Embryonic Exposure to Tryptophan Yields Bullying Victimization via Reprogramming the Microbiota-Gut-Brain Axis in a Chicken Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eggs Incubation and Embryonic Trp Exposure
2.2. Bird Management
2.3. Sample Collection
2.4. Aggressive Behavior Observation
2.5. Intestinal Histomorphological Analysis
2.6. 16S rRNA Microbiome Sequencing
2.7. ELISA
2.8. HPLC
2.9. Data Processing and Analysis
2.9.1. Bioinformatics Analysis
2.9.2. Physiological and Behavioral Data
3. Results
3.1. Embryonic Trp Exposure Reduced BW
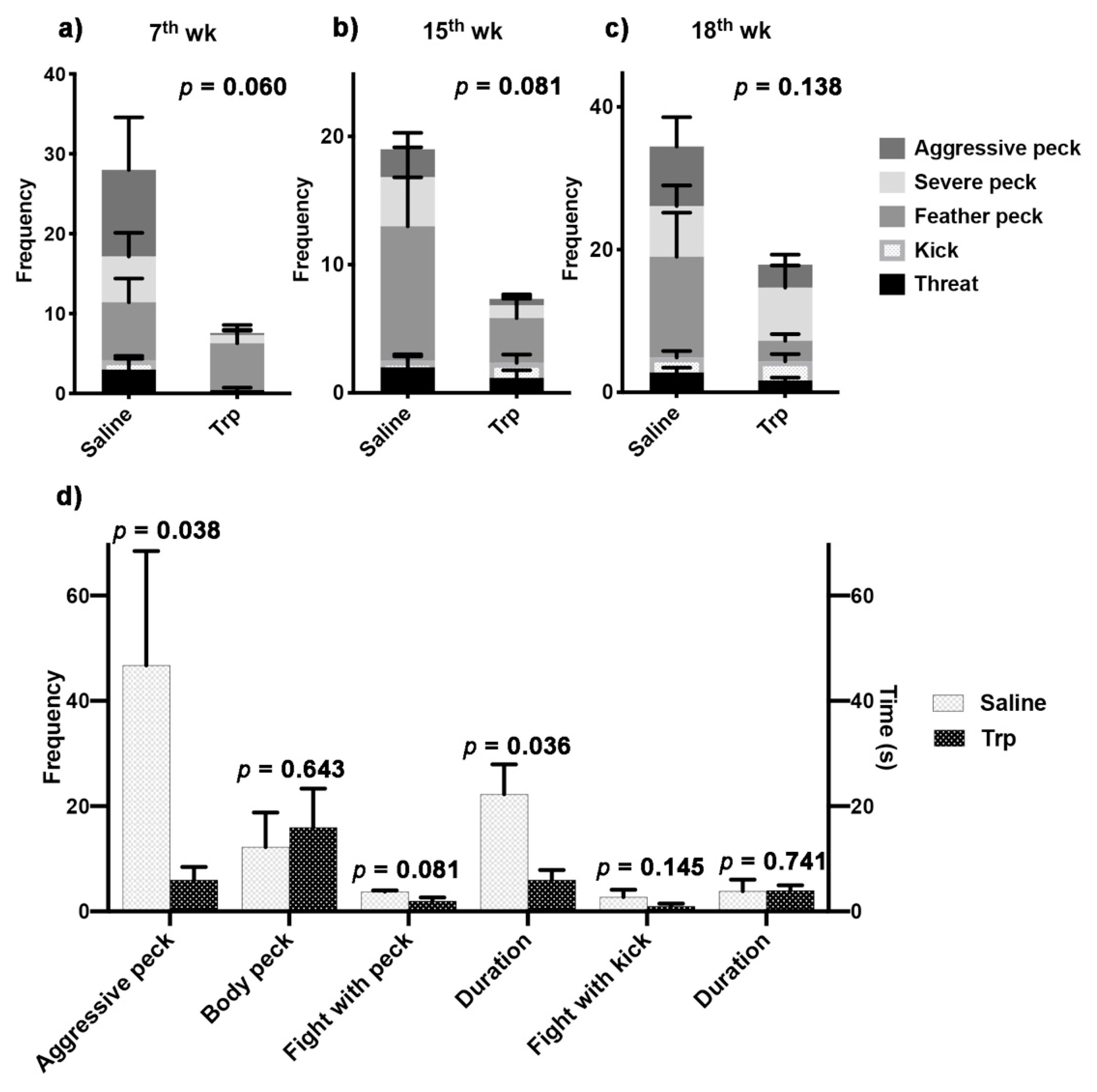
3.2. Embryonic Trp Exposure Reduced Aggressiveness
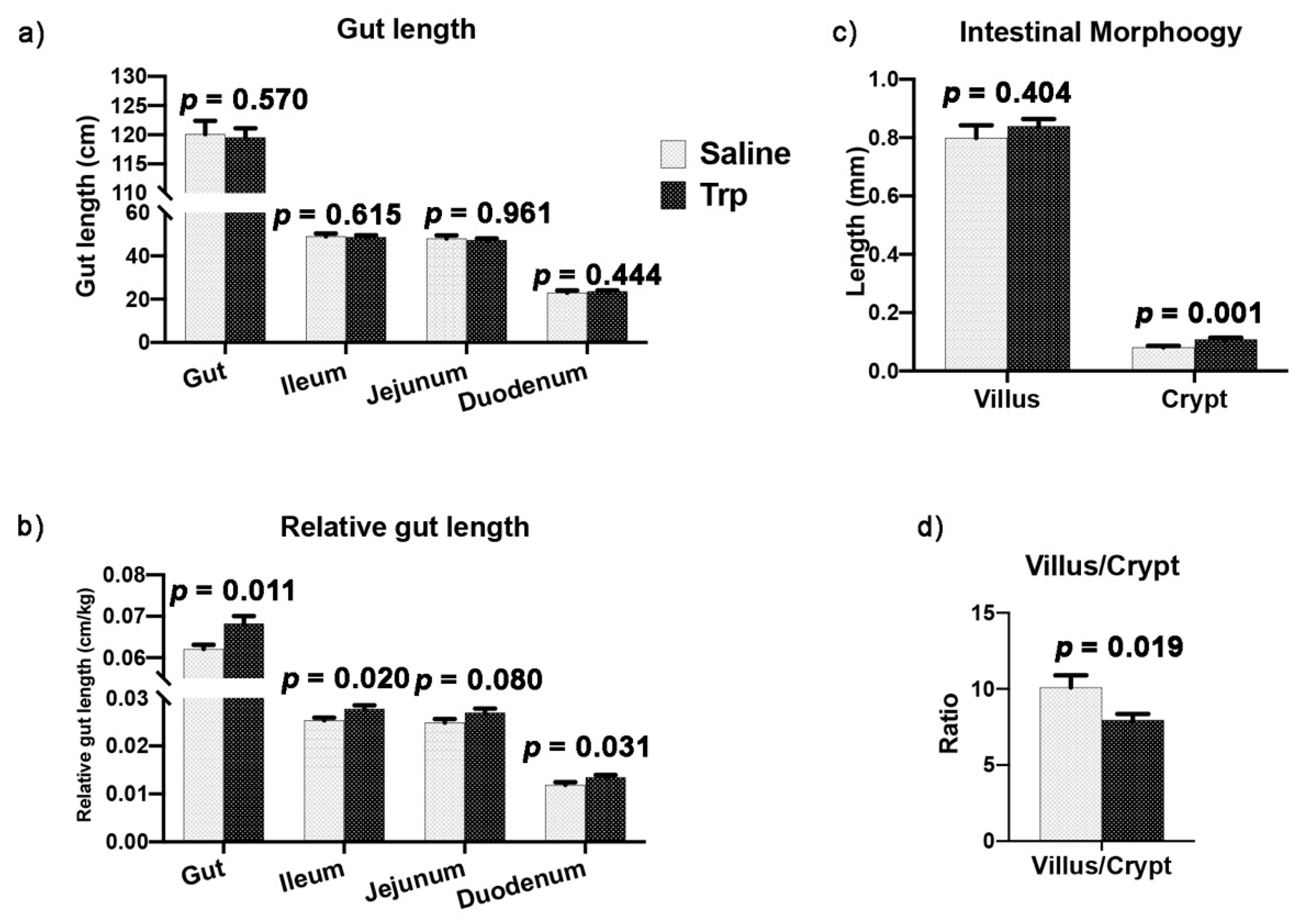
3.3. Embryonic Trp Exposure Altered Gut Morphology
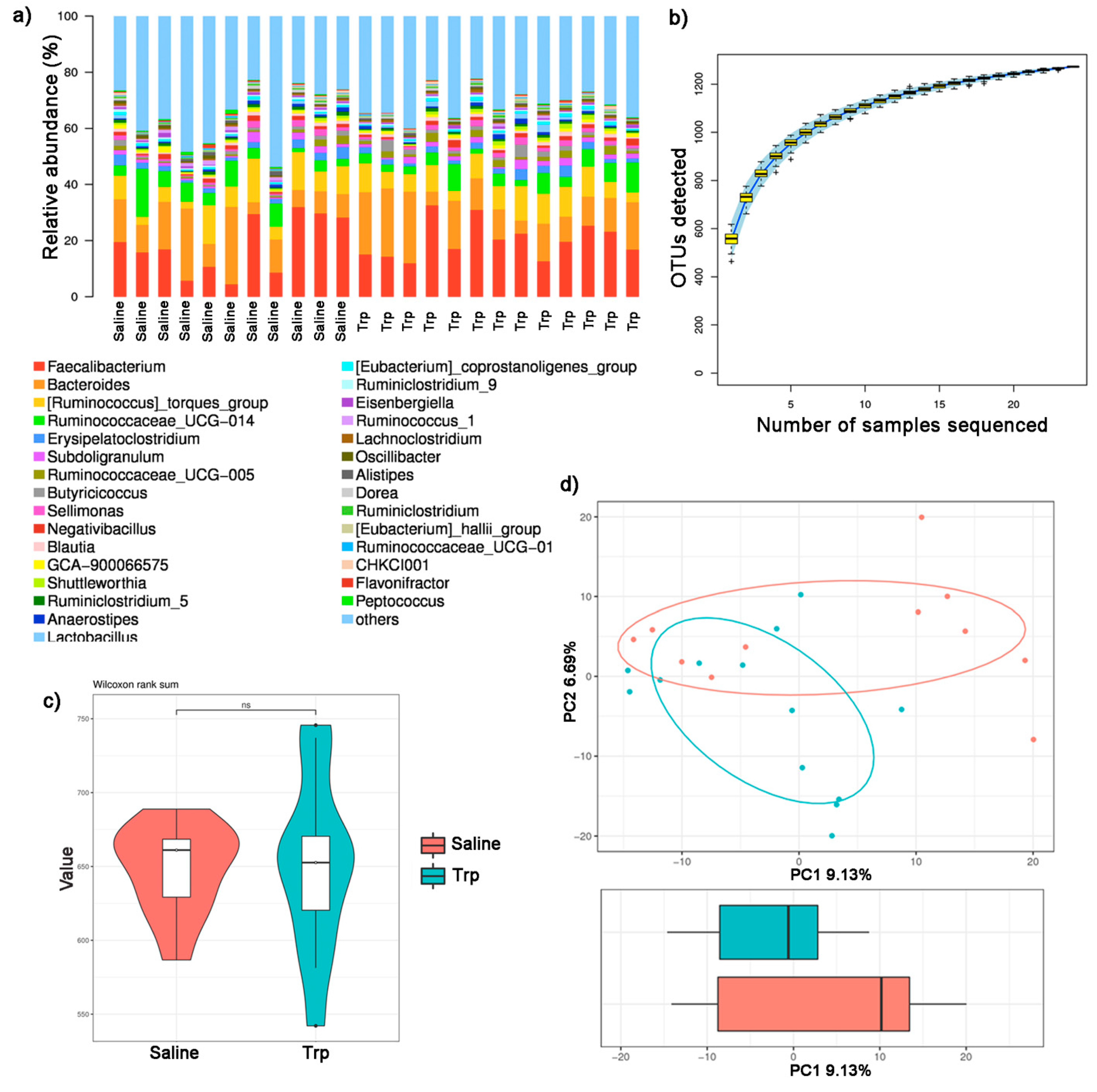
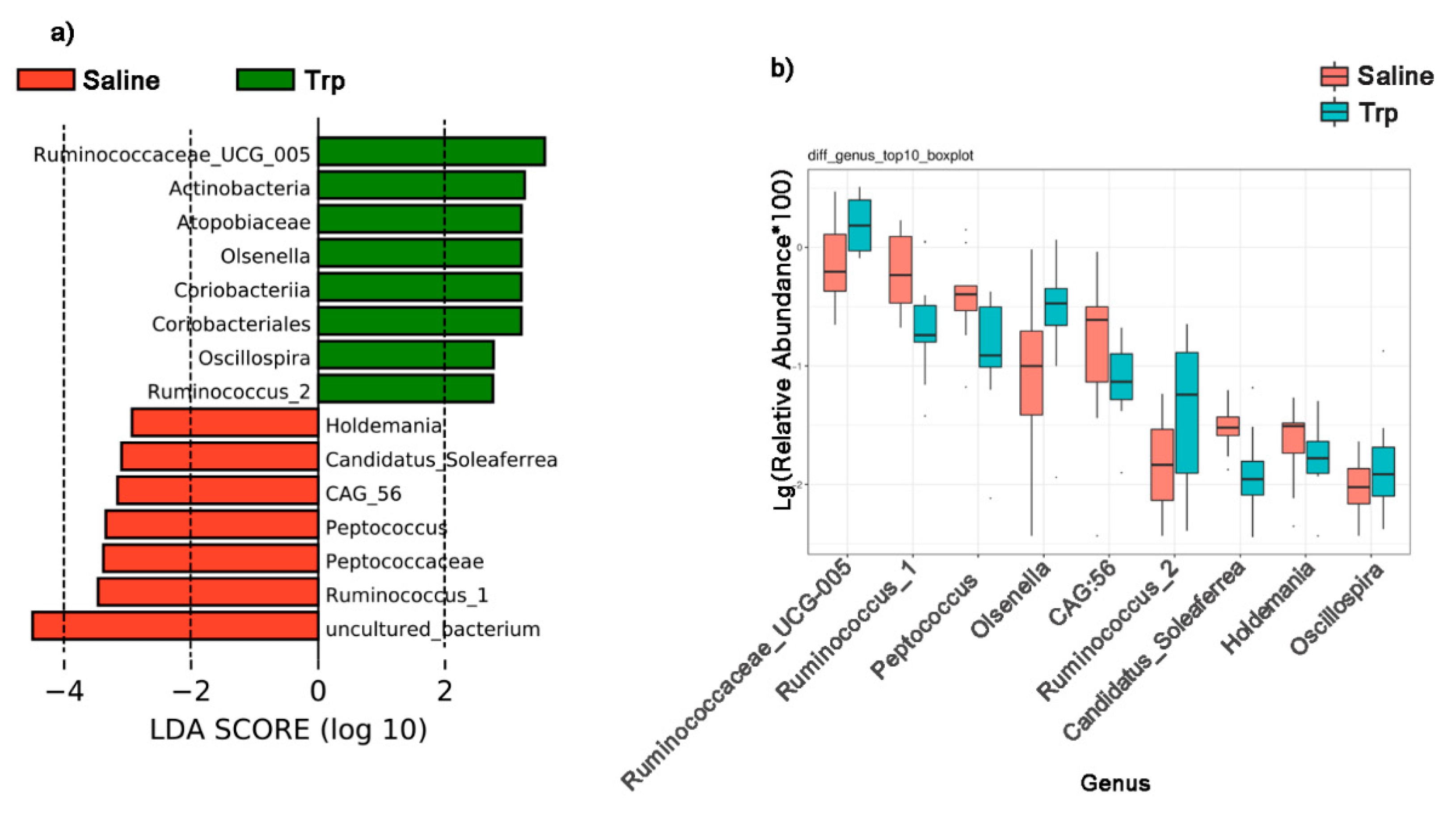
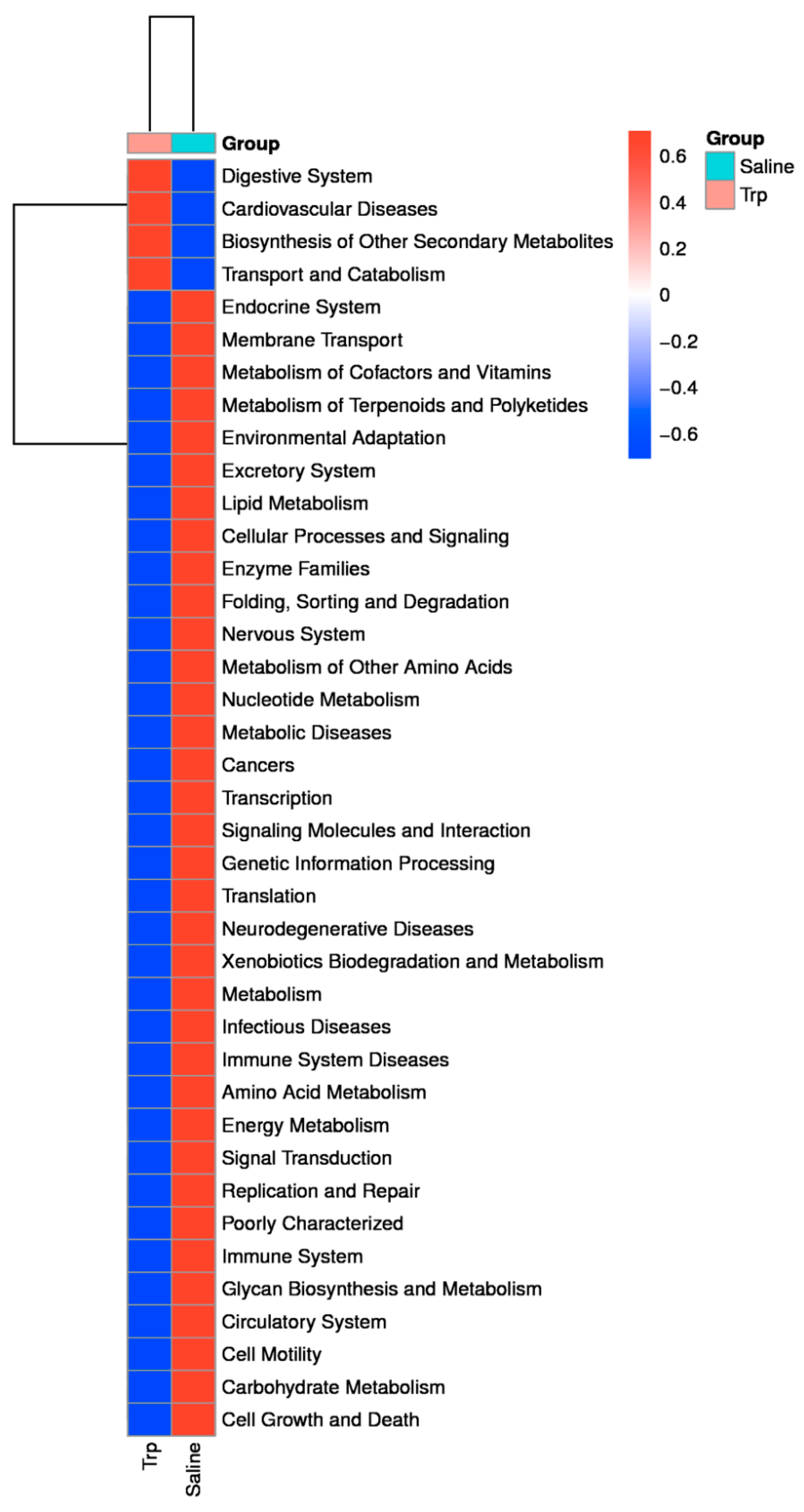
3.4. Embryonic Trp Exposure Altered Microbial Profile
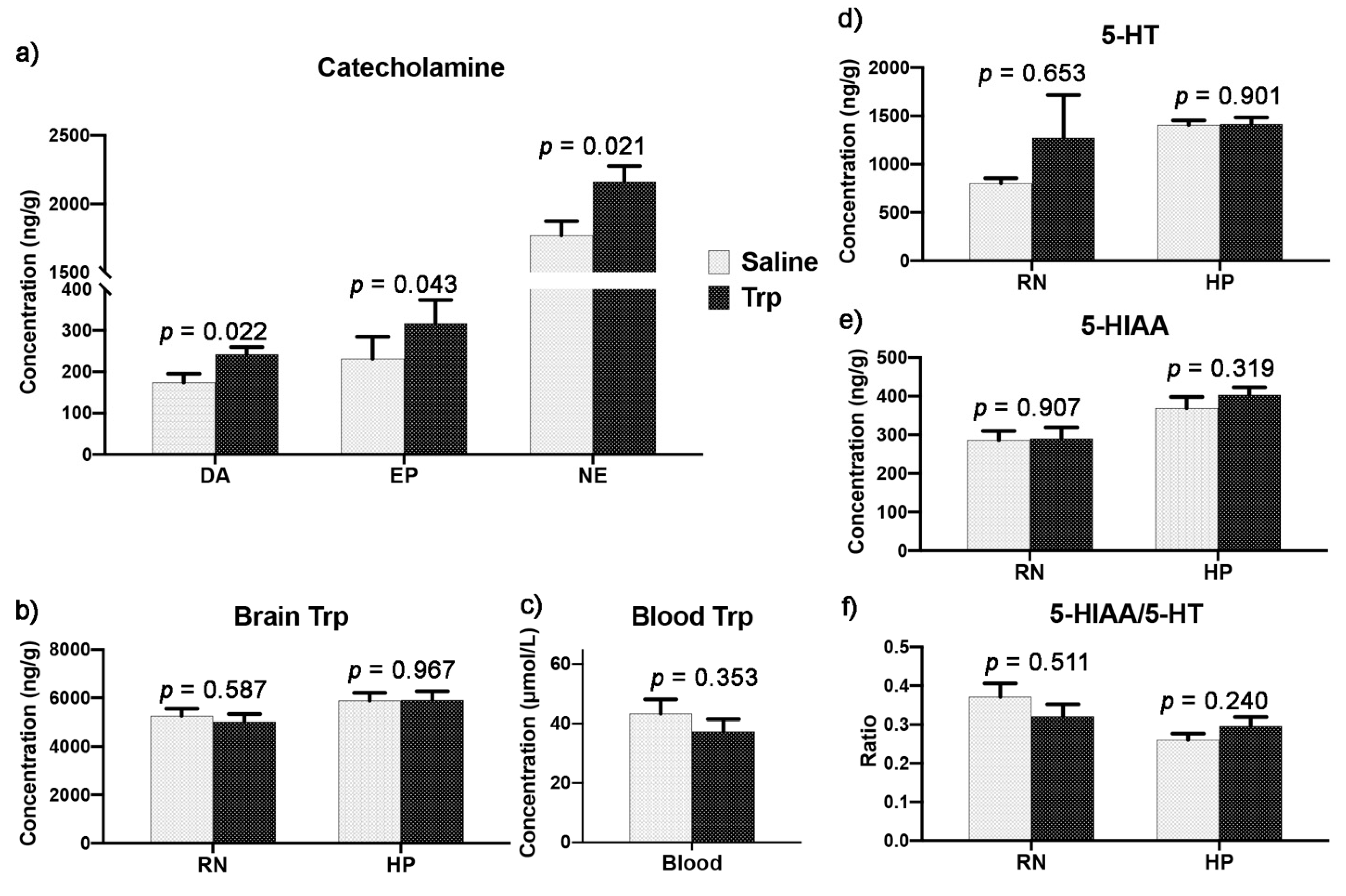
3.5. Embryonic Trp Exposure Affected Catecholamine and Serotonin Metabolism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ball, H.A.; Arseneault, L.; Taylor, A.; Maughan, B.; Caspi, A.; Moffitt, T.E. Genetic and environmental influences on victims, bullies and bully-victims in childhood. J. Child Psychol. Psychiatry 2008, 49, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.; Mundy, L.; Roberts, S.; Carta, R.; Waters, C.; Perra, O.; Jones, R.; Jones, I.; Goodyer, I.; Harold, G.; et