Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Lipoteichoic Acid Purification and Administration
2.3. Bone Marrow-Derived Dendritic Cell Differentiation and Stimulation
2.4. Expression of MHC Class-II and CD86 and Cytokine Secretion by LTA-Treated BMDC
2.5. Antigen-Specific T Cell Proliferation
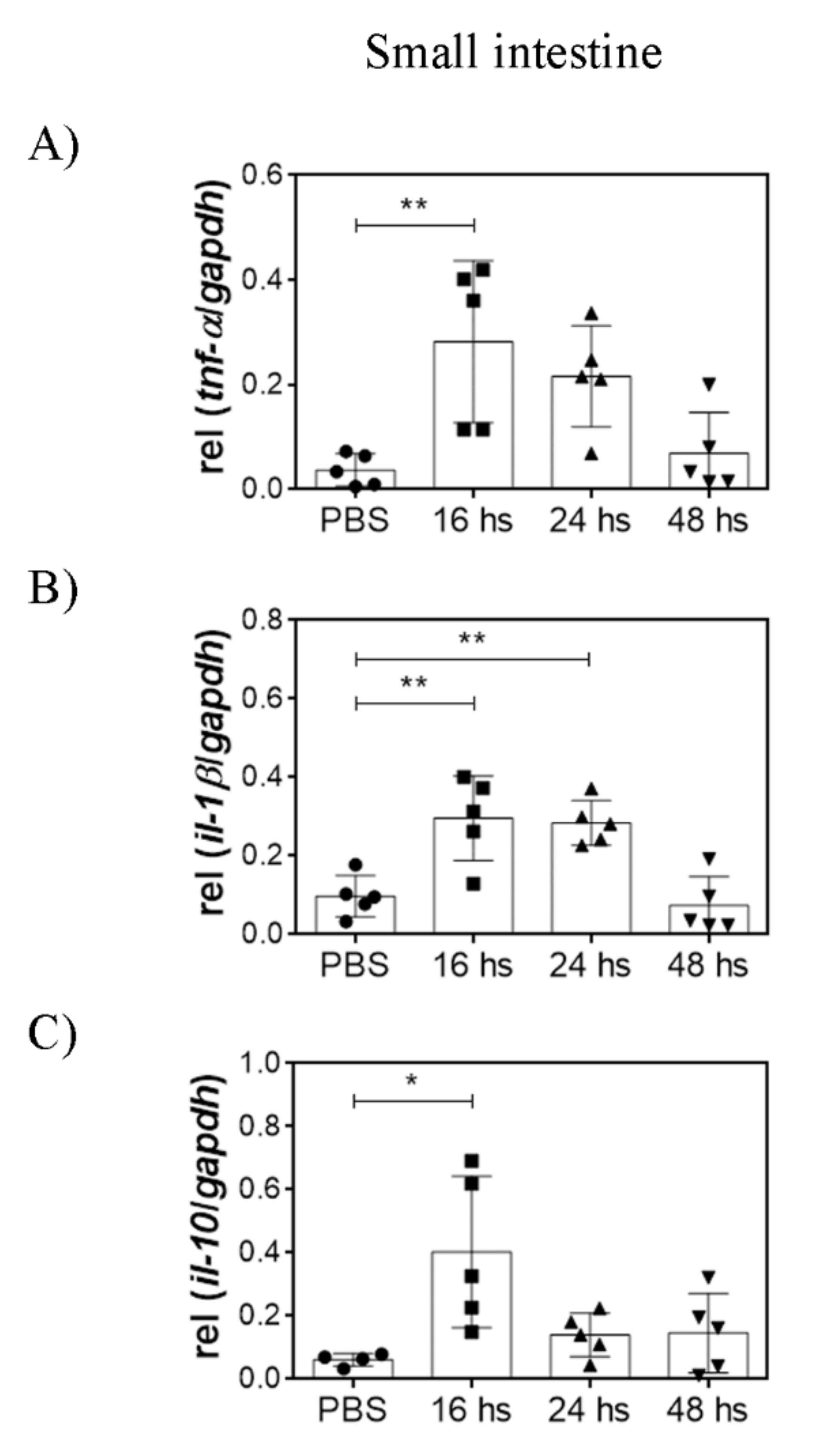
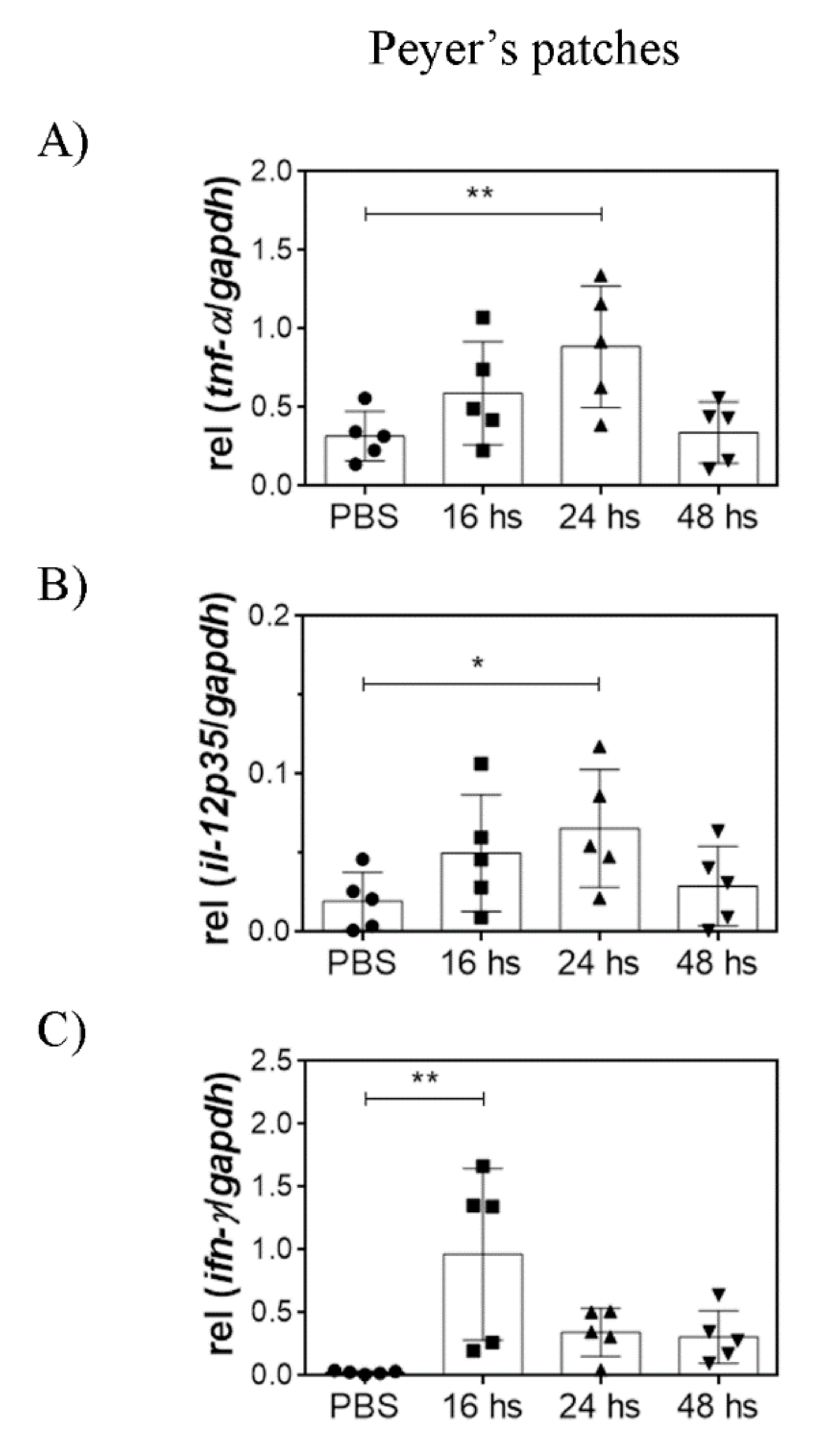
2.6. Analysis of Cytokine Transcripts in the GALT
2.7. Cell Phenotype in the GALT
2.8. Statistical Analysis
3. Results
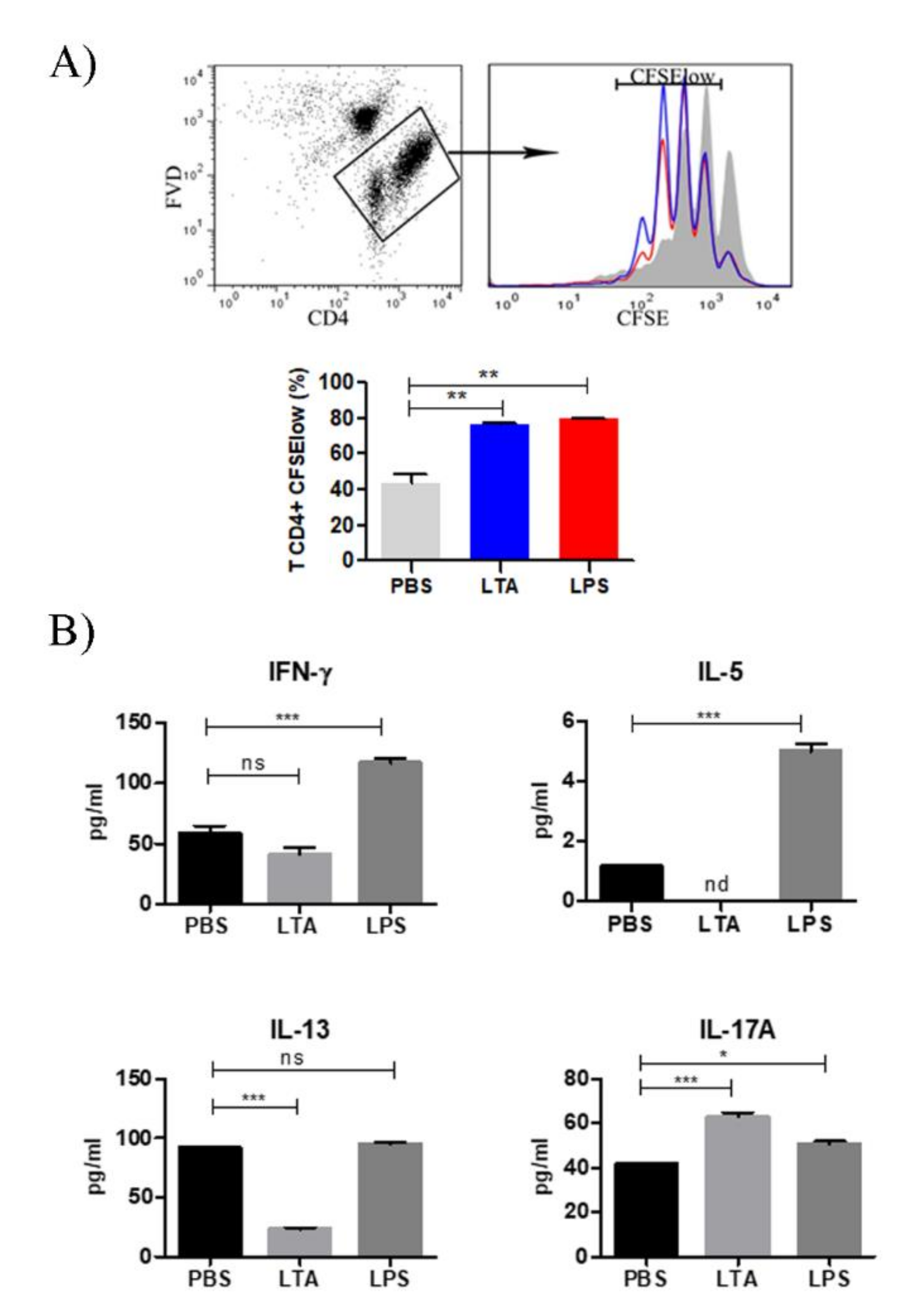
3.1. LTA from LGG Promotes the Activation of BMDC
3.2. LTA Treatment Increases T Cell Priming Capacity of BMDC
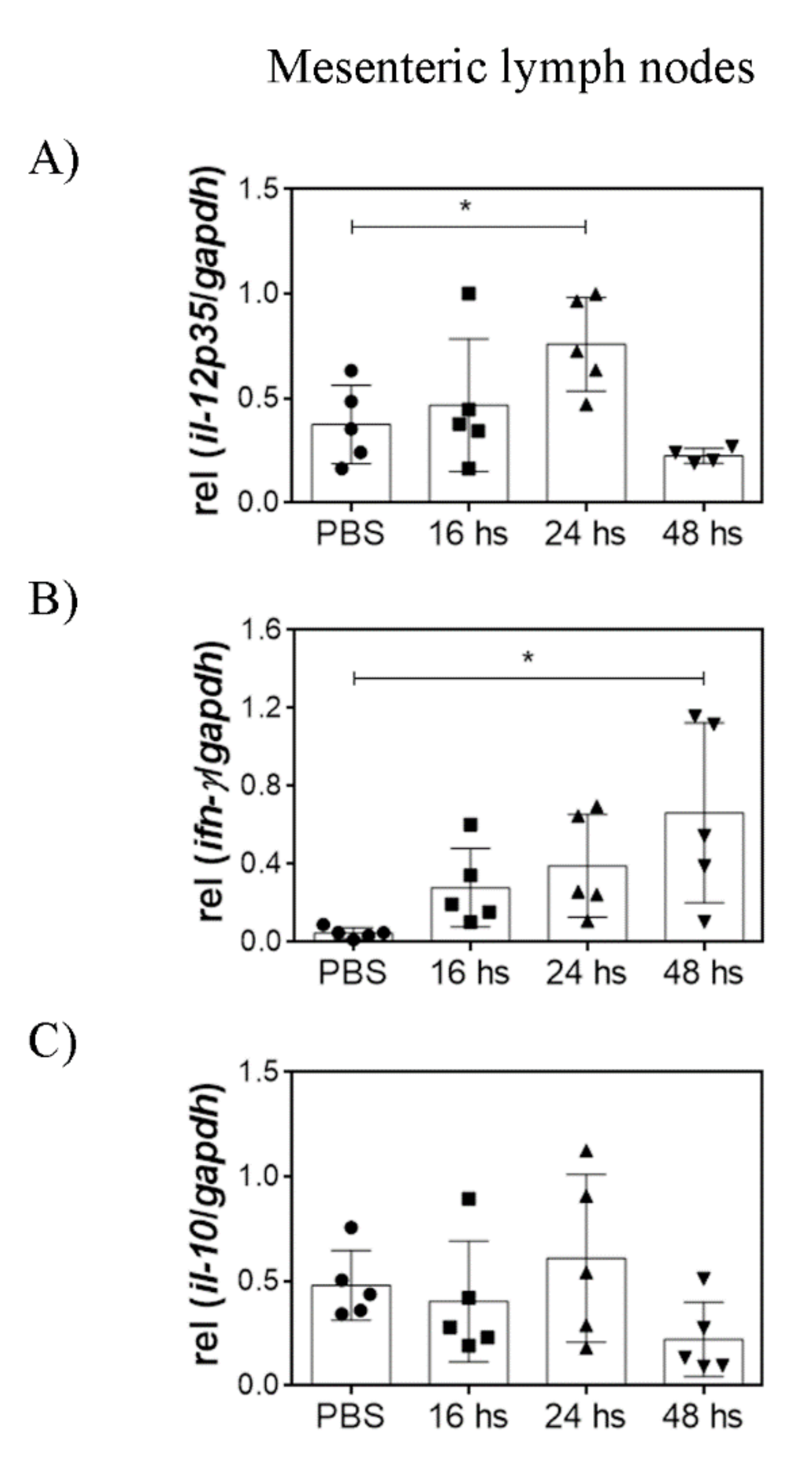
3.3. Oral LTA Modifies Cytokine Transcription in the GALT
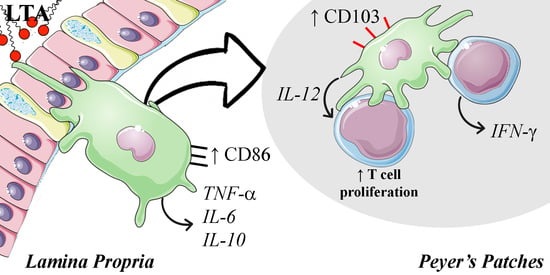
3.4. LTA Promotes the Reduction in Mature DC in the Lamina Propria, but It Increases the Population of CD103+ DC in the PP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corr, S.C.; Hill, C.; Gahan, C.G.M. Understanding the Mechanisms by Which Probiotics Inhibit Gastrointestinal Pathogens. Adv. Food Nutr. Res. 2009, 56, 1–15. [Google Scholar] [PubMed]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 Mucin Secretion Follows Adherence of Lactobacillus Strains to Intestinal Epithelial Cells in Vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.K.; Samak, G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [PubMed] [Green Version]
