Effects of Zn-Enriched Bifidobacterium longum on the Growth and Reproduction of Rats
Abstract
:1. Introduction
2. Materials and Methods
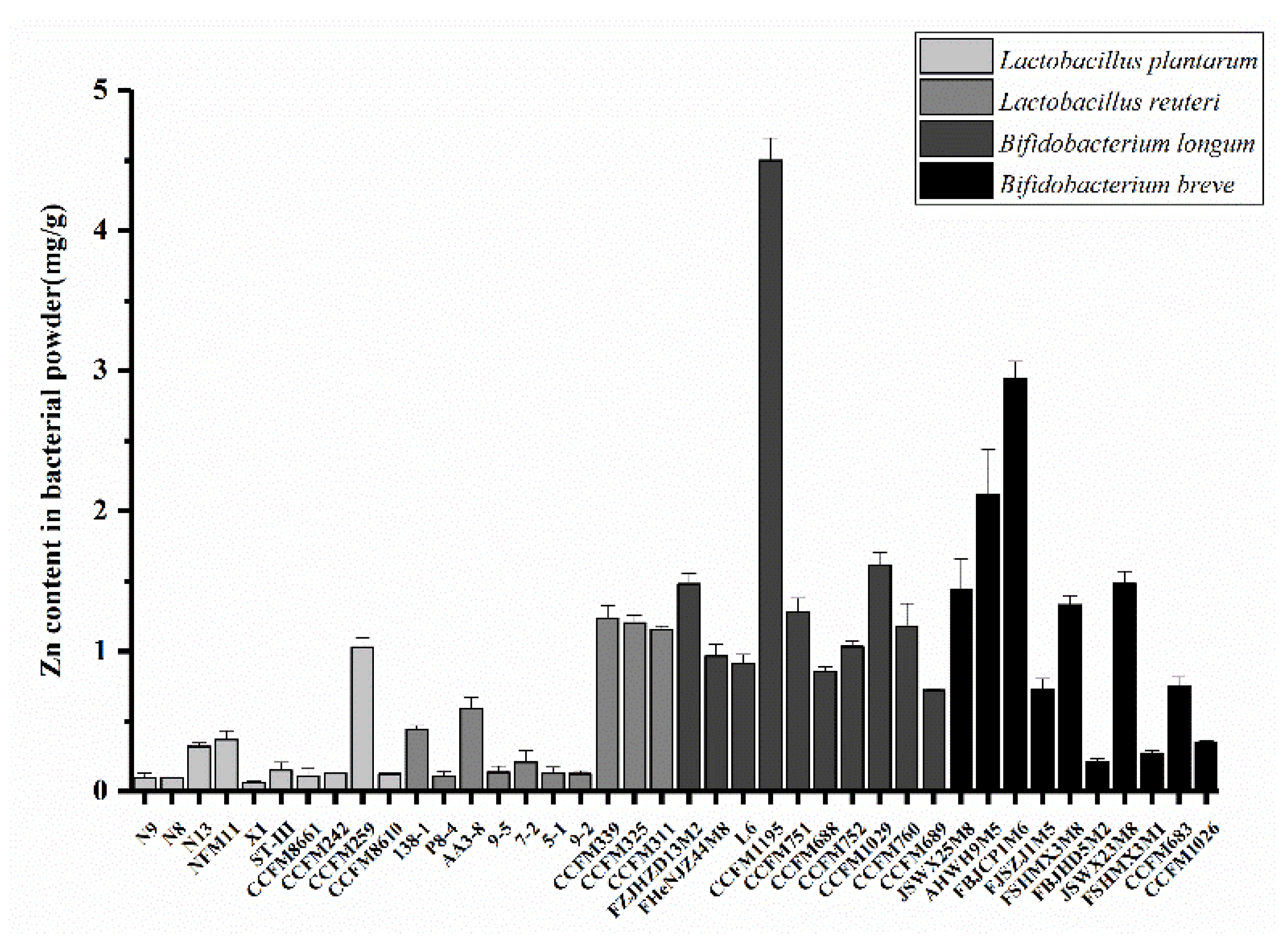