al. Known risk factors for violence predict 12-month-old infants’ aggressiveness with peers. Psychol. Sci. 2011, 22, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Lereya, S.T.; Wolke, D. Prenatal family adversity and maternal mental health and vulnerability to peer victimisation at school. J. Child Psychol. Psychiatry 2013, 54, 644–652. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal origins of adult disease. Fetal Matern. Med. Rev. 1994, 6, 71–80. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Miyagawa, K.; Tsuji, M.; Fujimori, K.; Saito, Y.; Takeda, H. Prenatal stress induces anxiety-like behavior together with the disruption of central serotonin neurons in mice. Neurosci. Res. 2011, 70, 111–117. [Google Scholar] [CrossRef]
- Keane, J.M.; Khashan, A.S.; McCarthy, F.P.; Kenny, L.C.; Collins, J.M.; O’Donovan, S.; Brown, J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Identifying a biological signature of prenatal maternal stress. JCI Insight 2021, 6, e143007. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Antonson, A.M.; Rajasekera, T.A.; Patterson, J.M.; Bailey, M.T.; Gur, T.L. Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe-and CCL2-dependent mechanisms. Transl. Psychiatry 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Cappadocia, M.C.; Weiss, J.A.; Pepler, D. Bullying experiences among children and youth with autism spectrum disorders. J. Autism Dev. Disord. 2012, 42, 266–277. [Google Scholar] [CrossRef]
