Abstract
Akkermansia muciniphila is well known for the amelioration of inflammatory responses and restoration of intestinal barrier function. The beneficial effect of A. muciniphila occurred through contacting Toll-like receptor 2 (TLR2) on intestinal epithelial cells by wall components. In this case, the downstream mechanism of pasteurized A. muciniphila stimulating TLR2 for ameliorated intestinal barrier function is worth investigating. In this study, we evaluated the effect of live and pasteurized A. muciniphila on protecting the barrier dysfunction of Caco-2 intestinal epithelial cells induced by lipopolysaccharide (LPS). We discovered that both live and pasteurized A. muciniphila could attenuate an inflammatory response and improve intestinal barrier integrity in Caco-2 monolayers. We demonstrated that A. muciniphila enhances AMP-activated protein kinase (AMPK) activation and inhibits Nuclear Factor-Kappa B (NF-κB) activation through the stimulation of TLR2. Overall, we provided a specific mechanism for the probiotic effect of A. muciniphila on the intestinal barrier function of Caco-2 cells.
1. Introduction
Akkermansia muciniphila is an anaerobe and Gram-positive bacterium, representing approximately 1% to 3% of the total intestinal microbiota (109 cfu/g) [1]. A. muciniphila generously distributes in the intestinal mucus layer and can contact epithelial cells at the villi tips more easily than other non-mucin-degrading bacteria [1,2]. A. muciniphila mainly uses intestinal mucins as its source of carbon and nitrogen and produces short-chain fatty acids, such as acetate and propionate [1]. Dietary supplementation enriches the abundance of A. muciniphila in mice, such as the dark sweet cherry [3], alpine bearberry, lingonberry, and cloudberry extracts [4], and inulin [5], fucoidan [6], polysaccharides from Gastrodia elata and Ophiopogon japonicas [7,8]. In addition, tryptophan and its derived metabolites could significantly promote the growth of A. muciniphila in vitro [9].
Recent studies indicate that supplementing with A. muciniphila can ameliorate metabolic syndrome, obesity, diabetes, and inflammatory bowel disease [10,11] in animals. A. muciniphila improved the dextran sulfate sodium (DSS)-induced colitis by reducing the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-2, IL-6, and IFN-γ, and chemokines MCP-1 and MIP-1, and by enhancing the anti-inflammatory cytokine IL-10 [12,13,14]. In addition, A. muciniphila decreased the inflammatory response by reducing the infiltration of macrophages and the population of CD8+ cytotoxic T lymphocytes in mice [15]. Moreover, A. muciniphila reduced chronic low-grade inflammation by decreasing plasma lipopolysaccharide (LPS)-binding protein (LBP) and leptin and by inhibiting c-Jun N-terminal kinase (JNK) and Nuclear Factor-Kappa B (NF-κB) activation in metabolically abnormal mice [16]. Several studies reported A. muciniphila can enhance intestinal barrier integrity [17,18,19]. For instance, A. muciniphila improved the transepithelial electrical resistance (TER) of the epithelial monolayer and up-regulated the expression of zonula occludens-2 (ZO-2) in Caco-2 cells’ monolayer [20,21]. Moreover, researchers observed that A. muciniphila reduced gut permeability via increased expression of tight junction proteins including occludin, junctional adhesion molecule-3, claudin-3, -4, and -15 in mice with DSS-induced colitis [18,19,22].
Currently, several studies suggest that, similar to live A. muciniphila, inactivated A. muciniphila could ameliorate several diseases [15,18]. Supplementation with pasteurized A. muciniphila can significantly decrease LPS, insulinemia, and plasma cholesterol of obese human volunteers in a proof-of-concept study [23]. Moreover, oral administration of pasteurized A. muciniphila delayed the onset of colitis-associated colorectal cancer by modulation of CD8+ T cells in mice [15]. Animal experiments suggest A. muciniphila exerted its beneficial effects through contacting the intestinal epithelial cell of the host directly [22,24], and a special protein Amuc_1100 isolated from the outer membrane of A. muciniphila, was verified to bind human TLR2 directly in vitro [18,25,26]. However, there is little knowledge about how A. muciniphila improves the intestinal barrier through modulating TLR2.
Research on the mechanism of A. muciniphila’s beneficial effects is deficient and warrants further study. AMP-activated protein kinase (AMPK) can act as an intracellular energy sensor for regulating energy homeostasis and metabolic stress and is activated by metformin and polyphenols [27,28,29]. Additionally, AMPK was found to promote the assembly of zonula occludens-1 (ZO-1) [30]. Therefore, we hypothesize A. muciniphila pretreatment may regulate tight junctions through the AMPK pathway in LPS-treated Caco-2 cells. We investigated the improving effect of live and pasteurized A. muciniphila on LPS-impaired barrier function in Caco-2 cell monolayers, respectively. We found that A. muciniphila may activate the AMPK and inhibit the NF-κB signaling pathway through modulating TLR2. This study provides a scientific basis for the understanding and application of A. muciniphila as probiotics.
2. Materials and Methods
2.1. Growth Conditions and Heat-Inactivation of Akkermansia muciniphila
Akkermansia muciniphila (DSM 22959) was purchased from the DSMZ (Braunschweig, Germany). The probiotic bacteria were cultured in Brain–Heart Infusion (Oxoid Ltd., Hampshire, United Kingdom) at 37 °C under strictly anaerobic conditions with H2/CO2/N2 (5/5/90, v/v/v) as a gas phase [18]. Cultures were centrifuged for 10 min at 16,000× g and the bacteria were washed two times with PBS. To obtain pasteurized A. muciniphila the part of live bacteria was inactivated by pasteurization for 30 min at 70 °C. The live or pasteurized A. muciniphila were resuspended in sterile DMEM medium under anaerobic conditions and the concentration was adjusted to 1 × 107 CFU/mL to treat Caco-2 cells.
