Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease
Abstract
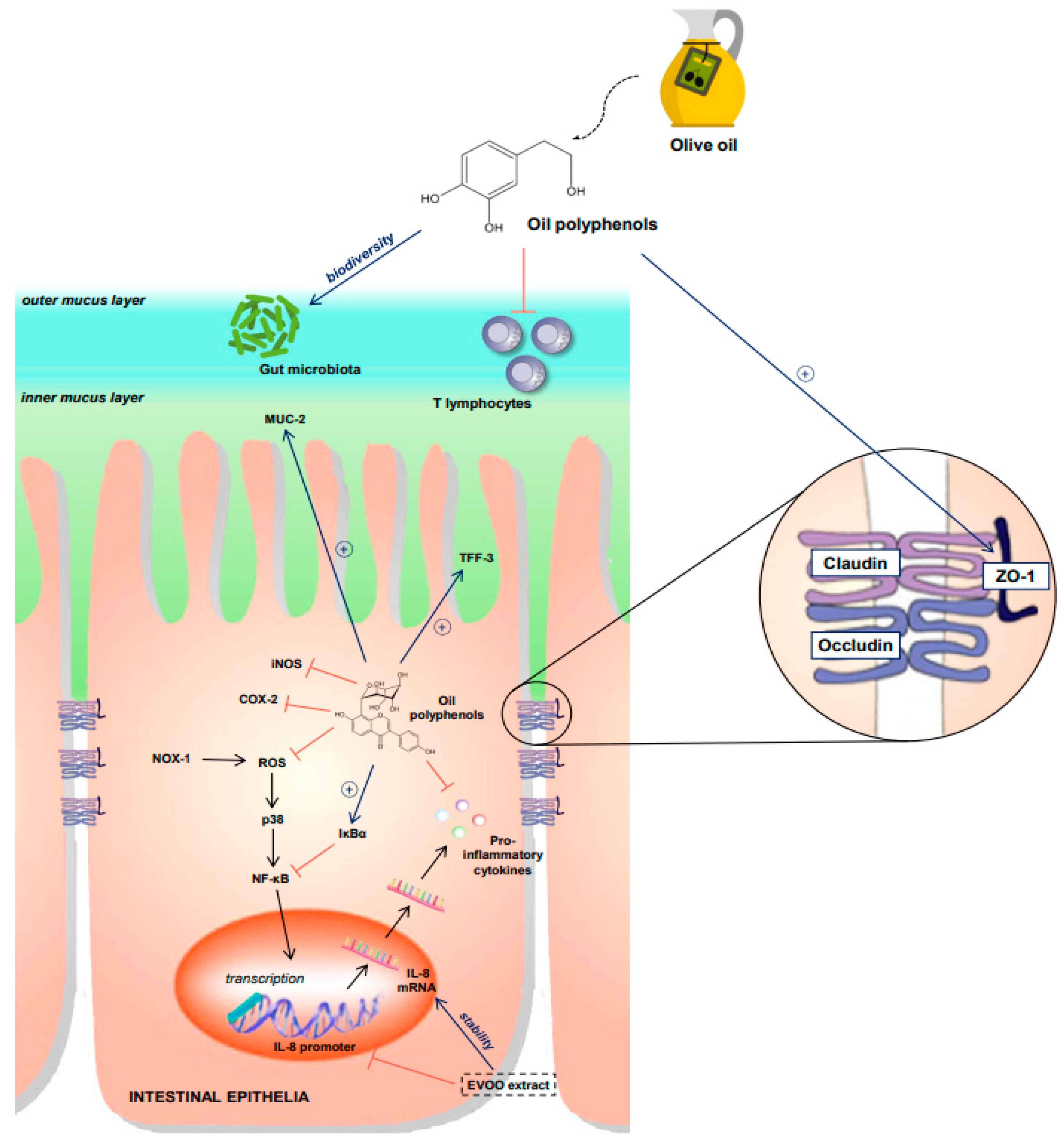
:1. Introduction
2. Olive Oil and IBD
2.1. Olive Oil Composition
2.2. Olive Oil and IBD
2.2.1. The Evidence In Vitro
2.2.2. Evidence from Animal Studies
2.2.3. Evidence from Human Studies
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, B. Chapter 1—Introduction and classification of inflammatory bowel diseases. In Atlas of Endoscopy Imaging in Inflammatory Bowel Disease; Shen, B., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–8. [Google Scholar]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zivkovic, P.M.; Matetic, A.; Hadjina, I.T.; Rusic, D.; Vilovic, M.; Supe-Domic, D.; Borovac, J.A.; Mudnic, I.; Tonkic, A.; Bozic, J. Serum Catestatin Levels and Arterial Stiffness Parameters Are Increased in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brnic, D.; Martinovic, D.; Zivkovic, P.M.; Tokic, D.; Vilovic, M.; Rusic, D.; Hadjina, I.T.; Libers, C.; Glumac, S.; Supe-Domic, D.; et al. Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 4866–4877. [Google Scholar] [CrossRef] [PubMed]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2019, 14, 4–22. [Google Scholar] [CrossRef]
- Ashton, J.; Gavin, J.; Beattie, R.M. Exclusive enteral nutrition in Crohn’s disease: Evidence and practicalities. Clin. Nutr. 2019, 38, 80–89. [Google Scholar] [CrossRef]
- Adamji, M.; Day, A.S. An overview of the role of exclusive enteral nutrition for complicated Crohn’s disease. Intest. Res. 2019, 17, 171–176. [Google Scholar] [CrossRef]
- Campmans-Kuijpers, M.J.E.; Dijkstra, G. Food and Food Groups in Inflammatory Bowel Disease (IBD): The Design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients 2021, 13, 1067. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Vilović, M.; Živković, P.; Hadjina, I.T.; Rušić, D.; Bukić, J.; Borovac, J.; Božić, J. Mediterranean Diet Adherence and Dietary Attitudes in Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 3429. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.L.; Pop, C.; Dumitrascu, D.L. Diet Advice for Crohn’s Disease: FODMAP and Beyond. Nutrients 2020, 12, 3751. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, A.V.; Palmiero, P.; Manfrini, O.; Puddu, P.E.; Nodari, S.; Cas, A.D.; Mercuro, G.; Scrutinio, D.; Palermo, P.; Sciomer, S.; et al. Mediterranean diet impact on cardiovascular diseases: A narrative review. J. Cardiovasc. Med. 2017, 18, 925–935. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Vrdoljak, J.; Kumric, M.; Kurir, T.T.; Males, I.; Martinovic, D.; Vilovic, M.; Bozic, J. Effects of Wine Components in Inflammatory Bowel Diseases. Molecules 2021, 26, 5891. [Google Scholar] [CrossRef]
- LaRussa, T.; Imeneo, M.; Luzza, F. Olive Tree Biophenols in Inflammatory Bowel Disease: When Bitter is Better. Int. J. Mol. Sci. 2019, 20, 1390. [Google Scholar] [CrossRef] [Green Version]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabbà, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blekas, G.; Tsimidou, M.; Boskou, D. Olive Oil Composition. In Olive Oil; AOCS Press: Urbana, IL, USA, 2006. [Google Scholar]
