Hepatic Steatosis Assessment as a New Strategy for the Metabolic and Nutritional Management of Duchenne Muscular Dystrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric and Biochemical Analysis
2.3. Ultrasound Examination for Hepatic Steatosis Assessment
2.4. Diagnostic Criteria for Metabolic Risk Factors
2.5. Statistical Analysis
3. Results
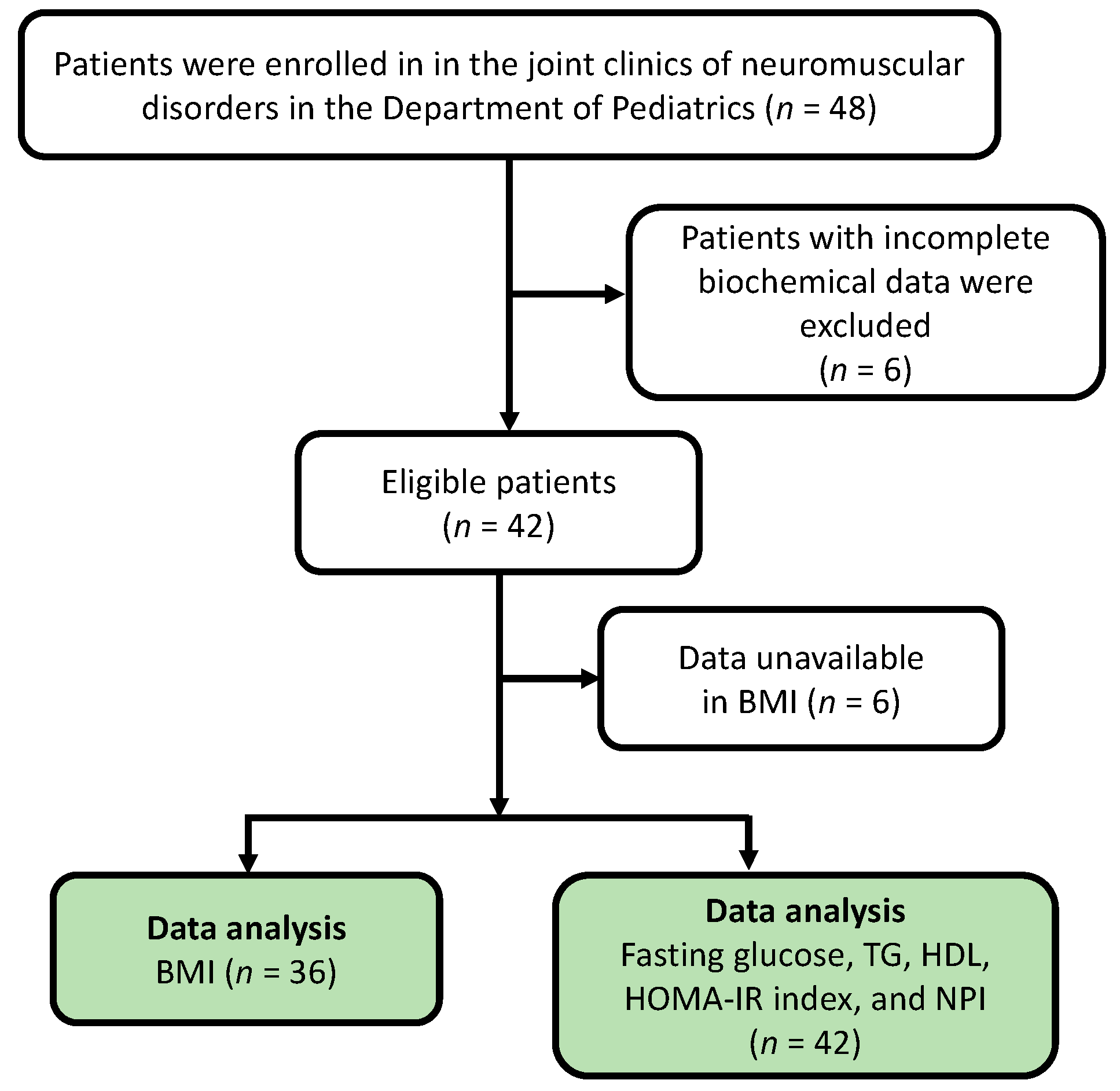
3.1. Participant Recruitment and Patient Demographics
3.2. Anthropometry and Biochemical Parameters
3.3. Hepatic Steatosis Assessment
3.4. Metabolic Risks
3.5. The Role of Steroid Treatment in Metabolic Risks and Hepatic Steatosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deenen, J.C.; Horlings, C.G.; Verschuuren, J.J.; Verbeek, A.L.; van Engelen, B.G. The epidemiology of neuromuscular disorders: A comprehensive overview of the literature. J. Neuromuscul. Dis. 2015, 2, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, E.P.; Brown, R.H., Jr.; Kunkel, L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- McDonald, C.M.; Abresch, R.T.; Carter, G.T.; Fowler, W.M., Jr.; Johnson, E.R.; Kilmer, D.D.; Sigford, B.J. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am. J. Phys. Med. Rehabil. 1995, 74, S70–S92. [Google Scholar] [CrossRef]
- Cheeran, D.; Khan, S.; Khera, R.; Bhatt, A.; Garg, S.; Grodin, J.L.; Morlend, R.; Araj, F.G.; Amin, A.A.; Thibodeau, J.T.; et al. Predictors of death in adults with duchenne muscular dystrophy-associated cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e006340. [Google Scholar] [CrossRef] [Green Version]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. Lancet Neurol. 2010, 9, 177–189. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Lamb, M.M.; West, N.A.; Ouyang, L.; Yang, M.; Weitzenkamp, D.; James, K.; Ciafaloni, E.; Pandya, S.; DiGuiseppi, C. Corticosteroid treatment and growth patterns in ambulatory males with Duchenne muscular dystrophy. J. Pediatr. 2016, 173, 207–213.e203. [Google Scholar] [CrossRef] [Green Version]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 2016, Cd003725. [Google Scholar] [CrossRef] [Green Version]
- Willig, T.N.; Carlier, L.; Legrand, M.; Rivière, H.; Navarro, J. Nutritional assessment in Duchenne muscular dystrophy. Dev. Med. Child. Neurol. 1993, 35, 1074–1082. [Google Scholar] [CrossRef]
- Fujita, T. Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol. Dial. Transplant. 2007, 22, 3102–3107. [Google Scholar] [CrossRef] [Green Version]