- Zinner, S.H.; Conelea, C.A.; Glew, G.M.; Woods, D.W.; Budman, C.L. Peer victimization in youth with Tourette syndrome and other chronic tic disorders. Child Psychiatry Hum. Dev. 2012, 43, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.M.; Fedina, C. Cyber bullying in ADHD and Asperger Syndrome populations. Res. Autism Spectr. Disord. 2011, 5, 1201–1208. [Google Scholar] [CrossRef]
- Law, K.P.; Han, T.-L.; Mao, X.; Zhang, H. Tryptophan and purine metabolites are consistently upregulated in the urinary metabolome of patients diagnosed with gestational diabetes mellitus throughout pregnancy: A longitudinal metabolomics study of Chinese pregnant women part 2. Clin. Chim. Acta 2017, 468, 126–139. [Google Scholar] [CrossRef]
- Badawy, A.A. The tryptophan utilization concept in pregnancy. Obstet. Gynecol. 2014, 57, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Roussin, L.; Prince, N.; Perez-Pardo, P.; Kraneveld, A.D.; Rabot, S.; Naudon, L. Role of the gut microbiota in the pathophysiology of autism spectrum disorder: Clinical and preclinical evidence. Microorganisms 2020, 8, 1369. [Google Scholar] [CrossRef]