- Bianco, A.; Melchioni, C.; Ramunno, A.; Romeo, G.; Uccella, N. Phenolic components of Olea Europaea—Isolation of tyrosol derivatives. Nat. Prod. Res. 2004, 18, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virruso, C.; Accardi, G.; Colonna-Romano, G.; Candore, G.; Vasto, S.; Caruso, C. Nutraceutical Properties of Extra-Virgin Olive Oil: A Natural Remedy for Age-Related Disease? Rejuvenation Res. 2014, 17, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef] [Green Version]
- De La Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef]
- Covas, M.I.; Miró-Casas, E.; Fitó, M.; Farré-Albadalejo, M.; Gimeno, E.; Marrugat, J.; de la Torre, R. Bioavailability of tyrosol, an antioxidant phenolic compound present in wine and olive oil, in humans. Drugs Exp. Clin. Res. 2003, 29, 203–206. [Google Scholar]
- Fitó, M.; De La Torre, R.; Farré-Albaladejo, M.; Khymenetz, O.; Marrugat, J.; Covas, M.-I. Bioavailability and antioxidant effects of olive oil phenolic compounds in humans: A review. Ann. Ist. Super. Sanità 2007, 43, 375–381. [Google Scholar]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef]
- Corona, G.; Tzounis, X.; Dessì, M.A.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The fate of olive oil polyphenols in the gastrointestinal tract: Implications of gastric and colonic microflora-dependent biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Cardeno, A.; Sanchez-Hidalgo, M.; Aparicio-Soto, M.; Alarcón-De-La-Lastra, C. Unsaponifiable fraction from extra virgin olive oil inhibits the inflammatory response in LPS-activated murine macrophages. Food Chem. 2014, 147, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Magnusson, M.; Strid, H.; de La Lastra, C.A.; Sánchez-Hidalgo, M.; Öhman, L. The unsaponifiable fraction of extra virgin olive oil promotes apoptosis and attenuates activation and homing properties of T cells from patients with inflammatory bowel disease. Food Chem. 2014, 161, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol Is the Major Anti-Inflammatory Compound in Aqueous Olive Extracts and Impairs Cytokine and Chemokine Production in Macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [Green Version]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.; Tuberoso, C.I.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols-induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef]
- Serra, G.; Deiana, M.; Spencer, J.P.E.; Corona, G. Olive Oil Phenolics Prevent Oxysterol-Induced Proinflammatory Cytokine Secretion and Reactive Oxygen Species Production in Human Peripheral Blood Mononuclear Cells, Through Modulation of p38 and JNK Pathways. Mol. Nutr. Food Res. 2017, 61, 1700283. [Google Scholar] [CrossRef] [Green Version]
- Guina, T.; Deiana, M.; Calfapietra, S.; Cabboi, B.; Maina, M.; Tuberoso, C.I.; Leonarduzzi, G.; Gamba, P.; Gargiulo, S.; Testa, G.; et al. The role of p38 MAPK in the induction of intestinal inflammation by dietary oxysterols: Modulation by wine phenolics. Food Funct. 2015, 6, 1218–1228. [Google Scholar] [CrossRef]
- Chiesi, C.; Fernandez-Blanco, C.; Cossignani, L.; Font, G.; Ruiz, M. Alternariol-induced cytotoxicity in Caco-2 cells. Protective effect of the phenolic fraction from virgin olive oil. Toxicon 2015, 93, 103–111. [Google Scholar] [CrossRef]