- Wells, J.M. Immunomodulatory Mechanisms of Lactobacilli. Microb. Cell Fact. 2011, 10, S17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraishi, T.; Yokota, S.I.; Fukiya, S.; Yokota, A. Structural Diversity and Biological Significance of Lipoteichoic Acid in Gram-Positive Bacteria: Focusing on Beneficial Probiotic Lactic Acid Bacteria. Biosci. Microbiota Food Health 2016, 35, 147–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claes, I.J.J.; Segers, M.E.; Verhoeven, T.L.A.; Dusselier, M.; Sels, B.F.; De Keersmaecker, S.C.J.; Vanderleyden, J.; Lebeer, S. Lipoteichoic Acid Is an Important Microbe-Associated Molecular Pattern of Lactobacillus Rhamnosus GG. Microb. Cell Fact. 2012, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.H.; Baik, J.E.; Yang, J.S.; Kang, S.S.; Im, J.; Yun, C.H.; Kim, D.W.; Lee, K.; Chung, D.K.; Ju, H.R.; et al. Differential Immunostimulatory Effects of Gram-Positive Bacteria Due to Their Lipoteichoic Acids. Int. Immunopharmacol. 2009, 9, 127–133. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, N.-R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic Acid Isolated from Lactobacillus Plantarum Inhibits Lipopolysaccharide-Induced TNF-α Production in THP-1 Cells and Endotoxin Shock in Mice. J. Immunol. 2008, 180, 2553–2561. [Google Scholar] [CrossRef] [Green Version]
- Molina, M.A.; Díaz, A.M.; Hesse, C.; Ginter, W.; Gentilini, M.V.; Nuñez, G.G.; Canellada, A.M.; Sparwasser, T.; Berod, L.; Castro, M.S.; et al. Immunostimulatory Effects Triggered by Enterococcus Faecalis CECT7121 Probiotic Strain Involve Activation of Dendritic Cells and Interferon-Gamma Production. PLoS ONE 2015, 10, e0127262. [Google Scholar] [CrossRef] [Green Version]