2.1. Screening of Strains with High Zn Enrichment
2.2. Morphological Analysis of Zn in Zn-Enriched Bacteria
2.3. Animal Experiment Design
2.4. Blood Measurements
2.4.1. Zn Content
2.4.2. Alkaline Phosphatase (ALP) Activity
2.4.3. Growth Hormone (GH) and Testosterone Levels
2.5. Tissue Zn Measurements
2.6. Microbial Profiling by 16S rRNA Sequencing
2.7. Statistical Analysis
3. Results
3.1. Zn Enrichment Ability of Different Bacterial Strains
3.2. Morphological Analysis of Zn in B. longum CCFM1195 + ZnO and Zn-Enriched B. longum CCFM1195
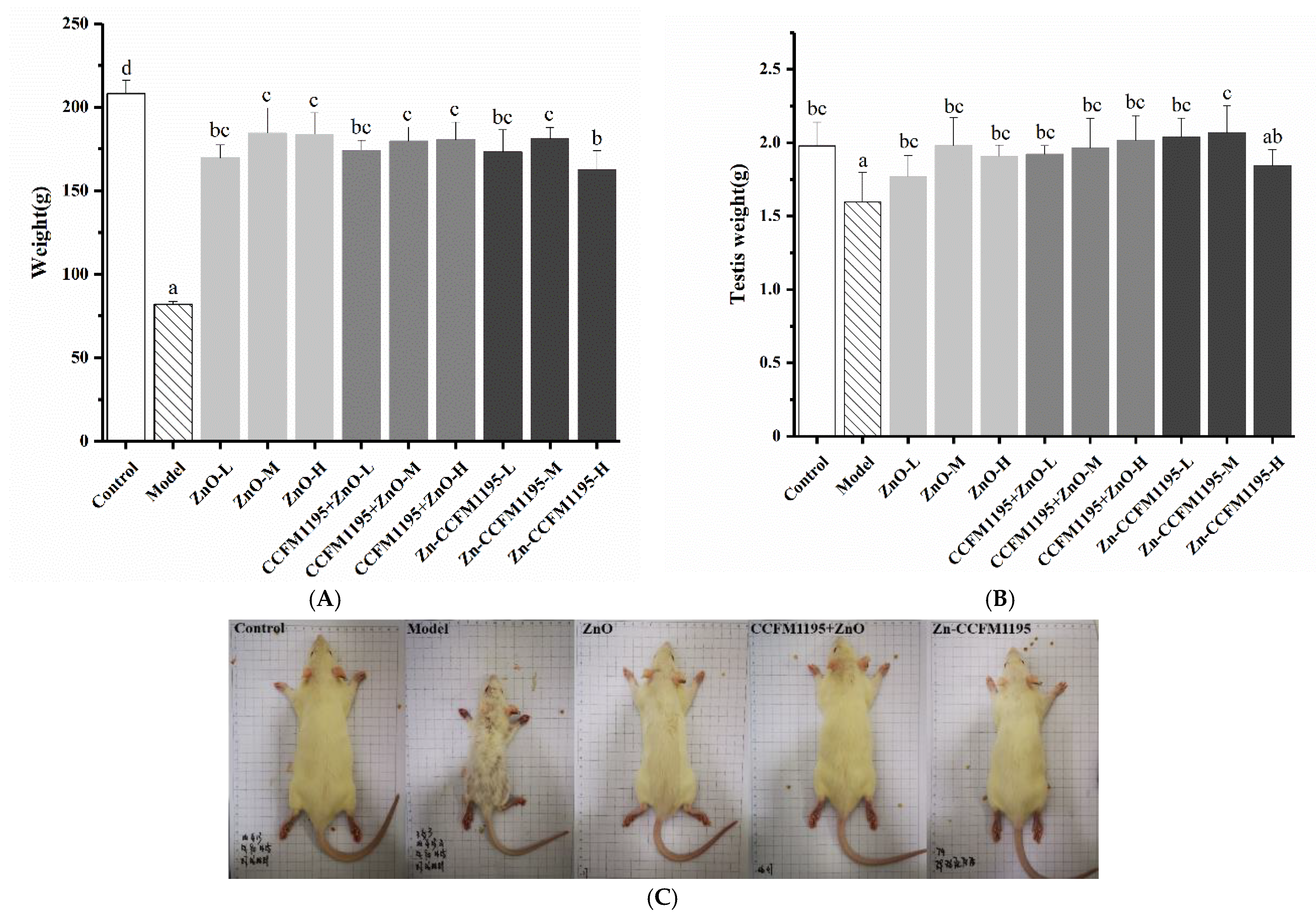