- Muto, E.; Dell’Agli, M.; Sangiovanni, E.; Mitro, N.; Fumagalli, M.; Crestani, M.; De Fabiani, E.; Caruso, D. Olive oil phenolic extract regulates interleukin-8 expression by transcriptional and posttranscriptional mechanisms in Caco-2 cells. Mol. Nutr. Food Res. 2015, 59, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Melis, M.P.; Corona, G.; Deiana, M. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites: Insight into the mechanism of action. Food Chem. Toxicol. 2019, 125, 520–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef]
- Borges, T.H.; Cabrera-Vique, C.; Seiquer, I. Antioxidant properties of chemical extracts and bioaccessible fractions obtained from six Spanish monovarietal extra virgin olive oils: Assays in Caco-2 cells. Food Funct. 2015, 6, 2375–2383. [Google Scholar] [CrossRef]
- Gill, C.I.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; et al. Potential anti-cancer effects of virgin olive oil phenolson colorectal carcinogenesis modelsin vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [CrossRef]
- Mateos, R.; Caro, G.P.; Bacon, J.R.; Bongaerts, R.; Sarriá, B.; Bravo, L.; Kroon, P.A. Anticancer Activity of Olive Oil Hydroxytyrosyl Acetate in Human Adenocarcinoma Caco-2 Cells. J. Agric. Food Chem. 2013, 61, 3264–3269. [Google Scholar] [CrossRef]
- Lopez de las Hazas, M.-C.; Piñol, C.; Macià, A.; Motilva, M.-J. Hydroxytyrosol and the Colonic Metabolites Derived from Virgin Olive Oil Intake Induce Cell Cycle Arrest and Apoptosis in Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6467–6476. [Google Scholar] [CrossRef]
- LaRussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef] [Green Version]
- Vezza, T.; Algieri, F.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Utrilla, M.P.; Talhaoui, N.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Rodriguez-Cabezas, M.E.; Monteleone, G.; et al. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017, 61, 1601066. [Google Scholar] [CrossRef]
- Kondreddy, V.K.R.; Naidu, K.A. Oleic acid, hydroxytyrosol and n-3 fatty acids collectively modulate colitis through reduction of oxidative stress and IL-8 synthesis; in vitro and in vivo studies. Int. Immunopharmacol. 2016, 35, 29–42. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Sanchez De Ibargüen, L.; Cárdeno, A.; Alarcon De La Lastra, C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2011, 51, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Hidalgo, M.S.; Soto, M.A.; de la Lastra, C.A. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J. Nutr. Biochem. 2013, 24, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Hidalgo, M.S.; Soto, M.A.; Villegas, I.; Rosillo, M.A.; de la Lastra, C.A. Dietary unsaponifiable fraction from extra virgin olive oil supplementation attenuates acute ulcerative colitis in mice. Eur. J. Pharm. Sci. 2013, 48, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Takashima, T.; Sakata, Y.; Iwakiri, R.; Shiraishi, R.; Oda, Y.; Inoue, N.; Nakayama, A.; Toda, S.; Fujimoto, K. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J. Nutr. Biochem. 2014, 25, 186–192. [Google Scholar] [CrossRef]