2.2. Cell Culture
The human Caco-2 cells line (1102HUM-NIFDC00087) were purchased from National Infrastructure of Cell Line Resource and cultured in Dulbecco’s modified Eagle’s medium (DMEM, 4.5 g/L glucose, Macgene, Beijing, China) supplemented with 10% (v/v) fetal bovine serum (FBS, Abwbio, AB-FBS0500, Shanghai, China) and 1% (v/v) Penicillin–Streptomycin (Gibco, Thermo Fisher Scientific, Rochester, NY, USA). The culture was kept at 37 °C in 95% air/5% CO2 and 95% humidity [31]. For expression of tight junction and inflammatory cytokine experiments, the Caco-2 cells were seeded at a density of 1 × 106 cells/well into 6-well plates and incubated for 2 days at 37 °C.
The designated concentrations of 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) (HY-13417, MedChemExpress, Monmouth Junction, NJ, USA) and dorsomorphin (HY-13418A, MedChemExpress) were used to investigate the cellular functions of AMPK [32], and C16H15NO4 (C29) (HY-100461, MedChemExpress) were used to inhibition the cellular functions of TLR2 [33].
2.3. Experiment Design
To investigate the protected effects of A. muciniphila on Caco-2 cells, an inoculum of 1 × 107 CFU/mL live or pasteurized A. muciniphila was added to the cells before being treated with LPS. Caco-2/A. muciniphila co-cultures were incubated for 6 h at 37 °C with 95% air/5% CO2. After removal of the bacteria and the Caco-2 cells were washed 2–3 times with PBS. The 5 μg/mL LPS (L-2880, Sigma-Aldrich, St Louis, MO, USA) were added to the basal and apical side of the culture plates in the Transwell cell culture system, and cells were incubated for 6 h. After then, the Caco-2 cells were washed once with PBS and collected for further experiments.
To build up of co-culture model of live or pasteurized A. muciniphila with Caco-2 cells, the cell viability was measured in different treatment groups. (A) The Caco-2 cells were co-cultured with 1 × 107 CFU, 1 × 108 CFU and 1 × 109 CFU live A. muciniphila for 6 h (Figure S1A). (B) The Caco-2 cells were co-cultured with 1 × 107 CFU live A. muciniphila for 4, 6, and 8 h (Figure S1B). (C) The Caco-2 cell was co-cultured with 1 × 107 CFU live A. muciniphila for 4, 6, and 8 h after being stimulated by 5 μg/mL LPS for 6 h (Figure S1C). Pasteurized A. muciniphila had no effect on the cell viability for each treatment (Figure S1A–C). Therefore, for the next experiments, 1 × 107 CFU live or pasteurized A. muciniphila was chosen to co-culture with Caco-2 cell for 6 h.
2.4. Measurement of TER
Intestinal barrier functions were evaluated by measuring the TER value in Caco-2 cell monolayers grown on polyester membranes in Transwell inserts [31]. The Caco-2 cells were seeded at a density of 5 × 105 cells/well on inserts in the Transwell system (0.4 μm pore size costar, corning Incorporated, Corning, NY, USA) and placed into 12-well tissue culture plates. The medium was changed every 2 days until 21 days later when full polarization of the Caco-2 cell monolayer was achieved. The changes of TER were measured with the MILLICELL®-ERS voltohmmeter system (Millipore, Burlington, MA, USA).
Before starting each experiment, TER increased until 14–21 days when a steady state of higher than 450 Ω·cm2 was reached, indicating development of functional polarity and an intact monolayer, which is referred to as completed tight junction formation and maximized integrity of barrier function. To investigate the protective effect of A. muciniphila on intestinal barrier function, the TER value was measured before addition of A. muciniphila to the Caco-2 cell monolayers and after cells were incubated with A. muciniphila treatment for 6 h, and after induced of LPS for 6 h. Values were corrected for background resistance due to the membrane insert and calculated as Ω·cm2. The technical replicates for TER measurement were 3 times.
2.5. Monolayer Paracellular Permeability Determination
The permeability of Caco-2 cell monolayer was determined by adding fluorescein isothiocyanate-dextran (FITC-dextran) as previously described with modifications [34]. The 100 μL 1 mg/mL FITC-dextran (20 kDa, Sigma-Aldrich, St Louis, MO, USA) was added to the apical side of the monolayer immediately after the last measurement of the TER value. Thereafter, the fluorescence intensity of the medium from each of the basolateral chambers was measured with excitation and emission wavelengths of 490 and 520 nm, respectively. The technical replicates for measurement were 3 times.
2.6. Transmission Electron Microscopy (TEM)
The TEM were used to investigate the adhesion properties of A. muciniphila with Caco-2 cells. Approximately 1 × 106 cells/well of Caco-2 cells were seeded in 6-well plates with or without cell slides. The Caco-2 cells were co-cultured with live or pasteurized A. muciniphila for 6 h at 37 °C and washed with PBS three times. The co-cultures without cell slides were fixed in 2.5% glutaraldehyde buffer solution and transferred to new 1.5 mL tubes for TEM [20]. The untreated Caco-2 cells were used as a control. Then, the samples were sent to Wuhan Servicebio Technology Co., Ltd. for further experiments.
2.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (Real-Time qPCR)
The real-time qPCR assay was performed as described previously [35]. At the end of co-culture, Caco-2 cells were homogenized with TRIzol reagent (Life Technologies, Carlsbad, CA, USA). Total RNA was isolated following the manufacturer’s instructions and was reverse transcribed using PrimeScript RT reagent Kit with gDNA Eraser (Takara, Kyoto, Japan). Quantitative real-time PCR was performed using SYBR Premix Ex Taq II Kits (Takara) in a Light Cycler 480 real-time PCR system (Roche, Basel, Switzerland). The reaction conditions included 40 cycles of three-stage PCR consisting of denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, and after an initial denaturation step at 95 °C for 10 min. The primers shown in Table S1 were used for PCR reactions. Expression levels of target genes were normalized to housekeeping gene GAPDH, and comparative quantification of gene expression was analyzed using the 2−ΔΔCt method.
2.8. Cytokines Assay by Enzyme-linked Immunosorbent Assay (ELISA)
The secretion of cytokines in cell culture supernatants was determined using ELISA kits, according to the manufacturer’s protocols. Briefly, the supernatants of co-cultures were centrifuged 10 min at 5000× g. ELISA kits for TNF-α (MM-0122H1), IL-1α (MM-0056H1), IL-1β (MM-0181H1), IL-6 (MM-0049H1), IL-8 (MM-1558H1), TGF-β (MM-1774H1), and IL-10 (MM-0066H1) were obtained from Meimian Industrial Co., Ltd. (Yancheng, China).