- Higazi, A.M.; Kamel, H.M.; Abdel-Naeem, E.A.; Abdullah, N.M.; Mahrous, D.M.; Osman, A.M. Expression analysis of selected genes involved in tryptophan metabolic pathways in Egyptian children with Autism Spectrum Disorder and learning disabilities. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-W.; Jiang, S.; Hu, J. Gut-Brain Axis: Probiotic, Bacillus subtilis, Prevents Aggression via the Modification of the Central Serotonergic System. In Oral Health by Using Probiotic Products; Mahmoudi, R., Moosazad, S., Aghaei, K., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Galley, J.D.; Chen, H.J.; Antonson, A.M.; Gur, T.L. Prenatal stress-induced disruptions in microbial and host tryptophan metabolism and transport. Behav. Brain Res. 2021, 414, 113471. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Chen, N.; Ray, C.; Brewer, G.; Kornitzer, J.; Steer, R.A. A gut feeling: A hypothesis of the role of the microbiome in attention-deficit/hyperactivity disorders. Child Neurol. Open 2018, 5, 2329048X18786799. [Google Scholar] [CrossRef] [Green Version]
- Usui, N.; Matsuzaki, H.; Shimada, S. Characterization of Early Life Stress-Affected Gut Microbiota. Brain Sci. 2021, 11, 913. [Google Scholar] [CrossRef]
- Rincel, M.; Aubert, P.; Chevalier, J.; Grohard, P.-A.; Basso, L.; de Oliveira, C.M.; Helbling, J.C.; Lévy, É.; Chevalier, G.; Leboyer, M. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav. Immun. 2019, 80, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Partrick, K.A.; Chassaing, B.; Beach, L.Q.; McCann, K.E.; Gewirtz, A.T.; Huhman, K.L. Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behav. Brain Res. 2018, 345, 39–48. [Google Scholar] [CrossRef]