- Schröder, N.W.J.; Morath, S.; Alexander, C.; Hamann, L.; Hartung, T.; Zähringer, U.; Göbel, U.B.; Weber, J.R.; Schumann, R.R. Lipoteichoic Acid (LTA) of Streptococcus Pneumoniae and Staphylococcus Aureus Activates Immune Cells via Toll-like Receptor (TLR)-2, Lipopolysaccharide-Binding Protein (LBP), and CD14, Whereas TLR-4 and MD-2 Are Not Involved. J. Biol. Chem. 2003, 278, 15587–15594. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Jang, S.; Jung, B.J.; Jang, K.S.; Kim, B.G.; Chung, D.K.; Kim, H. Differential Immune-Stimulatory Effects of LTAs from Different Lactic Acid Bacteria via MAPK Signaling Pathway in RAW 264.7 Cells. Immunobiology 2015, 220, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Weill, F.S.; Cela, E.M.; Paz, M.L.; Ferrari, A.; Leoni, J.; González Maglio, D.H. Lipoteichoic Acid from Lactobacillus Rhamnosus GG as an Oral Photoprotective Agent against UV-Induced Carcinogenesis. Br. J. Nutr. 2013, 109, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, A.D.; Campo, V.E.; Cela, E.M.; Morelli, A.E.; Shufesky, W.J.; Tckacheva, O.A.; Leoni, J.; Paz, M.L.; Larregina, A.T.; González Maglio, D.H. Oral Administration of Lipoteichoic Acid from Lactobacillus Rhamnosus GG Overcomes UVB-Induced Immunosuppression and Impairs Skin Tumor Growth in Mice. Eur. J. Immunol. 