2.9. Western Blot
Western blot analyses were performed as described previously [32]. Total proteins were extracted from cultured Caco-2 cells using RIPA lysis buffer (Thermo Fisher, Waltham, MA, USA) and sonicated at 4 °C. The protein concentrations in cell lysates were quantitated by the BCA protein assay kit (Huaxingbio, Beijing, China) according to the manufacturer’s protocol. Forty-microgram protein was loaded and separated by SDS-PAGE (Mini-Protean 4–12%, Huaxingbio, Beijing, China) and then transferred electrically to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked using 5% bovine serum albumin (BSA) or 5% nonfat milk in Tris-buffered saline and Tween 20 (TBST, Huaxingbio, Beijing, China) for 1 h at room temperature. Then, the membranes were probed with primary antibodies overnight at 4 °C. The primary antibodies included occludin (33-1500, Invitrogen, Thermo Fisher, Carlsbad, CA, USA), 1:1000; claudin-1 (51-9000, Invitrogen, Thermo Fisher, Carlsbad, CA, USA), 1:1000; claudin-2 (51-6100, Cell Signaling Technology, Danfoss, MA, USA), 1:500; ZO-1 (61-7300, Invitrogen, Thermo Fisher, Carlsbad, CA, USA), 1:1000; TLR2 (#2229, Cell Signaling Technology, Danfoss, MA, USA), 1:1000; TLR4 (ab13556, abcam, Cambridge, MA, USA), 1:1000; phosphorylated AMPK (p-AMPK) (Thr172) (#2535, Cell Signaling Technology, Danfoss, MA, USA), 1:1000; AMPK (#2532, Cell Signaling Technology, Danfoss, MA, USA), 1:1000; phosphorylated NF-κB (p-NF-κB) (#3033, Cell Signaling Technology, Danfoss, MA, USA), 1:500; NF-κB (#8242, Cell Signaling Technology, Danfoss, MA, USA), 1:1000; CDX2 (ab76541, abcam, Cambridge, MA, USA), 1:1000; and β-actin (A1978, Sigma-Aldrich, St Louis, MO, USA), 1:1000. After three washes with TBST, the membranes were incubated with the anti-rabbit (Huaxingbio, Beijing, China) or anti-mouse secondary antibodies (Huaxingbio, Beijing, China) at 1:5000 dilutions for 1.5 h at room temperature. Bands were detected by using an Amersham Imager 600 (GE Healthcare Life Sciences, Pittsburgh, PA, USA). Densitometry analyses were performed by using Image J software (version 1.46r, National Institute of Health, Bethesda, MD, USA). The technical replicates for immunoblot analysis were 3 times.
2.10. Statistical Analysis
All data were expressed as the mean ± S.D. Data of each test are consistent with normal distribution, statistical differences among different experimental groups were analyzed using one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. All analyses were performed using SPSS (version 21, IBM Japan Inc., Tokyo, Japan) and GraphPad Prism version 6.0 (San Diego, CA, USA). Values of p < 0.05 were considered to be statistically significant, p < 0.01 were considered to be statistically extremely significant.
3. Results
3.1. Akkermansia muciniphila Ameliorated the LPS-Induced Inflammation in Caco-2 Cells
LPS treatment could significantly increase the inflammation level of Caco-2 cells. Fortunately, the pretreatment with live or pasteurized A. muciniphila significantly decreased the mRNA expression and secretion of TNF-α, IL-1α, IL-1β, IL-6, and IL-8 (p < 0.01, p < 0.05, Figure 1B,C). Moreover, TGF-β secretion levels increased in cells treated with live or pasteurized A. muciniphila (p < 0.05), and mRNA levels increased only by pasteurized A. muciniphila (p < 0.05, Figure 1D,E). In addition, the mRNA expression and secretion of IL-10 increased with live A. muciniphila, whereas pasteurized A. muciniphila only increased the mRNA level (p < 0.01, Figure 1D,E). Correspondingly, we found that pretreatment with A. muciniphila can reverse the LPS-induced up-regulation in p-NF-κB level (Figure 1F,G), and there is a significant difference in the p-NF-κB level between the PAL and LPS groups (p < 0.05). Therefore, we suggest that the A. muciniphila showed a pre-protect effect on the inflammatory status in Caco-2 cells induced by LPS.
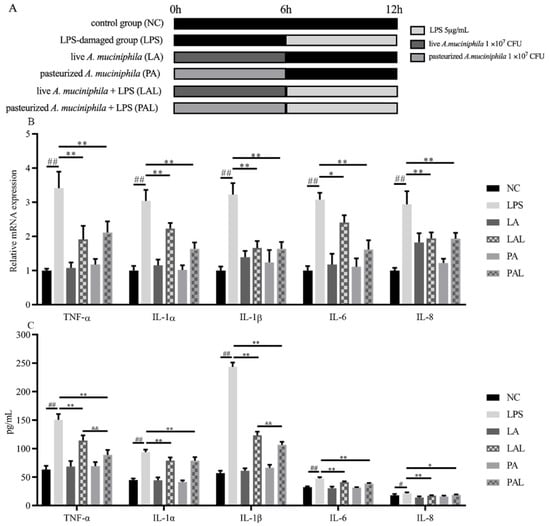
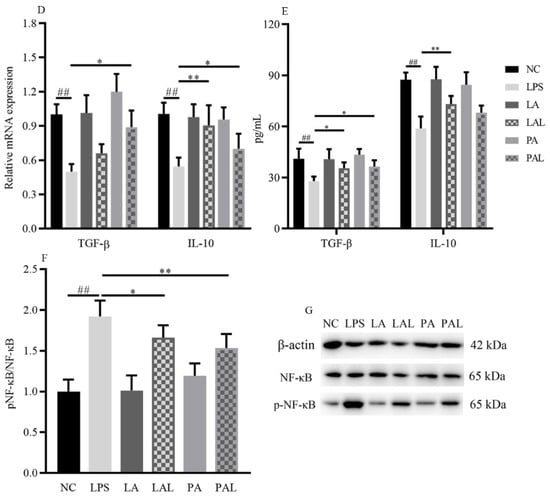
Figure 1.