- Soto, M.; Herzog, C.; Pacheco, J.A.; Fujisaka, S.; Bullock, K.; Clish, C.B.; Kahn, C.R. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol. Psychiatry 2018, 23, 2287–2301. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M.; Penders, J. Does a prenatal bacterial microbiota exist? Mucosal Immunol. 2017, 10, 598–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa-Salva, O.; Mayer, U.; Versace, E.; Hébert, M.; Lemaire, B.S.; Vallortigara, G. Sensitive periods for social development: Interactions between predisposed and learned mechanisms. Cognition 2021, 104552. [Google Scholar] [CrossRef]
- Lorenzi, E.; Pross, A.; Rosa-Salva, O.; Versace, E.; Sgadò, P.; Vallortigara, G. Embryonic exposure to valproic acid affects social predispositions for dynamic cues of animate motion in newly-hatched chicks. Front. Physiol. 2019, 10, 501. [Google Scholar] [CrossRef] [Green Version]
- Nishigori, H.; Kagami, K.; Takahashi, A.; Tezuka, Y.; Sanbe, A.; Nishigori, H. Impaired social behavior in chicks exposed to sodium valproate during the last week of embryogenesis. Psychopharmacology 2013, 227, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Daigle, C.L.; Rodenburg, T.B.; Bolhuis, J.E.; Swanson, J.C.; Siegford, J.M. Individual