2019, 49, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.; Geyer, A.; Hartung, T. Structure–Function Relationship of Cytokine Induction by Lipoteichoic Acid from Staphylococcus aureus. J. Exp. Med. 2001, 193, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janelsins, B.M.; Mathers, A.R.; Tkacheva, O.A.; Erdos, G.; Shufesky, W.J.; Morelli, A.E.; Larregina, A.T. Proinflammatory Tachykinins That Signal through the Neurokinin 1 Receptor Promote Survival of Dendritic Cells and Potent Cellular Immunity. Blood 2009, 113, 3017–3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.S.; Kim, G.; Ju, Y.J.; Cheon, I.S.; Hong, S.W.; Kim, D.W.; Park, B.C.; Han, S.H.; Yun, C.H. Distinct Pattern of Immune Tolerance in Dendritic Cells Treated with Lipopolysaccharide or Lipoteichoic Acid. Mol. Immunol. 2017, 91, 57–64. [Google Scholar] [CrossRef]
- Volz, T.; Kaesler, S.; Draing, C.; Hartung, T.; Röcken, M.; Skabytska, Y.; Biedermann, T. Induction of IL-10-Balanced Immune Profiles Following Exposure to LTA from Staphylococcus Epidermidis. Exp. Dermatol. 2018, 27, 318–326. [Google Scholar] [CrossRef]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented Foods: Patented Approaches and Formulations for Nutritional Supplementation and Health Promotion. Recent Patents Food Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and Other Fermented Foods as Sources of Health-Promoting Bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, A.; Paz, M.; Leoni, J.; González Maglio, D. Message in a Bottle: Dialog between Intestine and Skin Modulated by Probiotics. Int. J. Mol. Sci. 2017, 18, 1067. [Google Scholar] [CrossRef] [Green Version]