A. muciniphila inhibits LPS-induced inflammatory responses. (A) The experiment design of Caco-2 cells pretreatment with A. muciniphila. (B,C) The effect of A. muciniphila on expressions of TNF-α, IL-1α, IL-1β, IL-6, IL-8, (D,E) TGF-β, and IL-10 in Caco-2 cells. (F,G) A. muciniphila inhibits the activation of the NF-κB signaling pathway. ** p < 0.01, * p < 0.05, compared with LPS, ## p < 0.01, # p < 0.05, compared with NC group, && p < 0.01, LAL compared with PAL. Data are expressed as mean ± S.D. (n = 6). LPS, lipopolysaccharide. NF-κB, Nuclear Factor-Kappa B.
3.2. Akkermansia muciniphila Ameliorated the Increased Barrier Permeability of Caco-2 Cell Monolayer Induced by LPS
The barrier functions were evaluated by measuring the TER value and FITC-dextran in Caco-2 cell monolayers. Results showed that LPS treatment (p < 0.05, Figure 2A,B) reduced the TER value and raised FITC-dextran. However, both pretreatment with live and pasteurized A. muciniphila could recover the LPS-induced increase in barrier permeation in Caco-2 monolayers (p < 0.01, Figure 2A,B).
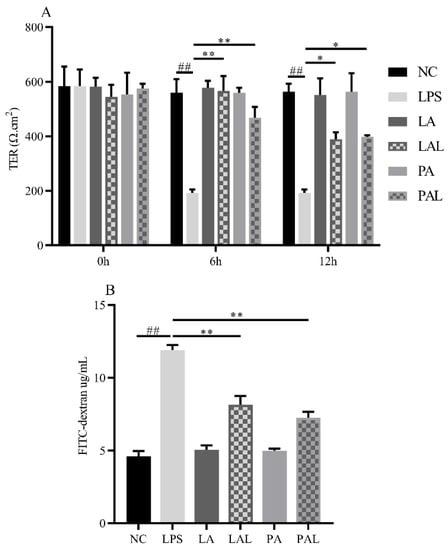
Figure 2.
A. muciniphila improves the barrier function dysfunction induced by LPS. (A) The TER value and (B) FITC-dextran value of Caco-2 cell monolayers. ** p < 0.01, * p < 0.05, compared with LPS group. ## p < 0.01, compared with NC group. Data are expressed as mean ± S.D. (n = 6). TER, transepithelial electrical resistance. FITC-dextran, fluorescein isothiocyanate-dextran. NC, control group. LPS, lipopolysaccharide-damaged group. LA, live A. muciniphila. PA, pasteurized A. muciniphila. LAL, live A. muciniphila + LPS. PAL, pasteurized A. muciniphila + LPS.
3.3. Akkermansia muciniphila Improved the Expression of ZO-1 and Decreased the Expression of Claudin-2
The expression level of tight junction proteins can be used to evaluate the barrier integrity of the Caco-2 cell monolayer in this study. On the one hand, the pretreatment with live and pasteurized A. muciniphila significantly up-regulated the LPS-induced decline of the mRNA expression of claudin1 (p < 0.01, p < 0.05), whereas the mRNA expression levels of ZO-1 and claudin4 were increased by pretreatment with pasteurized A. muciniphila (p < 0.01, p < 0.05, Figure 3A). Furthermore, we observed the LPS-induced down-regulation of ZO-1 protein expression was rapidly enhanced by pretreatment with live and pasteurized A. muciniphila, respectively (p < 0.01, Figure 3B,C). On the other hand, the pretreatment with A. muciniphila consistently down-regulated the LPS-induced increase in claudin2 mRNA and protein expression levels. This suggests A. muciniphila can maintain barrier integrity by modulating the expression levels of tight junction proteins.
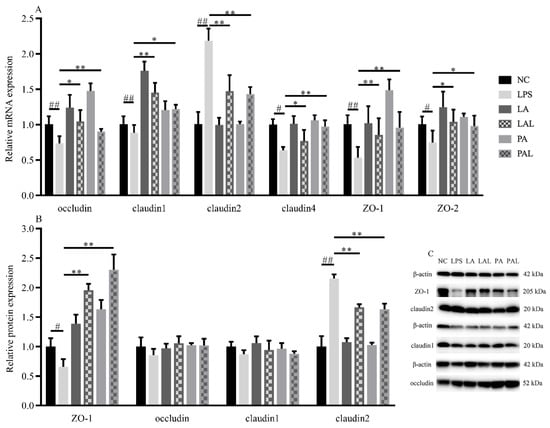
Figure 3.
A. muciniphila affects the expression of tight junction proteins. (A) The level of relative mRNA expression, (B,C) and protein expression of tight junction proteins. ** p < 0.01, * p < 0.05, compared with LPS group. ## p < 0.01, # p < 0.05, compared with NC group. Data are expressed as mean ± S.D. (n = 6). NC, control group. LPS, lipopolysaccharide-damaged group. LA, live A. muciniphila. PA, pasteurized A. muciniphila. LAL, live A. muciniphila + LPS. PAL, pasteurized A. muciniphila + LPS.
3.4. Akkermansia muciniphila Regulated TLR2 and Phosphorylated AMPK Expression in LPS Caco-2 Cells
TLRs are involved in the promotion of barrier function, and TLR2 especially plays an important role in the recognition of A. muciniphila to epithelial cells. We applied TEM to observe how epithelial cells were recognized with A. muciniphila in co-cultured Caco-2 cells and to determine the expression of TLRs. TEM results showed that A. muciniphila adheres to the membrane of Caco-2 cells treated with both live and pasteurized A. muciniphila (Figure 4A). Additionally, the LPS-induced decline of TLR2 mRNA expression was significantly recovered in cells treated with pasteurized A. muciniphila (p < 0.05, Figure 4B), but the levels of TLR1 and TLR4 mRNA in all treatments did not significantly change (p < 0.05, Figure 4B). Consistently, TLR2 protein level showed an up-regulation in LPS-damaged Caco-2 cells by both A. muciniphila pretreatment but TLR1 and TLR4 were not affected (p < 0.01, p < 0.05, Figure 4C,F).