- Caterson, I.D.; Hubbard, V.; Bray, G.A.; Grunstein, R.; Hansen, B.C.; Hong, Y.; Labarthe, D.; Seidell, J.C.; Smith, S.C., Jr. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group III: Worldwide comorbidities of obesity. Circulation 2004, 110, e476–e483. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.R.; Hadjiyannakis, S.; McMillan, H.J.; Noritz, G.; Ward, L.M. Obesity and endocrine management of the patient with duchenne muscular dystrophy. Pediatrics 2018, 142, S43–S52. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.; Sanchez, R.; Escobar, R.E.; Cruz-Guzmán Odel, R.; López-Alarcón, M.; Bernabe García, M.; Coral-Vázquez, R.; Matute, G.; Velázquez Wong, A.C. Evidence of insulin resistance and other metabolic alterations in boys with Duchenne or Becker muscular dystrophy. Int. J. Endocrinol. 2015, 2015, 867273. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Cruz, M.; Cruz-Guzmán, O.R.; Escobar, R.E.; López-Alarcón, M. Leptin and metabolic syndrome in patients with Duchenne/Becker muscular dystrophy. Acta Neurol. Scand. 2016, 133, 253–260. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liao, Y.Y.; Yeh, C.K.; Yang, K.C.; Tsui, P.H. Ultrasound entropy imaging of nonalcoholic fatty liver disease: Association with metabolic syndrome. Entropy 2018, 20, 893. [Google Scholar] [CrossRef] [Green Version]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005, 115, e500–e503. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.A.; Cruz, J.F.; Macena, L.B.; de Santana, D.S.; Oliveira, C.C.; Lima, S.O.; Franca, A.V. Association of the nonalcoholic hepatic steatosis and its degrees with the values of liver enzymes and homeostasis model assessment-insulin resistance index. Gastroenterol. Res. 2015, 8, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Isaksen, V.T.; Larsen, M.A.; Goll, R.; Florholmen, J.R.; Paulssen, E.J. Hepatic steatosis, detected by hepatorenal index in ultrasonography, as a predictor of insulin resistance in obese subjects. BMC Obes. 2016, 3, 39. [Google Scholar] [CrossRef]
- Leite, N.C.; Salles, G.F.; Araujo, A.L.; Villela-Nogueira, C.A.; Cardoso, C.R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009, 29, 113–119. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Veropalumbo, C.; Del Giudice, E.; Capuano, G.; Gentile, C.; Di Cosmo, N.; Vajro, P. Duchenne and Becker muscular dystrophy presenting as nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Wan, Y.L.; Tai, D.I.; Tseng, J.H.; Wang, C.Y.; Tsai, Y.W.; Lin, Y.R.; Chang, T.Y.; Tsui, P.H. Considerations of ultrasound scanning approaches in non-alcoholic fatty liver disease assessment through acoustic structure quantification. Ultrasound Med. Biol. 2019, 45, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.H.; Hsieh, C.S.; Lai, M.W.; Chen, C.C.; Chao, H.C.; Yeh, H.Y.; Lai, H.H.; Tsui, P.H. Detection of pediatric hepatic steatosis through ultrasound backscattering analysis. Eur. Radiol. 2021, 31, 3216–3225. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.C.; Tsui, P.H.; Lin, C.W.; Lu, C.H.; Lin, C.Y.; Shieh, J.Y.; Lu, F.L.; Ee, T.W.; Wu, K.W.; Lee, W.T. Evaluation of muscular changes by ultrasound Nakagami imaging in Duchenne muscular dystrophy. Sci. Rep. 2017, 7, 4429. [Google Scholar] [CrossRef] [Green Version]