3.3. Apparent Indicators of Growth and Reproduction
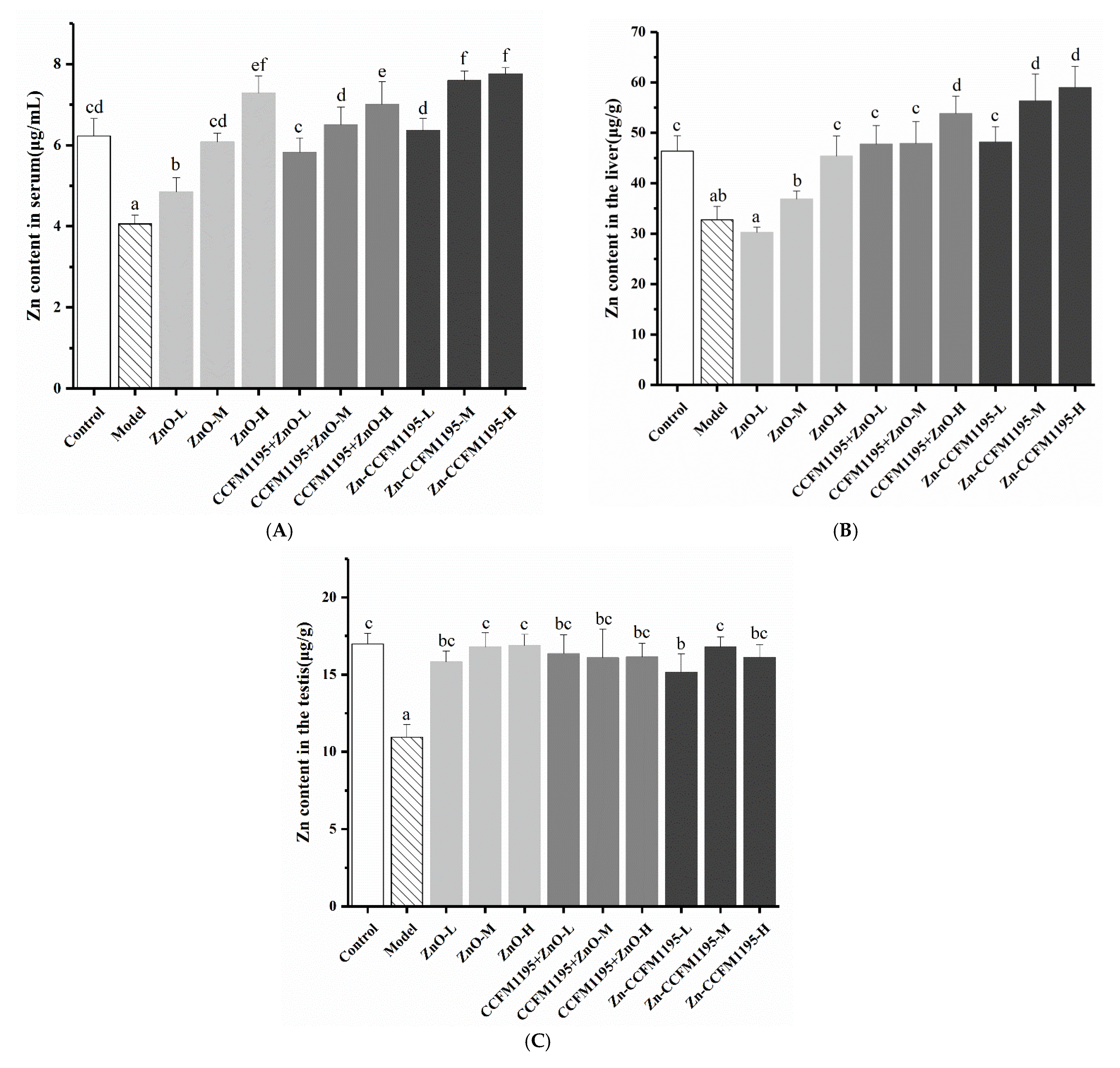
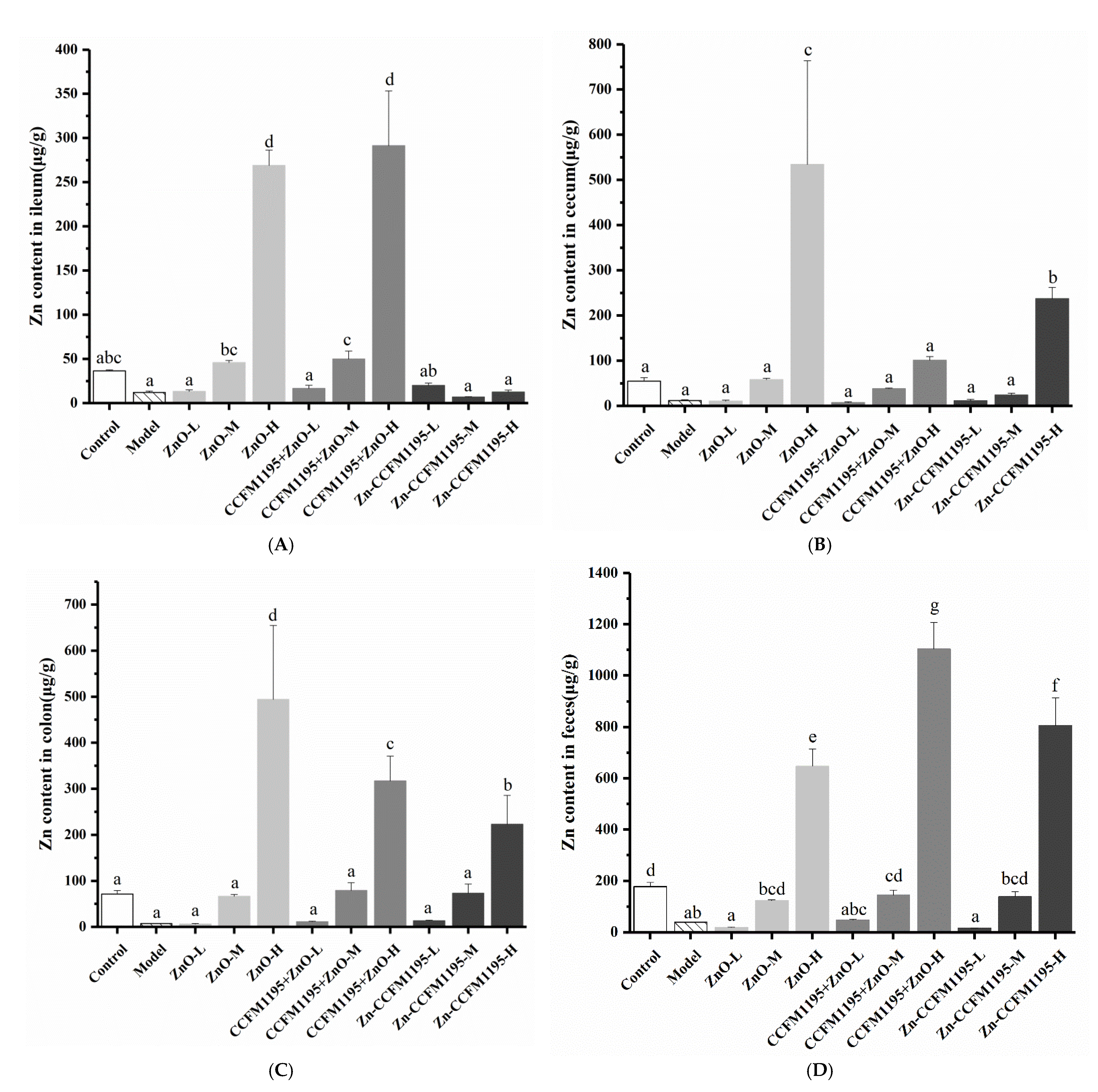
3.4. Zn Content in Tissue