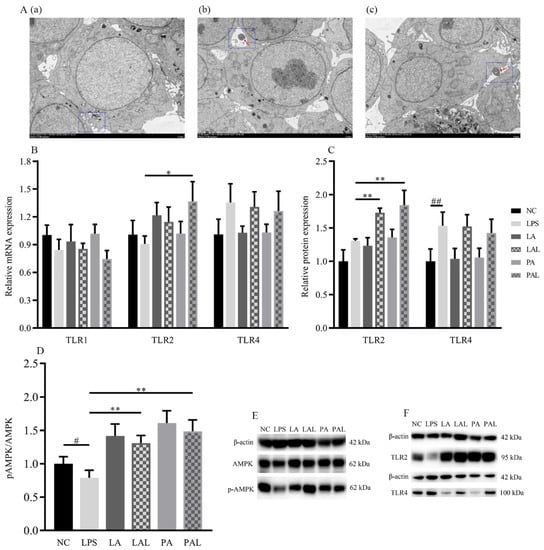
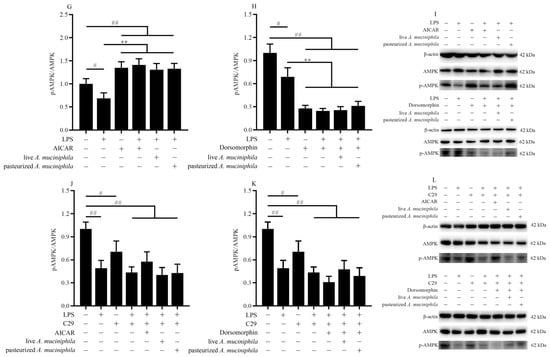
Figure 4.
The activation of AMPK was mediated by A. muciniphila through TLR2 in LPS Caco-2 cells. (A(a–c)) The TEM images of A. muciniphila attaching to Caco-2 cells. The red arrows indicate A. muciniphila. The blue circles indicate cross-section between cells and A. muciniphila. Scale bars, 5 μm. (B,C,F) The expression of TLR2, TLR4, (D,E) and activation of AMPK in Caco-2 cells treated with A. muciniphila. (G–I) The activation of AMPK in Caco-2 cells treated with A. muciniphila, AICAR, or dorsomorphin. (J–L) The activation of AMPK in Caco-2 cells treated with A. muciniphila, C29, AICAR, or dorsomorphin. ** p < 0.01, * p < 0.05, compared with LPS group; ## p < 0.01, # p < 0.05, compared with NC group. Data are expressed as mean ± S.D. (n = 6). NC, control group. LPS, lipopolysaccharide-damaged group. LA, live A. muciniphila. PA, pasteurized A. muciniphila. LAL, live A. muciniphila + LPS. PAL, pasteurized A. muciniphila + LPS. AMPK, AMP-activated protein kinase. TLR, Toll-like receptor. AICAR, 5-Aminoimidazole-4-carboxamide ribonucleotide. C29, C16H15NO4.
Moreover, pretreatment with live or pasteurized A. muciniphila significantly increased the level of phosphorylated AMPK (p-AMPK) (p < 0.01, Figure 4D,E). We further treated Caco-2 cells with AMPK-activator AICAR and inhibitor dorsomorphin (Figure 4G–I) to verify the regulation of A. muciniphila on the AMPK pathway. We found that AICAR treatment showed similar p-AMPK activation to A. muciniphila treatment (p < 0.05, Figure 4G,I), whereas the A. muciniphila-induced recovery of p-AMPK in the LPS group was diminished by dorsomorphin (p < 0.01, Figure 4H,I).
Then, we treated Caco-2 cells with TLR2 inhibitor C29 (Figure 4J–L) to determine whether AMPK activation is dependent on TLR2. Results showed that the influence of A. muciniphila on p-AMPK was only observed in cells without C29 treatment (p < 0.01), suggesting A. muciniphila modulates phosphorylation of AMPK by regulating TLR2 expression.
These results indicated that TLR2 and the AMPK signaling pathway were regulated by pretreatment with A. muciniphila and that TLR2 participates in the activation of AMPK.
3.5. Akkermansia muciniphila Increased the Level of ZO-1 and Decreased Claudin2 through TLR/AMPK Signaling
Because AMPK is known to facilitate tight junction assembly, we next determined the effect of A. muciniphila on tight junction assembly through the AMPK pathway in Caco-2 cells treated with AICAR or dorsomorphin. Pretreatment with A. muciniphila increased the level of ZO-1 similar to AICAR (p < 0.01) and the effect was diminished after being treated with dorsomorphin (p < 0.01, Figure 5A–C). The expression of claudin2 was decreased in all groups treated with dorsomorphin (p < 0.01, Figure 5B,C). This indicated that inhibition of AMPK significantly eliminated the ability of A. muciniphila to ameliorate the level of ZO-1 decrease and claudin2 increase in LPS-induced Caco-2 cells.
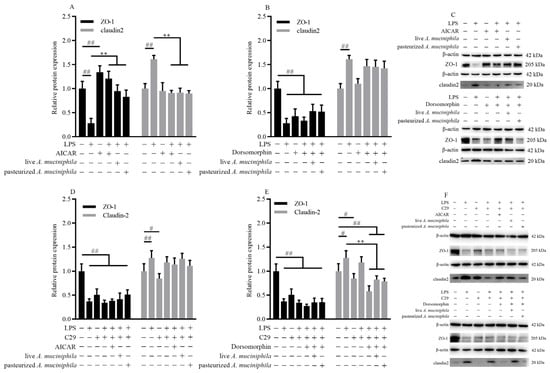
Figure 5.
A. muciniphila mediated the expression of ZO-1 and claudin2 through activation of the AMPK signaling pathway dependent on TLR2 in Caco-2 cells. (A–C) The ZO-1 and claudin2 protein expressions were treated with AICAR or dorsomorphin in Caco-2 cells. (D–F) The level of ZO-1 and claudin2 treated with C29, AICAR, or dorsomorphin in Caco-2 cells induced by pretreatment with A. muciniphila. ** p < 0.01, compared with LPS group; ## p < 0.01, # p < 0.05, compared with NC group. Data are expressed as mean ± S.D. (n = 6). LPS, lipopolysaccharide. AICAR, 5-Aminoimidazole-4-carboxamide ribonucleotide. C29, C16H15NO4. ZO, zonula occludens.