- Weihe, P.; Weihrauch-Blüher, S. Metabolic syndrome in children and adolescents: Diagnostic criteria, therapeutic options and perspectives. Curr. Obes. Rep. 2019, 8, 472–479. [Google Scholar] [CrossRef]
- Chen, W.; Chang, M.H. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr. Neonatol. 2010, 51, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, W.; Moreno, L.A.; Mårild, S.; Molnár, D.; Siani, A.; De Henauw, S.; Böhmann, J.; Günther, K.; Hadjigeorgiou, C.; Iacoviello, L.; et al. Metabolic syndrome in young children: Definitions and results of the IDEFICS study. Int. J. Obes. 2014, 38, S4–S14. [Google Scholar] [CrossRef] [Green Version]
- Salera, S.; Menni, F.; Moggio, M.; Guez, S.; Sciacco, M.; Esposito, S. Nutritional challenges in Duchenne muscular dystrophy. Nutrients 2017, 9, 594. [Google Scholar] [CrossRef] [Green Version]
- Shimizu-Fujiwara, M.; Komaki, H.; Nakagawa, E.; Mori-Yoshimura, M.; Oya, Y.; Fujisaki, T.; Tokita, Y.; Kubota, N.; Shimazaki, R.; Sato, K.; et al. Decreased resting energy expenditure in patients with Duchenne muscular dystrophy. Brain Dev. 2012, 34, 206–212. [Google Scholar] [CrossRef]
- van den Engel-Hoek, L.; de Groot, I.J.; Sie, L.T.; van Bruggen, H.W.; de Groot, S.A.; Erasmus, C.E.; van Alfen, N. Dystrophic changes in masticatory muscles related chewing problems and malocclusions in Duchenne muscular dystrophy. Neuromuscul. Disord. 2016, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]
- van den Engel-Hoek, L.; Erasmus, C.E.; Hendriks, J.C.; Geurts, A.C.; Klein, W.M.; Pillen, S.; Sie, L.T.; de Swart, B.J.; de Groot, I.J. Oral muscles are progressively affected in Duchenne muscular dystrophy: Implications for dysphagia treatment. J. Neurol. 2013, 260, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Samuels, E.; Mullins, L. Nutrition Considerations in Duchenne muscular dystrophy. Nutr. Clin. Pract. 2015, 30, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.V.; Singh, Y.; Kumar, R.Y.; Kumar, S.; Kunwar, B.; Kumar, Y. Study of lipid profile levels in malnourished and healthy children: A case control study acquired pneumonia in children. Pediatr. Rev. 2018, 5, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Saure, C.; Caminiti, C.; Weglinski, J.; de Castro Perez, F.; Monges, S. Energy expenditure, body composition, and prevalence of metabolic disorders in patients with Duchenne muscular dystrophy. Diabetes Metab. Syndr. 2018, 12, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, L.; Rajpal, A.; Ismail-Beigi, F. Glucocorticoid-induced fatty liver disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1133–1145. [Google Scholar] [CrossRef] [Green Version]