- Schwandner, R.; Dziarski, R.; Wesche, H.; Rothe, M.; Kirschning, C.J. Peptidoglycan- and Lipoteichoic Acid-Induced Cell Activation Is Mediated by Toll-like Receptor 2. J. Biol. Chem. 1999, 274, 17406–17409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y.; et al. Lipoteichoic Acid Is Involved in the Ability of the Immunobiotic Strain Lactobacillus Plantarum CRL1506 to Modulate the Intestinal Antiviral Innate Immunity Triggered by TLR3 Activation. Front. Immunol. 2020, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Abedin-Do, A.; Taherian-Esfahani, Z.; Ghafouri-Fard, S.; Ghafouri-Fard, S.; Motevaseli, E. Immunomodulatory Effects of Lactobacillus Strains: Emphasis on Their Effects on Cancer Cells. Immunotherapy 2015, 7, 1307–1329. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Strobl, H.; Schuler, G.; Romani, N. GM-CSF Monocyte-Derived Cells and Langerhans Cells as Part of the Dendritic Cell Family. Front. Immunol. 2017, 8, 1388. [Google Scholar] [CrossRef]
- Price, A.E.; Shamardani, K.; Lugo, K.A.; Deguine, J.; Roberts, A.W.; Lee, B.L.; Barton, G.M. A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity 2018, 49, 560–575.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deetz, C.O.; Hebbeler, A.M.; Propp, N.A.; Cairo, C.; Tikhonov, I.; Pauza, C.D. Gamma Interferon Secretion by Human Vgamma2Vdelta2 T Cells after Stimulation with Antibody against the T-Cell Receptor plus the Toll-Like Receptor 2 Agonist Pam3Cys. Infect. Immun. 2006, 74, 4505–4511. [Google Scholar] [CrossRef] [Green Version]