As TLR2 is demonstrated to mediate the activation of AMPK, we investigated the effect of TLR2 and AMPK on the assembly of ZO-1 and claudin2 using Caco-2 cells treated with C29. C29 notably reduced the protein expression of ZO-1 but this decrease was not affected by subsequent treatment with AICAR or A. muciniphila (p < 0.01, Figure 5D–F). The improvement of ZO-1 by A. muciniphila in LPS-induced Caco-2 cells was eliminated after treatment with C29 (p < 0.01, p < 0.05, Figure 5E,F). The reduction of increased claudin2 expression by treatment with AICAR and A. muciniphila in LPS-induced cells was eliminated after treatment with C29 (p < 0.01, p < 0.05, Figure 5D,F). Results suggested that stimulation of TLR2 is important for tight junction assembly through AMPK activation in cells treated with A. muciniphila.
Together, these results showed that the activation of the AMPK signaling pathway through TLR2 was associated with increasing the level of ZO-1 and decreasing claudin2 by pretreatment with live or pasteurized A. muciniphila.
3.6. Akkermansia muciniphila Regulated Caudal Type Homeobox 2 (CDX2) Expression in LPS Caco-2 Cells
CDX2 is important in the assembly of tight junction proteins [30]. To determine the effect of CDX2 on ZO-1 and claudin2 assembly, the expression of CDX2 was measured. The Western blot analysis revealed that the level of CDX2 decreased markedly in LPS-treated Caco-2 cells (p < 0.01, Figure 6A,B), but live or pasteurized A. muciniphila reversed the protein expression of CDX2 significantly to the level found in the NC group (p < 0.01, Figure 6A,B). Moreover, pretreatment with pasteurized A. muciniphila showed higher up-regulation of CDX2 compared with live A. muciniphila groups (p < 0.05, Figure 6A,B). The results suggest A. muciniphila can improve transcription factor CDX2 in tight junction proteins.
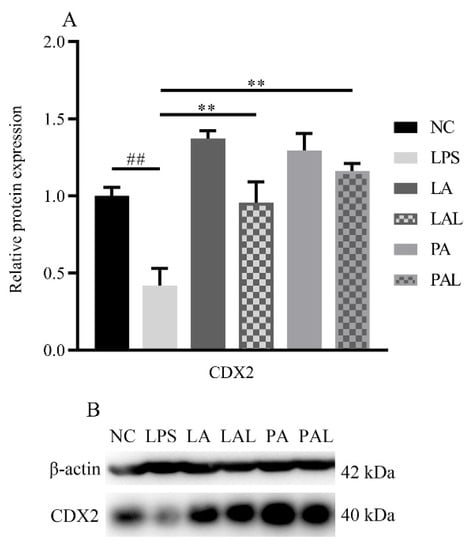
Figure 6.
A. muciniphila mediated the expression of CDX2. (A,B) The protein expression of CDX2 in Caco-2 cells. ** p < 0.01, compared with LPS group. ## p < 0.01, compared with NC group. Data are expressed as mean ± S.D. (n = 6). NC, control group. LPS, lipopolysaccharide-damaged group. LA, live A. muciniphila. PA, pasteurized A. muciniphila. LAL, live A. muciniphila + LPS. PAL, pasteurized A. muciniphila + LPS. CDX2, Caudal type homeobox 2.
4. Discussion
Mounting evidence indicates that A. muciniphila is beneficial for maintaining intestinal barrier integrity and immunologic response [13,19,24]. Previous studies have revealed that A. muciniphila activates TLR2 in vivo and in vitro [18,25]. However, the modulating mechanism of A. muciniphila is still obscure. In this study, we demonstrated that both live and pasteurized A. muciniphila strains can prevent LPS-induced epithelial barrier dysfunction and inflammatory response in Caco-2 monolayers through activating AMPK and inhibiting NF-κB. These findings provide a theoretical basis for understanding the mechanisms and application of pasteurized A. muciniphila for disease pre-protection.
Studies demonstrate the anti-inflammatory effect of A. muciniphila in vivo and in vitro [13,14]. In our study, the mRNA expression and secretion of pro-inflammatory cytokines, such as TNF-α, IL-1α, IL-1β, IL-6, and IL-8 was decreased, and anti-inflammatory cytokines TGF-β and IL-10 were increased in cells pretreated with A. muciniphila. Moreover, pasteurized and live A. muciniphila showed a similar effect in preventing inflammation disorders implementation. Consistently, we found that pretreatment with A. muciniphila significantly inhibited the DSS-induced activation of NF-κB in triggering inflammatory reactions [36,37], which was dependent on TLR2 stimulation. It is reported that the modulatory effect of NF-κB activated in the intestinal barrier is regulated by the recognition of TLR2 or TLR4 [38,39]. Moreover, the TLR2 was activated with Amuc_1100 of A. muciniphila in the human TLR2 and Caco-2 cell lines, respectively [18,26]. Therefore, we determined the mRNA and protein expression of TLR2 and TLR4 in this study. Our results showed that A. muciniphila indeed contacts Caco-2 cells, and pretreatment with pasteurized A. muciniphila significantly increased TLR2 expression, while TLR4 expression did not significantly change. This indicated that the anti-inflammatory effects of A. muciniphila are related to the stimulation of TLR2, not TLR4.
Previous studies demonstrated that pasteurized A. muciniphila improved the expression of tight junctions, such as ZO-2, occludin, and claudin4, through interaction with TLR2 [18,25]. It is known that the maintenance of barrier integrity and their functions are determined by tight junction proteins [25,40], whose assembly can be regulated by the AMPK pathway [41,42]. Further, we determined the effect of pretreatment with A. muciniphila on AMPK phosphorylation in Caco-2 cells treated with LPS. In the treatment with the TLR2-inhibitor, we observed that C29 inhibited the activation of AMPK, which was not alleviated by the AICAR treatment, thus indicating the activation of AMPK is dependent on the TLR2. Additionally, the AMPK activated by A. muciniphila also vanished after the C29 treatment. Therefore, the TLR2 plays a key role in activating the AMPK pathway in Caco-2 cells treated with A. muciniphila.