- van Zutphen, T.; Ciapaite, J.; Bloks, V.W.; Ackereley, C.; Gerding, A.; Jurdzinski, A.; de Moraes, R.A.; Zhang, L.; Wolters, J.C.; Bischoff, R.; et al. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J. Hepatol. 2016, 65, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Deo, M.G.; Ramalingaswami, V. Mechanism of fatty liver in protein deficiency. An experimental study in the rhesus monkey. Gastroenterology 1972, 62, 445–451. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.K.; Cripe, L.; Herron, A.R.; Kennedy, A.; Kinnett, K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled attenuation parameter (CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography assessment of liver fibrosis: Society of radiologists in ultrasound consensus conference statement. Radiology 2015, 276, 845–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Stage | Clinical Symptoms | Age (Years) Median (Range) | No. of Subjects (With Steroid Use) |
|---|---|---|---|
| Stage 1 | Early ambulatory: Showing a Gowers’ sign (patients need to support themselves with hands to get up from the floor), waddling type walking (gait), and walking on their toes. Late ambulatory: Walking becomes increasingly difficult (labored gait) and climbing stairs and getting up from the floor are more problematic. | 8.5 (3–18.5) | 21 (16) |
| Stage 2 | Early non-ambulatory: Patients start to need to use a wheelchair; they may be able to wheel the chair themselves and typically their postures can be maintained even scoliosis is possible. | 15.5 (8.9–18.3) | 14 (9) |
| Stage 3 | Late non-ambulatory: Upper limb function and maintenance of good posture are increasingly difficult, and complications are more likely. | 18.1(14.1–26.2) | 7 (1) |
| Parameters | Stage 1 Ambulatory (n = 21) | Stage 2 Early Non-Ambulatory (n = 14) | Stage 3 Late Non-Ambulatory (n = 7) | p Value |
|---|---|---|---|---|
| BMI (kg/m2) | 18.87 ± 3.58 | 23.39 ± 5.30 1 | 14.94 ± 4.29 ** | 0.02 |
| Fasting glucose (mg/dL) | 86.66 ± 7.15 | 85.14 ± 9.44 | 83.57 ± 6.18 | 0.64 |
| TG (mg/dL) | 130.66 ± 42.65 **,1 | 86.07 ± 20.87 * | 66.85 ± 24.01 * | <0.001 |
| HDL (mg/dL) | 53.76 ± 10.95 | 50.07 ± 11.75 | 48.71 ± 9.74 | 0.46 |
| HOMA-IR | 1.23 ± 0.91 | 2.47 ± 2.21 1 | 0.79 ± 0.26 ** | 0.006 |
| NPI | 0.61 ± 0.11 **,1 | 0.74 ± 0.07 * | 0.74 ± 0.08 * | <0.001 |