- Cariello, M.; Contursi, A.; Gadaleta, R.M.; Piccinin, E.; De Santis, S.; Piglionica, M.; Spaziante, A.F.; Sabbà, C.; Villani, G.; Moschetta, A. Extra-Virgin Olive Oil from Apulian Cultivars and Intestinal Inflammation. Nutrients 2020, 12, 1084. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Fidalgo, S.; Villegas, I.; Cárdeno, A.; Talero, E.; Sánchez-Hidalgo, M.; Motilva, V.; De La Lastra, C.A. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clin. Nutr. 2010, 29, 663–673. [Google Scholar] [CrossRef]
- do Nascimento, R.D.P.; Lima, A.V.; Oyama, L.M.; Paiotti, A.P.R.; Cardili, L.; Martinez, C.A.R.; Pereira, J.A.; Silva, M.F.; Garofolo, I.C.; Silveira, V.L.F.; et al. Extra virgin olive oil and flaxseed oil have no preventive effects on DSS-induced acute ulcerative colitis. Nutrients 2020, 74, 110731. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020, 586, 119515. [Google Scholar] [CrossRef]
- Voltes, A.; Bermúdez, A.; Rodríguez-Gutiérrez, G.; Reyes, M.L.; Olano, C.; Fernández-Bolaños, J.; de la Portilla, F. Anti-Inflammatory Local Effect of Hydroxytyrosol Combined with Pectin-Alginate and Olive Oil on Trinitrobenzene Sulfonic Acid-Induced Colitis in Wistar Rats. J. Investig. Surg. 2020, 33, 8–14. [Google Scholar] [CrossRef]
- Camuesco, D.; Galvez, J.; Nieto, A.; Comalada, M.; Rodriguez-Cabezas, M.E.; Concha, A.; Xaus, J.; Zarzuelo, A. Dietary Olive Oil Supplemented with Fish Oil, Rich in EPA and DHA (n-3) Polyunsaturated Fatty Acids, Attenuates Colonic Inflammation in Rats with DSS-Induced Colitis. J. Nutr. 2005, 135, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigagli, E.; Toti, S.; Lodovici, M.; Giovannelli, L.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Dietary Extra-Virgin Olive Oil Polyphenols Do Not Attenuate Colon Inflammation in Transgenic HLAB-27 Rats but Exert Hypocholesterolemic Effects through the Modulation of HMGCR and PPAR-α Gene Expression in the Liver. Lifestyle Genom. 2018, 11, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, G.; Hiane, P.A.; Freitas, K.D.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.D.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrypnik, K.; Bogdański, P.; Łoniewski, I.; Regula, J.; Suliburska, J. Effect of probiotic supplementation on liver function and lipid status in rats. Acta Sci. Pol. Technol. Aliment. 2015, 17, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 2411–2425. [Google Scholar] [CrossRef] [Green Version]
- Farràs, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef]
- Daniel, K.; Vitetta, L.; Singh, M.A.F. Effects of olives and their constituents on the expression of ulcerative colitis: A systematic review of randomised controlled trials. Br. J. Nutr. 2021, 1–19. [Google Scholar] [CrossRef]
- Taylor, L.; Almutairdi, A.; Shommu, N.; Fedorak, R.; Ghosh, S.; Reimer, R.A.; Panaccione, R.; Raman, M. Cross-Sectional Analysis of Overall Dietary Intake and Mediterranean Dietary Pattern in Patients with Crohn’s Disease. Nutrients 2018, 10, 1761. [Google Scholar] [CrossRef] [Green Version]
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Alicic, D.; Martinovic, D.; Rusic, D.; Zivkovic, P.M.; Hadjina, I.T.; Vilovic, M.; Kumric, M.; Tokic, D.; Supe-Domic, D.; Lupi-Ferandin, S.; et al. Urotensin II levels in patients with inflammatory bowel disease. World J. Gastroenterol. 2021, 27, 6142–6153. [Google Scholar] [CrossRef] [PubMed]