ZO-1 can link the transmembrane proteins with the actin cytoskeleton in the cell and recruitment of other tight junction proteins to maintain intestinal function [43]. On the contrary, claudin2 is a pore-forming protein correlated with epithelial leakiness [44]. The up-regulation of ZO-1 and the down-regulation of claudin2 were simultaneously mediated by AMPK activation in mice and epithelial cell models [41,45,46]. In this study, we found the pretreatment with A. muciniphila resulted in a similar trend with that of the AICAR pretreatment. Consistent with that finding, dorsomorphin treatment eliminated the above effect, which was also diminished by the C29 pretreatment, suggesting that A. muciniphila may stimulate TLR2 and activate the AMPK pathway to regulate the protein expression of ZO-1 and claudin2. Additionally, A. muciniphila pretreatment up-regulated the CDX2 protein expression, which was eliminated by dorsomorphin. This means AMPK may promote ZO-1 assembly via CDX2 [30]. Consequently, pretreatment with A. muciniphila reversed the barrier dysfunction of LPS-induced Caco-2 cell monolayers significantly, including an increase in TER value and a decrease in FITC-dextran permeation.
5. Conclusions
The present study indicated that pretreatment with live and pasteurized A. muciniphila can attenuate intestinal barrier dysfunction in LPS-induced Caco-2 cells by restoring the inflammatory cytokines toward the unchallenged control and by facilitating the assembly of tight junctions. The protective effects of A. muciniphila on barrier function were proved to ameliorate inflammation disorders by inhibition of the NF-κB pathway and to increase tight junctions via CDX2 mediation by activation of the AMPK pathway, both dependent on TLR2. Our findings revealed a specific mechanism responsible for the protective effect of A. muciniphila on the intestinal barrier function of Caco-2 cells (Figure 7). However, further research is needed to clarify how A. muciniphila stimulated TLR2 and to explain the minor difference in effect on claudin1 mRNA expression between pasteurized and live A. muciniphila.
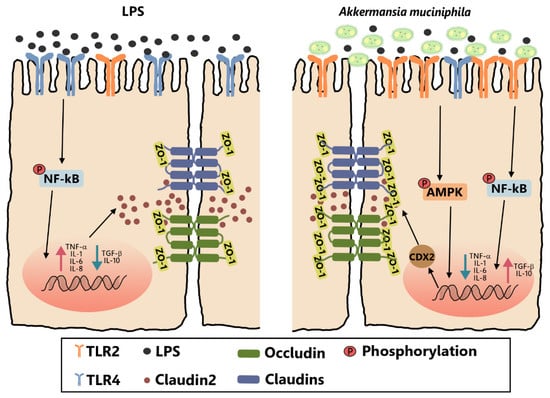
Figure 7.
Pretreatment with live and pasteurized A. muciniphila can attenuate intestinal barrier dysfunction in LPS-induced Caco-2 cells by restoring the inflammatory response and by facilitating the assembly of tight junctions. The protective effects of A. muciniphila on barrier function were proved to ameliorate inflammation disorders by inhibition of the NF-κB pathway and to increase tight junctions via CDX2 mediation by activation of the AMPK pathway, both dependent on TLR2. TLR2, Toll-like receptor 2. LPS, lipopolysaccharide. ZO, zonula occludens. CDX2, Caudal type homeobox 2. AMPK, AMP-activated protein kinase. NF-κB, Nuclear Factor-Kappa B.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14040764/s1, Table S1: Real-time PCR primers used in this study. Figure S1: The survival rate of Caco-2 cell viability co-culture with (A) 1 × 107 CFU, 1 × 108 CFU and 1 × 109 CFU A. muciniphila for 6 h, (B) 1 × 107 CFU A. muciniphila for 4, 6, 8 h, and (C) 1 × 107 CFU A. muciniphila for 4, 6 and 8 h after stimulated by LPS (5 μg/mL).
Author Contributions
Conceptualization, M.S., Y.Y. and F.C.; methodology, M.S.; software, M.S.; validation, Y.Y. and L.D.; formal analysis, M.S., Y.Y. and F.C.; investigation, M.S.; resources, F.C.; data curation, Y.Y., C.M. and F.C.; writing—original draft preparation, M.S.; writing—review and editing, C.M., L.D. and F.C.; visualization, M.S.; supervision, Y.Y.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the 2115 Talent Development Program of China Agricultural University.
Institutional Review Board Statement
Not applicable.
