporters in Bile Acid—Treated Animals Receiving Parenteral Nutrition. J. Parenter. Enter. Nutr. 2016, 41, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladurner, A.; Zehl, M.; Grienke, U.; Hofstadler, C.; Faur, N.; Pereira, F.C.; Berry, D.; Dirsch, V.M.; Rollinger, J.M. Allspice and Clove As Source of Triterpene Acids Activating the G Protein-Coupled Bile Acid Receptor TGR5. Front. Pharmacol. 2017, 8, 468. [Google Scholar] [CrossRef] [Green Version]
- Loubinoux, J.; Valente, F.M.; Pereira, I.A.; Costa, A.; Grimont, P.A.; Le Faou, A.E. Reclassification of the only species of the genus Desulfomonas, Desulfomonas pigra, as Desulfovibrio piger comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D.; Shanahan, F.; Marchesi, J.R. Culture-independent analysis of desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. FEMS Microbiol. Ecol. 2009, 69, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Takada, K.; Nakane, T.; Masuda, K.; Ishii, H. Ursolic acid and oleanolic acid, members of pentacyclic triterpenoid acids, suppress TNF-α-induced E-selectin expression by cultured umbilical vein endothelial cells. Phytomedicine 2010, 17, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Saaby, L.; Jäger, A.K.; Moesby, L.; Hansen, E.W.; Christensen, S.B. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.). Phytother. Res. 2011, 25, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Q.; Hu, Q.; Wang, B.; Cui, W.; Wu, F.; Ding, Y. Oleanolic acid alleviates diabetic rat carotid artery injury through the inhibition of NLRP3 inflammasome signaling pathways. Mol. Med. Rep. 2017, 16, 8413–8419. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Castellano, J.M.; Espinosa, J.M.; Perona, J.S. Modulation of Lipid Transport and Adipose Tissue Deposition by Small Lipophilic Compounds. Front. Cell Dev. Biol. 2020, 8, 555359. [Google Scholar] [CrossRef]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.Z.; Wan, Z.X. The effect of oleanolic acid on acute hepatitis (70 cases). Hum. Med. 1980, 7, 50–52. [Google Scholar]
- Minich, D.M.; Bland, J.S.; Katke, J.; Darland, G.; Hall, A.; Lerman, R.H.; Lamb, J.; Carroll, B.; Tripp, M. Clinical safety and efficacy of NG440: A novel combination of rho iso-alpha acids from hops, rosemary, and oleanolic acid for inflammatory conditions. Can. J. Physiol. Pharmacol. 2007, 85, 872–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Lozano, J.M.; Rada, M.; Lapetra, J.; Guinda, Á.; Jiménez-Rodríguez, M.C.; Cayuela, J.A.; Ángel-Lugo, A.; Vilches-Arenas, Á.; Gómez-Martín, A.M.; Ortega-Calvo, M.; et al. Prevention of type 2 diabetes in prediabetic patients by using functional olive oil enriched in oleanolic acid: The PREDIABOLE study, a randomized controlled trial. Diabetes Obes. Metab. 2019, 21, 2526–2534. [Google Scholar] [CrossRef]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic Acid Exerts a Neuroprotective Effect Against Microglial Cell Activation by Modulating Cytokine Release and Antioxidant Defense Systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef] [Green Version]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef] [Green Version]


| OA Dose (mg) | OA Formulation | Participants | n | Cmax (ng/mL) | tmax (h) | t1/2 (h) | AUC0→t (ng h/mL) | CL (L/h) | Vd (L) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 40 | capsules | healthy males | 18 | 12.1 ± 6.8 | 5.2 ± 2.9 | 8.7 ± 6.1 | 114.3 ± 74.9 | 555.3 ± 347.7 | 3371.1 ± 1990.1 | [80] |
| 20 | normal tablets | healthy males | 18 | 18.9 ± 8.0 | 2.9 ± 1.2 | 12.3 ± 9.0 | 112.2 ± 56.6 | No data available | No data available | [81] |
| 20 | dispersable tablets | healthy males | 18 | 17.8 ± 7.5 | 2.5 ± 1.0 | 16.1 ± 12.1 | 109.7 ± 41.6 | No data available | No data available | [81] |
| 30 | OA-enriched aPOO | healthy males | 9 | 598.2 ± 176.7 | 3.0 ± 0.8 | 4.6 ± 0.1 | 3025.2 ± 847.1 | 35.1 ± 4.2 | 81.4 ± 9.7 | [82] |
| 4.7 | bFOO | healthy adults (6 m, 6 f) | 12 | 5.1 ± 2.1 | 4 (1–6) c | 4.1 ± 0.2 | 28.2 ± 9.4 | No data available | No data available | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. https://doi.org/10.3390/nu14030623
Castellano JM, Ramos-Romero S, Perona JS. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients. 2022; 14(3):623. https://doi.org/10.3390/nu14030623
Chicago/Turabian StyleCastellano, José M., Sara Ramos-Romero, and Javier S. Perona. 2022. "Oleanolic Acid: Extraction, Characterization and Biological Activity" Nutrients 14, no. 3: 623. https://doi.org/10.3390/nu14030623