consistency of feather pecking behavior in laying hens: Once a feather pecker always a feather pecker? Front. Vet. Sci. 2015, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Poe, S. Test of von Baer’s law of the conservation of early development. Evolution 2006, 60, 2239–2245. [Google Scholar] [CrossRef]
- Rashidi, H.; Sottile, V. The chick embryo: Hatching a model for contemporary biomedical research. BioEssays 2009, 31, 459–465. [Google Scholar] [CrossRef]
- Lee, S.; La, T.-M.; Lee, H.-J.; Choi, I.-S.; Song, C.-S.; Park, S.-Y.; Lee, J.-B.; Lee, S.-W. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Huwaida, E.; Sakin, M.; Elagib, H.A.; Bakhiet, E.; Dousa, B.; Elamin, K. Effect of egg weight and egg shell thickness on hatchability and embryonic mortality of Cobb broiler breeder eggs. Glob. J. Anim. Sci. Res. 2015, 3, 186–190. [Google Scholar]
- Nayak, N.; Rajini, R.A.; Ezhilvalavan, S.; Kirubaharan, J.J.; Sahu, A.R.; Manimaran, K. Effect of in ovo feeding of arginine and/or tryptophan on hatchability and small intestinal morphology in broiler chicken. Indian J. Poult. Sci. 2015, 50, 18–23. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef] [PubMed]
- Hy-Line W-36 Commercial Layers Management Guide. Available online: https://www.hyline.com/filesimages/Hy-Line-Products/Hy-Line-Product-PDFs/W-36/36%20COM%20ENG.pdf (accessed on 1 January 2020).