- Ismaili, J.; Olislagers, V.; Poupot, R.; Fournié, J.-J.; Goldman, M. Human Gamma Delta T Cells Induce Dendritic Cell Maturation. Clin. Immunol. 2002, 103, 296–302. [Google Scholar] [CrossRef]
- Devilder, M.-C.; Allain, S.; Dousset, C.; Bonneville, M.; Scotet, E. Early Triggering of Exclusive IFN-γ Responses of Human Vγ9Vδ2 T Cells by TLR-Activated Myeloid and Plasmacytoid Dendritic Cells. J. Immunol. 2009, 183, 3625–3633. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, C.; Wagner, C.; Bonnardel, J.; Gorvel, J.P.; Lelouard, H. The Peyer’s Patch Mononuclear Phagocyte System at Steady State and during Infection. Front. Immunol. 2017, 8, 1254. [Google Scholar] [CrossRef]
- Schulz, O.; Jaensson, E.; Persson, E.K.; Liu, X.; Worbs, T.; Agace, W.W.; Pabst, O. Intestinal CD103+, but Not CX3CR1+, Antigen Sampling Cells Migrate in Lymph and Serve Classical Dendritic Cell Functions. J. Exp. Med. 2009, 206, 3101–3114. [Google Scholar] [CrossRef]
- Stagg, A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerovic, V.; Houston, S.A.; Scott, C.L.; Aumeunier, A.; Yrlid, U.; Mowat, A.M.; Milling, S.W.F. Intestinal CD103—Dendritic Cells Migrate in Lymph and Prime Effector T Cells. Mucosal Immunol. 2013, 6, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.G.; Yin, X.; Swaminathan, A.; Eisenbarth, S.C. Antigen-Presenting Cells in Food Tolerance and Allergy. Front. Immunol. 2021, 11, 3362. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Reis e Sousa, C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gen | 5′-3′ Forward Primer Sequence | 5′-3′ Reverse Primer Sequence |