Acknowledgments
We thank Wuhan Servicebio Technology Co., Ltd., for the technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Van Herreweghen, F.; De Paepe, K.; Marzorati, M.; Van de Wiele, T. Mucin as a Functional Niche is a More Important Driver of In Vitro Gut Microbiota Composition and Functionality than Supplementation of Akkermansia muciniphila. Appl. Environ. Microbiol. 2020, 87, e02647-20. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Lage, N.N.; Mertens-Talcott, S.; Talcott, S.; Chew, B.; Dowd, S.E.; Kawas, J.R.; Noratto, G.D. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. PeerJ 2018, 6, e4195. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Varin, T.V.; Le Barz, M.; Pilon, G.; Dudonne, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic berry extracts target the gut-liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2021, 60, 2217–2230. [Google Scholar] [CrossRef]
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Huo, J.; Lei, M.; Zhou, Y.; Zhong, X.; Liu, Y.; Hou, J.; Long, H.; Zhang, Z.; Tian, M.; Xie, C.; et al. Structural characterization of two novel polysaccharides from Gastrodia elata and their effects on Akkermansia muciniphila. Int. J. Biol. Macromol. 2021, 186, 501–509. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wu, F.; Wang, X.; Feng, Y.; Wang, Y. MDG, an Ophiopogon japonicus polysaccharide, inhibits non-alcoholic fatty liver disease by regulating the abundance of Akkermansia muciniphila. Int. J. Biol. Macromol. 2022, 196, 23–34. [Google Scholar] [CrossRef]
- Yin, J.; Song, Y.; Hu, Y.; Wang, Y.; Zhang, B.; Wang, J.; Ji, X.; Wang, S. Dose-Dependent Beneficial Effects of Tryptophan and Its Derived Metabolites on Akkermansia In Vitro: A Preliminary Prospective Study. Microorganisms 2021, 9, 1511. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, M.; Ou, Z.; Wu, D.; Deng, L.; Lu, Z.; Zhang, J.; Deng, G.; Chen, S.; et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe 2019, 61, 102138. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar]
- Wu, W.; Lv, L.; Shi, D.; Ye, J.; Fang, D.; Guo, F.; Li, Y.; He, X.; Li, L. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Front. Microbiol. 2017, 8, 1804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, X.; Liu, L.; Voglmeir, J.; Zhong, X.; Yu, Q. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium. Vet. Res. 2020, 51, 34. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia Muciniphila Protects against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/-Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Liu, J.H.; Yue, T.; Luo, Z.W.; Cao, J.; Yan, Z.Q.; Jin, L.; Wan, T.F.; Shuai, C.J.; Wang, Z.G.; Zhou, Y.; et al. Akkermansia muciniphila promotes type H vessels formation and bone fracture healing by reducing gut permeability and inflammation. Dis. Models Mech. 2020, 13, dmm043620. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietila, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, A.; Ava, B.; Arefeh, S.; Sara, A.B.; Mehdi, D.; Shohre, K.; Fatemeh, R.J.; Abolfazl, F.; Farzam, V.; Davar, S.S. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol. Hepatol. Bed Bench 2019, 12, 163–168. [Google Scholar]
- Wang, J.; Xiang, R.; Wang, R.; Zhang, B.; Gong, W.; Zhang, J.; Zhang, M.; Wang, M. The variable oligomeric state of Amuc_1100 from Akkermansia muciniphila. J. Struct. Biol. 2020, 212, 107593. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.; Conlon, M.A.; Topping, D.L. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients 2016, 8, 272. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Liu, Q.; Shan, T.; Wang, Y. Metformin Protects against LPS-Induced Intestinal Barrier Dysfunction by Activating AMPK Pathway. Mol. Pharm. 2018, 15, 3272–3284. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Lu, X.; Liang, Y.; Mao, Y.; Loor, J.J.; Yang, Z. Metformin activated AMPK signaling contributes to the alleviation of LPS-induced inflammatory responses in bovine mammary epithelial cells. BMC Vet. Res. 2021, 17, 97. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.-J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
- Kong, C.; Cheng, L.; Krenning, G.; Fledderus, J.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P. Human Milk Oligosaccharides Mediate the Crosstalk between Intestinal Epithelial Caco-2 Cells and Lactobacillus Plantarum WCFS1 in an In Vitro Model with Intestinal Peristaltic Shear Force. J. Nutr. 2020, 150, 2077–2088. [Google Scholar] [CrossRef]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Adachi, T.; Shimomura, Y.; Matsushita, H.; Nguyen, T.T.T.; Koike, K.; Ikeda, A.; Takashima, S.; et al. Berberine improved experimental chronic colitis by regulating interferon-γ- and IL-17A-producing lamina propria CD4+ T cells through AMPK activation. Sci. Rep. 2019, 9, 11934. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Z.; Zhang, X.; Zhu, F.; Liu, Y.; Jin, C.; Du, X.; Xu, C.; Chen, Y.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, Z.; Ji, Y.; Sun, K.; Dai, Z.; Wu, G. L-Glutamine Enhances Tight Junction Integrity by Activating CaMK Kinase 2-AMP-Activated Protein Kinase Signaling in Intestinal Porcine Epithelial Cells. J. Nutr. 2016, 146, 501–508. [Google Scholar] [CrossRef]
- Ren, C.; Dokter-Fokkens, J.; Figueroa Lozano, S.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Lactic Acid Bacteria May Impact Intestinal Barrier Function by Modulating Goblet Cells. Mol. Nutr. Food Res. 2018, 62, e1700572. [Google Scholar] [CrossRef]
- Shaukat, A.; Guo, Y.F.; Jiang, K.; Zhao, G.; Wu, H.; Zhang, T.; Yang, Y.; Guo, S.; Yang, C.; Zahoor, A.; et al. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced Acute Lung Injury through attenuating NF-kappaB and MAPK activation. Microb. Pathog. 2019, 132, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.F.; Zhao, G.; Deng, G.Z.; Wu, H.C.; Yin, N.N.; Chen, X.Y.; Qiu, C.W.; Peng, X.L. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-kappaB pathway. Acta Pharmacol. Sin. 2017, 38, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Hsu, W.F.; Chang, J.S.; Shih, C.K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar]
- Pattanaik, K.P.; Ganguli, G.; Naik, S.K.; Sonawane, A. Mycobacterium tuberculosis EsxL induces TNF-alpha secretion through activation of TLR2 dependent MAPK and NF-kappaB pathways. Mol. Immunol. 2021, 130, 133–141. [Google Scholar] [CrossRef]
- Nakahara, T.; Nishitani, Y.; Nishiumi, S.; Yoshida, M.; Azuma, T. Astilbin from Engelhardtia chrysolepis enhances intestinal barrier functions in Caco-2 cell monolayers. Eur. J. Pharmacol. 2017, 804, 46–51. [Google Scholar] [CrossRef][Green Version]
- Zhu, M.J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Cantley, L.C. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. USA 2007, 104, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Matsui, T.; Nakayama, M.; Furuse, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004, 279, 44785–44794. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.J.; Deng, Z.B.; Liu, J.N.; Qiu, J.J.; Guo, L.; Feng, P.P.; Sui, J.R.; Chen, D.P.; Guo, H.S. Enhancement of epithelial cell autophagy induced by sinensetin alleviates epithelial barrier dysfunction in colitis. Pharmacol. Res. 2019, 148, 104461. [Google Scholar] [CrossRef]
- Park, H.Y.; Kunitake, Y.; Hirasaki, N.; Tanaka, M.; Matsui, T. Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Biosci. Biotechnol. Biochem. 2015, 79, 130–137. [Google Scholar] [CrossRef]
- Bibi, S.; Kang, Y.; Du, M.; Zhu, M.J. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis. J. Nutr. Biochem. 2018, 51, 40–46. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).