- Huang, X.; Kuang, S.; Applegate, T.J.; Lin, T.-L.; Cheng, H.-W. The development of the serotonergic and dopaminergic systems during chicken mid-late embryogenesis. Mol. Cell. Endocrinol. 2019, 493, 110472. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.; Maas, D.A.; Fricke, K.; de Vries, R.B.; Poelmans, G.; Glennon, J.C. Aggressive behavior in transgenic animal models: A systematic review. Neurosci. Biobehav. Rev. 2018, 91, 198–217. [Google Scholar] [CrossRef]
- Dennis, R.L.; Fahey, A.G.; Cheng, H.W. Alterations to embryonic serotonin change aggression and fearfulness. Aggress. Behav. 2013, 39, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Mohammed, A.; Jacobs, J.; Cramer, T.; Cheng, H. Effect of synbiotics on thyroid hormones, intestinal histomorphology, and heat shock protein 70 expression in broiler chickens reared under cyclic heat stress. Poult. Sci. 2020, 99, 142–150. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, K.; Gorley, R. PRIMER v5: User Manual/Tutorial; Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Coşkun, Ş.; Özer, Ç.; Gönül, B.; Take, G.; Erdoğan, D. The effect of repeated tryptophan administration on body weight, food intake, brain lipid peroxidation and serotonin immunoreactivity in mice. Mol. Cell. Biochem. 2006, 286, 133–138. [Google Scholar] [CrossRef]
- Tsuji, A.; Nakata, C.; Sano, M.; Fukuwatari, T.; Shibata, K. L-tryptophan metabolism in pregnant mice fed a high L-tryptophan diet and the effect on maternal, placental, and fetal growth. Int. J. Tryptophan Res. 2013, 6, S12715. [Google Scholar] [CrossRef]
- Young, S.N. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos. Trans. R. Soc. 2013, 368, 20110375. [Google Scholar] [CrossRef] [Green Version]
- Methiwala, H.N.; Vaidya, B.; Vamsi, K.; Bishnoi, M.; Sharma, S.S.; Kondepudi, K.K. Gut microbiota in mental health and depression: Role of pre/pro/synbiotics in their modulation. Food Funct. 2021. [Google Scholar] [CrossRef] [PubMed]
- De Haas, E.N.; Newberry, R.C.; Edgar, J.; Riber, A.B.; Estevez, I.; Ferrante, V.; Hernandez, C.E.; Kjaer, J.B.; Ozkan, S.; Dimitrov, I. Prenatal and Early Postnatal Behavioural Programming in Laying Hens, With Possible Implications for the Development of Injurious Pecking. Front. Vet. Sci. 2021, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. In Seminars in Fetal and Neonatal Medicine; WB Saunders: Philadelphia, PA, USA, 2016; pp. 410–417. [Google Scholar]
- Kuo, P.-H.; Chung, Y.-C.E. Moody microbiome: Challenges and chances. J. Formos. Med. Assoc. 2019, 118, S42–S54. [Google Scholar] [CrossRef] [PubMed]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Lasheras, I.; Seral, P.; Latorre, E.; Barroso, E.; Gracia-García, P.; Santabárbara, J. Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian J. Psychiatr. 2020, 47, 101874. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jensen, R.L.; Højberg, O.; Canibe, N.; Jensen, B.B. Olsenella scatoligenes sp. nov., a 3-methylindole-(skatole) and 4-methylphenol-(p-cresol) producing bacterium isolated from pig faeces. Int. J. Syst. Evol. 2015, 65, 1227–1233. [Google Scholar] [CrossRef] [Green Version]
- Osadchiy, V.; Labus, J.S.; Gupta, A.; Jacobs, J.; Ashe-McNalley, C.; Hsiao, E.Y.; Mayer, E.A. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE 2018, 13, e0201772. [Google Scholar] [CrossRef] [Green Version]
- Saffarian, A.; Mulet, C.; Regnault, B.; Amiot, A.; Tran-Van-Nhieu, J.; Ravel, J.; Sobhani, I.; Sansonetti, P.J.; Pedron, T. Crypt-and mucosa-associated core microbiotas in humans and their alteration in colon cancer patients. MBio 2019, 10, e01315–e01319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria–From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The protective role of butyrate against obesity and obesity-related diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Duran, N.L.; Olson, S.L.; Hajal, N.J.; Felt, B.T.; Vazquez, D.M. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. J. Abnorm. Child Psychol. 2009, 37, 169–182. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA axis as target for depression outdated, or is there a new hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Buske-Kirschbaum, A.; Trikojat, K.; Tesch, F.; Schmitt, J.; Roessner, V.; Luksch, H.; Rösen-Wolff, A.; Plessow, F. Altered hypothalamus-pituitary-adrenal axis function: A relevant factor in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder? Psychoneuroendocrinology 2019, 105, 178–186. [Google Scholar] [CrossRef]
- Schalbroeck, R.; van Velden, F.H.; de Geus-Oei, L.-F.; Yaqub, M.; van Amelsvoort, T.; Booij, J.; Selten, J.-P. Striatal dopamine synthesis capacity in autism spectrum disorder and its relation with social defeat: An [18 F]-FDOPA PET/CT study. Transl. Psychiatry 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.L.; Kroeger, R.A.; Stroope, S. Autism spectrum disorder symptoms and bullying victimization among children with autism in the United States. J. Autism Dev. Disord. 2020, 50, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Nicola, S.; Taha, S.; Kim, S.; Fields, H. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience 2005, 135, 1025–1033. [Google Scholar] [CrossRef]
- Dennis, R. Adrenergic and noradrenergic regulation of poultry behavior and production. Domest. Anim. Endocrinol. 2016, 56, S94–S100. [Google Scholar] [CrossRef]
- Yu, C.; Garcia-Olivares, J.; Candler, S.; Schwabe, S.; Maletic, V. New insights into the mechanism of action of viloxazine: Serotonin and norepinephrine modulating properties. J. Exp. Pharmacol. 2020, 12, 285. [Google Scholar] [CrossRef]
- Bacchini, D.; Affuso, G.; Trotta, T. Temperament, ADHD and peer relations among schoolchildren: The mediating role of school bullying. Aggress. Behav. 2008, 34, 447–459. [Google Scholar] [CrossRef]