| No. of Subjects Who Fulfill the Criteria/No. of Subjects (%) | No. of Subjects Who Fulfill the Criteria/No. of Ambulatory Subjects (Stage 1) (%) | No. of Subjects Who Fulfill the Criteria/No. of Subjects at Stage 2 (%) | No. of Subjects Who Fulfill the Criteria/No. of Subjects at Stage 3 (%) | |
|---|---|---|---|---|
| BMI ≥ 85th percentile | 16/42 (38.09%) | 12/21 (52.38%) | 4/12 (33.33%) | 0/3 (0%) |
| Fasting glucose ≥ 110 (mg/dL) | 0/42 (0%) | 0/21 (0%) | 0/14 (0%) | 0/7 (0%) |
| TG ≥ 150 (mg/dL) | 6/42 (14.28%) | 6/21 (28.57%) | 0/14 (0%) | 0/7 (0%) |
| HDL < 35 (mg/dL) | 2/42 (4.76%) | 0/21 (0%), | 2/14 (14.28%) | 0/7 (0%) |
| HOMA-IR > 3.16 | 2/42 (4.76%) | 0/21 (0%) | 2/14 (14.28%) | 0/7 (0%) |
| NPI > 0.73 | 17/42 (40.48%) | 3/21 (14.28%) | 10/14 (71.43%) | 4/7 (57.14%) |
| Without Steroid Treatment | With Steroid Treatment | p Value | |
|---|---|---|---|
| Ambulatory subjects (n = 21) | 5 | 16 | |
| BMI (kg/m2) | 16.71 ± 1.11 | 19.55 ± 3.84 | 0.24 |
| AC glucose (mg/dL) | 88.61 ± 7.98 | 86.06 ± 7.03 | 0.60 |
| TG (mg/dL) | 130.61 ± 39.81 | 130.68 ± 44.76 | 0.78 |
| HDL (mg/dL) | 48.20 ± 10.32 | 55.50 ± 10.86 | 0.16 |
| HOMA-IR | 0.91 ± 0.51 | 1.34 ± 0.99 | 0.46 |
| NPI | 0.52 ± 0.06 | 0.63 ± 0.10 | 0.02 ** |
| Non-ambulatory subjects (n = 21) | 11 | 10 | |
| BMI (kg/m2) | 17.44 ± 4.54 | 23.94 ± 5.88 | 0.06 |
| AC glucose (mg/dL) | 85.09 ± 5.94 | 84.10 ± 10.76 | 0.18 |
| TG (mg/dL) | 72.90 ± 22.57 | 87.10 ± 22.83 | 0.18 |
| HDL (mg/dL) | 47.01 ± 4.95 | 52.50 ± 14.79 | 0.13 |
| HOMA-IR | 1.35 ± 1.14 | 2.54 ± 2.51 | 0.06 |
| NPI | 0.74 ± 0.05 | 0.74 ± 0.08 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-C.; Tsui, P.-H.; Wang, C.-Y.; Chien, Y.-H.; Weng, H.-L.; Yang, C.-Y.; Weng, W.-C. Hepatic Steatosis Assessment as a New Strategy for the Metabolic and Nutritional Management of Duchenne Muscular Dystrophy. Nutrients 2022, 14, 727. https://doi.org/10.3390/nu14040727
Tang Y-C, Tsui P-H, Wang C-Y, Chien Y-H, Weng H-L, Yang C-Y, Weng W-C. Hepatic Steatosis Assessment as a New Strategy for the Metabolic and Nutritional Management of Duchenne Muscular Dystrophy. Nutrients. 2022; 14(4):727. https://doi.org/10.3390/nu14040727
Chicago/Turabian StyleTang, Ya-Chun, Po-Hsiang Tsui, Chiao-Yin Wang, Yin-Hsiu Chien, Hui-Ling Weng, Chung-Yi Yang, and Wen-Chin Weng. 2022. "Hepatic Steatosis Assessment as a New Strategy for the Metabolic and Nutritional Management of Duchenne Muscular Dystrophy" Nutrients 14, no. 4: 727. https://doi.org/10.3390/nu14040727
APA StyleTang, Y.-C., Tsui, P.-H., Wang, C.-Y., Chien, Y.-H., Weng, H.-L., Yang, C.-Y., & Weng, W.-C. (2022). Hepatic Steatosis Assessment as a New Strategy for the Metabolic and Nutritional Management of Duchenne Muscular Dystrophy. Nutrients, 14(4), 727. https://doi.org/10.3390/nu14040727