- Morvaridi, M.; Jafarirad, S.; Seyedian, S.S.; Alavinejad, P.; Cheraghian, B. The effects of extra virgin olive oil and canola oil on inflammatory markers and gastrointestinal symptoms in patients with ulcerative colitis. Eur. J. Clin. Nutr. 2020, 74, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Castañer, O.; Solà, R.; Motilva, M.J.; Castell, M.; Pérez-Cano, F.J.; Fitó, M. Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function. Nutrients 2016, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; De La Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2015, 56, 119–131. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- de Silva, P.S.; Luben, R.; Shrestha, S.S.; Khaw, K.T.; Hart, A.R. Dietary arachidonic and oleic acid intake in ulcerative colitis etiology: A prospective cohort study using 7-day food diaries. Eur. J. Gastroenterol. Hepatol. 2014, 26, 11–18. [Google Scholar] [CrossRef]
- Cainzos-Achirica, M.; Glassner, K.; Zawahir, H.S.; Dey, A.K.; Agrawal, T.; Quigley, E.M.; Abraham, B.P.; Acquah, I.; Yahya, T.; Mehta, N.N.; et al. Inflammatory Bowel Disease and Atherosclerotic Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 2895–2905. [Google Scholar] [CrossRef]
- Setyawan, J.; Mu, F.; Zichlin, M.L.; Billmyer, E.; Downes, N.; Yang, H.; Azimi, N.; Strand, V.; Yarur, A. Risk of Thromboembolic Events and Associated Healthcare Costs in Patients with Inflammatory Bowel Disease. Adv. Ther. 2021, 39, 738–753. [Google Scholar] [CrossRef]
| Study | Cell Type | Intervention | Results |
|---|---|---|---|
| Chiesi et al. [42] | Caco-2 cells stimulated with alternariol | EVOO extract, Oleuropein, Tyrosol | ↓ ROS ↓ cytotoxicity |
| Muto et al. [43] | Caco-2 cells stimulated with LPS or IL-1β | EVOO phenolic extract | ↓ IL-8 expression and secretion |
| Serelli et al. [44] | Caco-2 cells treated with LPS | HT and Tyr metabolites | ↓ degradation of IĸBα ↓ iNOS expression |
| Di Nunzio et al. [45] | Caco-2 cells treated with IL-1β | Polyphenols sourced from olive pomace | ↓ IL-8 |
| Incani et al. [46] | Caco-2 cells treated with tert-butyl hydroperoxide (TBH) or a mixture of oxysterols | Preincubation with the phenolic extracts | ↓ ROS |
| Borges et al. [47] | Caco-2 cell cultures | Six Spanish monovarietal EVOOs (EVOOs’ bioaccessible fractions (BF) after in vitro digestion | ↑ phenolic count and anti-oxidant activity ↓ ROS |
| Gill et al. [48] | In vitro model of colon carcinogenesis (HT-29 cells treated with hydrogen peroxide, Caco-2 cells, HT115 cells) | EVOO phenols | anti-genotoxic effect ↑ barrier function in Caco-2 cells ↓ HT115 cell invasion and attachment |
| Larrussa et al. [51] | Ex vivo organ culture of mucosal explants from UC patients | Oleuropein | ↓ COX 2 and IL-17 expression ↓ infiltration of CD3, CD 4 and CD20 cells ↑ mucosal healing |
| Vezza et al. [52] | Ex vivo colon cultures from CD patients, DSS and DNBS mice colitis models | Olive leaf extract | ↓ expression of IL-1β, TNF-α, and iNOS ↑ epithelial barrier (ZO-1, MUC-2, and TFF-3) |