- Billes, S.K.; Cowley, M.A. Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology 2007, 32, 822–834. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.-Y.; Palmiter, R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 1995, 83, 1197–1209. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-J.; Meguid, M.M.; Chai, J.-K.; Chen, C.; Oler, A. Bilateral hypothalamic dopamine infusion in male Zucker rat suppresses feeding due to reduced meal size. Pharmacol. Biochem. Behav. 1997, 58, 631–635. [Google Scholar] [CrossRef]
- Thomas, S.A.; Palmiter, R.D. Disruption of the dopamine Β-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav. Neurosci. 1997, 111, 579. [Google Scholar] [CrossRef] [PubMed]
- Leshem, R.; Bar-Oz, B.; Diav-Citrin, O.; Gbaly, S.; Soliman, J.; Renoux, C.; Matok, I. Selective serotonin reuptake inhibitors (SSRIs) and Serotonin Norepinephrine Reuptake Inhibitors (SNRIs) During Pregnancy and the Risk for Autism spectrum disorder (ASD) and Attention deficit hyperactivity disorder (ADHD) in the Offspring: A True Effect or a Bias? A Systematic Review & Meta-Analysis. Reprod. Toxicol. 2020, 97, 3. [Google Scholar] [CrossRef]
- Kuikka, J.T.; Tammela, L.; Karhunen, L.; Rissanen, A.; Bergström, K.A.; Naukkarinen, H.; Vanninen, E.; Karhu, J.; Lappalainen, R.; Repo-Tiihonen, E. Reduced serotonin transporter binding in binge eating women. Psychopharmacology 2001, 155, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H. Anorexia nervosa, obessional behavior, and serotonin. Psychopharmacol. Bull. 1997, 33, 335. [Google Scholar]
- Berthoud, H.-R.; Albaugh, V.L.; Neuhuber, W.L. Gut-brain communication and obesity: Understanding functions of the vagus nerve. J. Clin. Investig. 2021, 131, e143770. [Google Scholar] [CrossRef]
- de Weerth, C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Wassum, K.M.; Izquierdo, A. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev. 2015, 57, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.M.; Kemp, A.H.; Felmingham, K.; Barton, M.; Olivieri, G.; Peduto, A.; Gordon, E.; Bryant, R.A. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 2006, 29, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.D.; Caldji, C.; Champagne, F.; Plotsky, P.M.; Meaney, M.J. The role of corticotropin-releasing factor–norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol. Psychiatry 1999, 46, 1153–1166. [Google Scholar] [CrossRef]
- Glass, M.J.; Billington, C.J.; Levine, A.S. Naltrexone administered to central nucleus of amygdala or PVN: Neural dissociation of diet and energy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R86–R92. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Sammartino, F.; Young, N.A.; Corrigan, J.; Krishna, V.; Rezai, A.R. Anatomic review of the ventral capsule/ventral striatum and the nucleus accumbens to guide target selection for deep brain stimulation for obsessive-compulsive disorder. World Neurosurg. 2019, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Liu, J.-J.; Xia, J.; Liu, J.; Mirabella, V.; Pang, Z.P. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 2015, 12, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestler, E.J. Role of the brain’s reward circuitry in depression: Transcriptional mechanisms. Int. Rev. Neurobiol. 2015, 124, 151–170. [Google Scholar] [CrossRef] [Green Version]


| Behaviors | Description | |
|---|---|---|
| Aggressive peck | Forceful pecks to the head or neck of the other bird | |
| Body peck | Severe peck | Vigorous pecks to at the feather on the wing, back, or tail, that tend to pull, break or remove the feathers |
| Feather peck | Gentle pecks/nibbles at the feathers on the wing, back, or tail | |
| Kick | Forceful extension of leg to make contact with the other bird | |
| Threat | Standing with the neck erected and hackle feathers raised in front of the other bird | |
| Fight with peck | Similar to threat, but at least one aggressive peck is involved | |
| Fight with kick | Similar to threat, but at least one kick is involved | |
| n | F | p | |
|---|---|---|---|
| Aggressive behaviors (7th week) | 7 | 4.31 | 0.060 |
| Aggressive peck | 7 | 2.6 | 0.133 |
| Sever peck | 7 | 2.38 | 0.149 |
| Feather peck | 7 | 0.15 | 0.709 |
| Kick | 7 | 4.27 | 0.061 |
| Threat | 7 | 3.48 | 0.087 |
| Aggressive behaviors (15th week) | 6 | 3.77 | 0.081 |
| Aggressive peck | 6 | 1.59 | 0.236 |
| Sever peck | 6 | 1.38 | 0.268 |
| Feather peck | 6 | 2.91 | 0.119 |
| Kick | 6 | 0.66 | 0.437 |
| Threat | 6 | 0.63 | 0.444 |
| Aggressive behaviors (18th week) | 9 | 2.43 | 0.138 |
| Aggressive peck | 9 | 1.38 | 0.257 |
| Sever peck | 9 | 0.01 | 0.938 |
| Feather peck | 9 | 3.23 | 0.091 |
| Kick | 9 | 0.17 | 0.688 |
| Threat | 9 | 1.95 | 0.182 |
| Method Name | ANOSIM |
|---|---|
| Test statistic name | R |
| Sample size | 24 |
| Number of groups | 2 |
| Test statistic | 0.120826542 |
| p-value | 0.036 |
| Number of permutations | 999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Hu, J.; Peng, H.; Cheng, H.-w. Embryonic Exposure to Tryptophan Yields Bullying Victimization via Reprogramming the Microbiota-Gut-Brain Axis in a Chicken Model. Nutrients 2022, 14, 661. https://doi.org/10.3390/nu14030661
Huang X, Hu J, Peng H, Cheng H-w. Embryonic Exposure to Tryptophan Yields Bullying Victimization via Reprogramming the Microbiota-Gut-Brain Axis in a Chicken Model. Nutrients. 2022; 14(3):661. https://doi.org/10.3390/nu14030661
Chicago/Turabian StyleHuang, Xiaohong, Jiaying Hu, Haining Peng, and Heng-wei Cheng. 2022. "Embryonic Exposure to Tryptophan Yields Bullying Victimization via Reprogramming the Microbiota-Gut-Brain Axis in a Chicken Model" Nutrients 14, no. 3: 661. https://doi.org/10.3390/nu14030661