|---|---|---|
| IL-12p35 | TACTAGAGAGACTTCTTCCACAACAAGAG | TCTGGTACATCTTCAAGTCCTCATAGA |
| IFN-γ | TGCATCTTGGCTTTGCAGCTCTTC | GGGTTGTTGACCTCAAACTTGGCA |
| IL-10 | ATGCTGCCTGCTCTTACTGACTG | CCCAAGTAACCCTTAAAGTCCTGC |
| TNF-α | GGCAGGTCTACTTTGGAGTCATTGC | ACATTCGAGGCTCCAGTGAATTCGG |
| IL-6 | CGTGGAAATGAGAAAAGAGTTGTGC | ATGCTTAGGCATAACGCACTAGGT |
| TGF-β | ACTGGAGTTGTACGGCAGTG | GGATCCACTTCCAACCCAGG |
| IL-1β | TGCCACCTTTTGACAGTGATG | AAGGTCCACGGGAAAGACAC |
| GAPDH | TGAAGGTCGGTGTGAACGG | CGTGAGTGGAGTCATACTGGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, A.D.; Leoni, J.; Paz, M.L.; González Maglio, D.H. Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut. Nutrients 2022, 14, 723. https://doi.org/10.3390/nu14030723
Friedrich AD, Leoni J, Paz ML, González Maglio DH. Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut. Nutrients. 2022; 14(3):723. https://doi.org/10.3390/nu14030723
Chicago/Turabian StyleFriedrich, Adrián D., Juliana Leoni, Mariela L. Paz, and Daniel H. González Maglio. 2022. "Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut" Nutrients 14, no. 3: 723. https://doi.org/10.3390/nu14030723
APA StyleFriedrich, A. D., Leoni, J., Paz, M. L., & González Maglio, D. H. (2022). Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut. Nutrients, 14(3), 723. https://doi.org/10.3390/nu14030723