| Study | Cell Type | Intervention | Results |
|---|---|---|---|
| Reddy et al. [53] | DSS-colitis rat model, and Caco-2 cells treated with t-butyl hydroperoxide | Oleic acid and HT, fish oil, MCT | Synergistic anti-inflammatory effect between olive oil PP and fish oil; MCT increased disease activity |
| Sanchez-Fidalgo et al. [54] | Chronic mouse DSS-colitis model | EVOO diet enriched with HT | ↓ disease activity index ↑ histological signs ↓ 50% reduction in mortality ↓ TNF-α, iNOS, and p38MAPK ↑ IL-10 |
| Sanchez-Fidalgo et al. [55] | Chronic mouse DSS-colitis model | EVOO enriched with polyphenols | ↓ MCP-1, TNF-α, COX-2 and iNOS expression |
| Sanchez-Fidalgo et al. [56] | Acute ulcerative colitis model in mice | EVOO’s unsaponifiable fraction (UF) | ↓ MCP-1 and TNF-α levels, iNOS, COX-2, and p38MAPK ↑ IκB expression |
| Takashima et al. [57] | Chronic DSS-induced colitis in rats | EVOO diet (5% of weight) | ↓ STAT3, pSTAT3, COX-2 and iNOS ↓ cell proliferation (PCNA) ↑ apoptosis (caspase-3) |
| Carrielo et al. [58] | Mouse model of DSS-induced colitis | EVOO from 4 Apulian cultivars | ↑ intestinal morphology ↓ body-weight loss ↓ rectal bleeding ↓ IL-1β, TGFβ, IL-6 gene expression |
| Sanchez-Fidalgo et al. [59] | DSS-colitis-associated colon carcinogenesis in mice | EVOO enriched diet | ↓ incidence and multiplicity of tumours |
| Nascimento et al. [60] | Mouse model of DSS-induced colitis | EVOO and flaxseed oil | ↓ IL-6 No differences in disease activity index (DAI), histopathological score, interleukin (IL)-1β, and iNOS between the DSS and treatment groups |
| Huguet-Casquero et al. [61] | Activated macrophages (J774), murine model of acute colitis | Oleuropein (OLE) and OLE loaded with nanostructured lipid carriers | ↓ MPO activity, TNF-α and IL-6 |
| Voltes et al. [62] | Wistar rat model of TNBS-induced colitis | Intrarectal administration of aqueous solution containing olive oil and HT | ↓ inflammatory infiltrate |
| Bigagli et al. [64] | Colitis induced in HLA-B27 transgenic rats | AIN-76 diet containing 10% corn oil or extra-virgin olive oil with high (EVOO) or low phenolic content (ROO) | No differences in disease signs like diarrhoea, myeloperoxidase activity, and mucosal injury |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Tomic, I.J.; Krnic, M.; Ticinovic Kurir, T.; Bozic, J. Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients 2022, 14, 757. https://doi.org/10.3390/nu14040757
Vrdoljak J, Kumric M, Vilovic M, Martinovic D, Tomic IJ, Krnic M, Ticinovic Kurir T, Bozic J. Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients. 2022; 14(4):757. https://doi.org/10.3390/nu14040757
Chicago/Turabian StyleVrdoljak, Josip, Marko Kumric, Marino Vilovic, Dinko Martinovic, Iris Jeroncic Tomic, Mladen Krnic, Tina Ticinovic Kurir, and Josko Bozic. 2022. "Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease" Nutrients 14, no. 4: 757. https://doi.org/10.3390/nu14040757
APA StyleVrdoljak, J., Kumric, M., Vilovic, M., Martinovic, D., Tomic, I. J., Krnic, M., Ticinovic Kurir, T., & Bozic, J. (2022). Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients, 14(4), 757. https://doi.org/10.3390/nu14040757