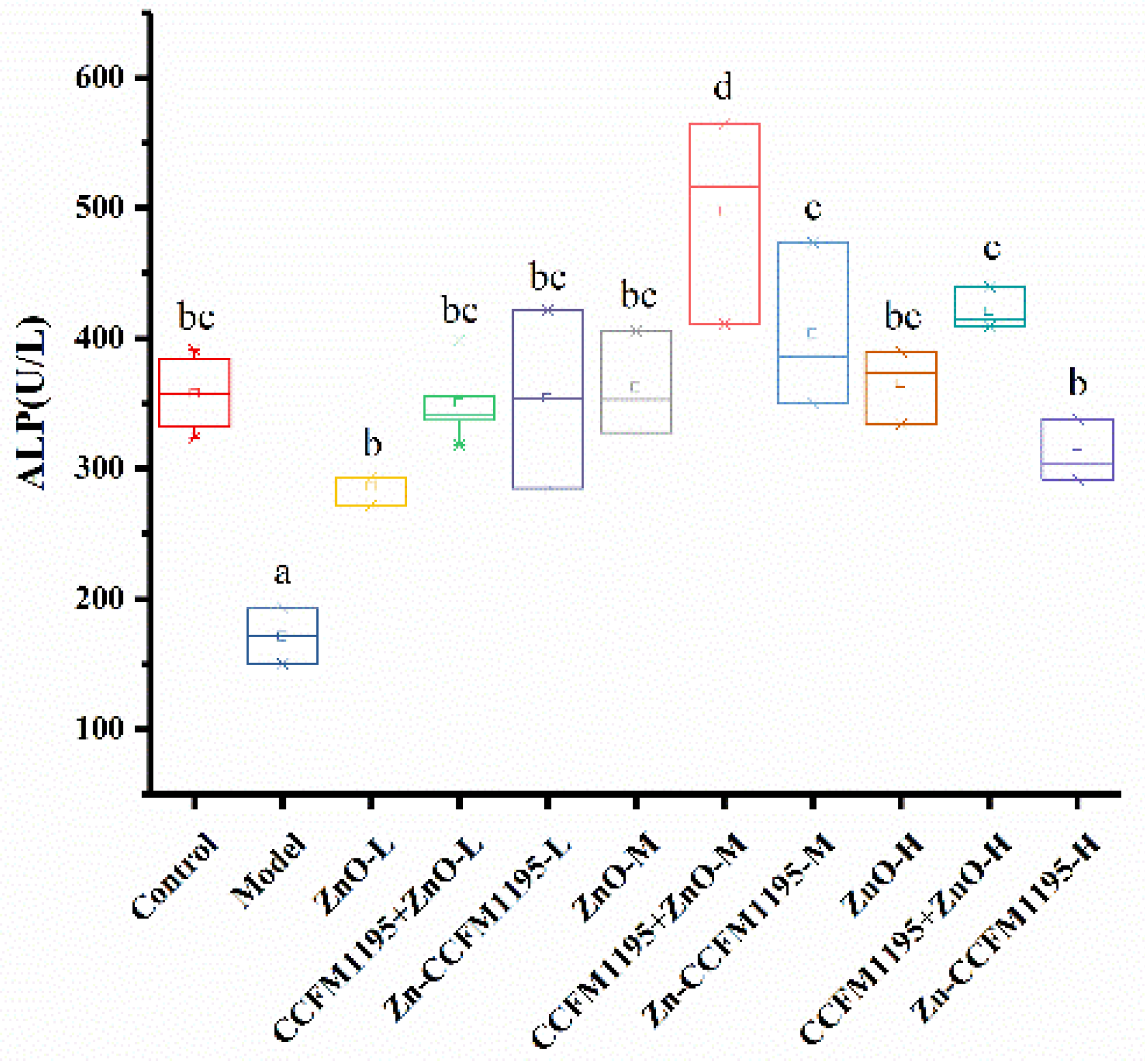
3.5. Activity Differences of Zn-Related Enzymes
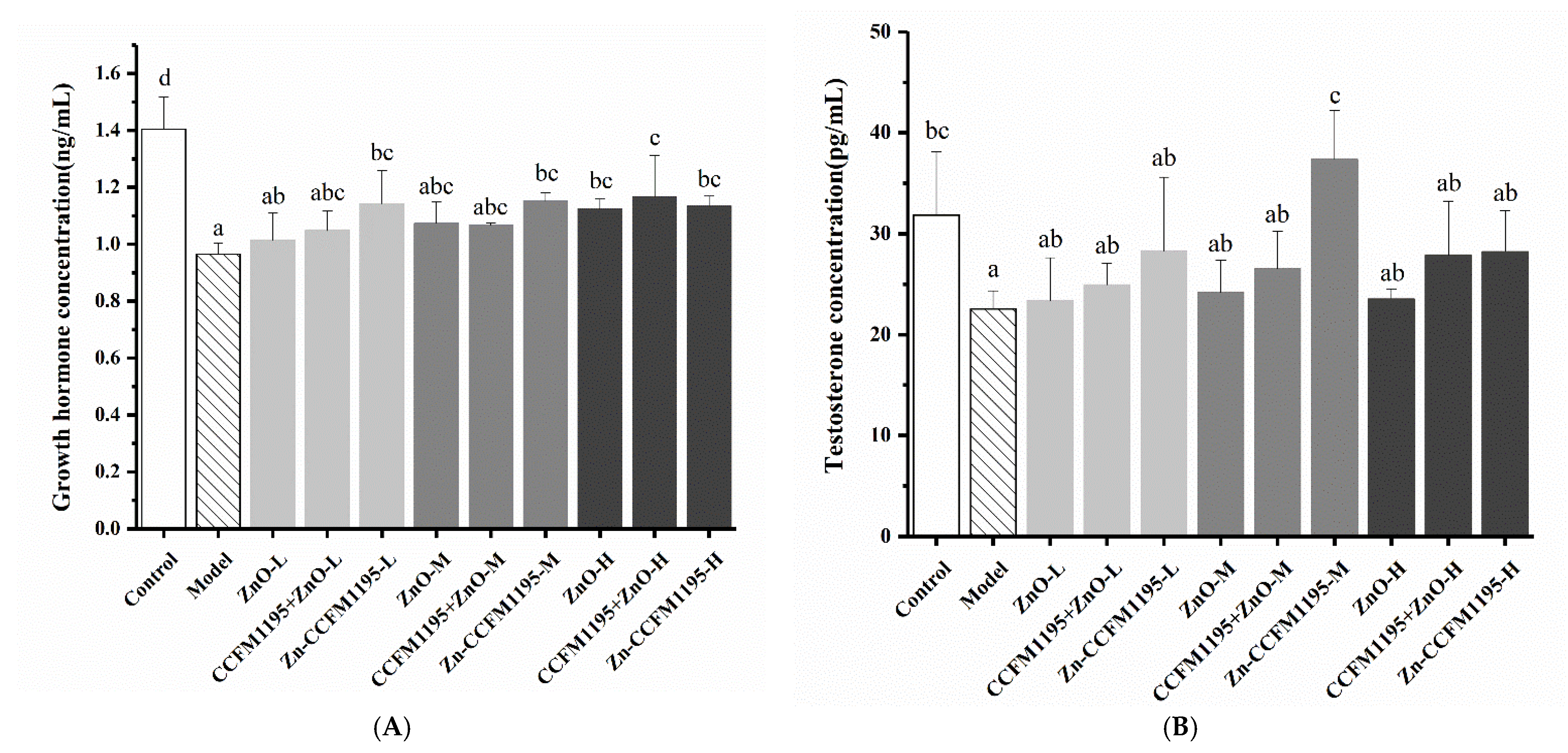
3.6. GH and Testosterone in Serum
3.7. Zn Content in the Intestinal Tract
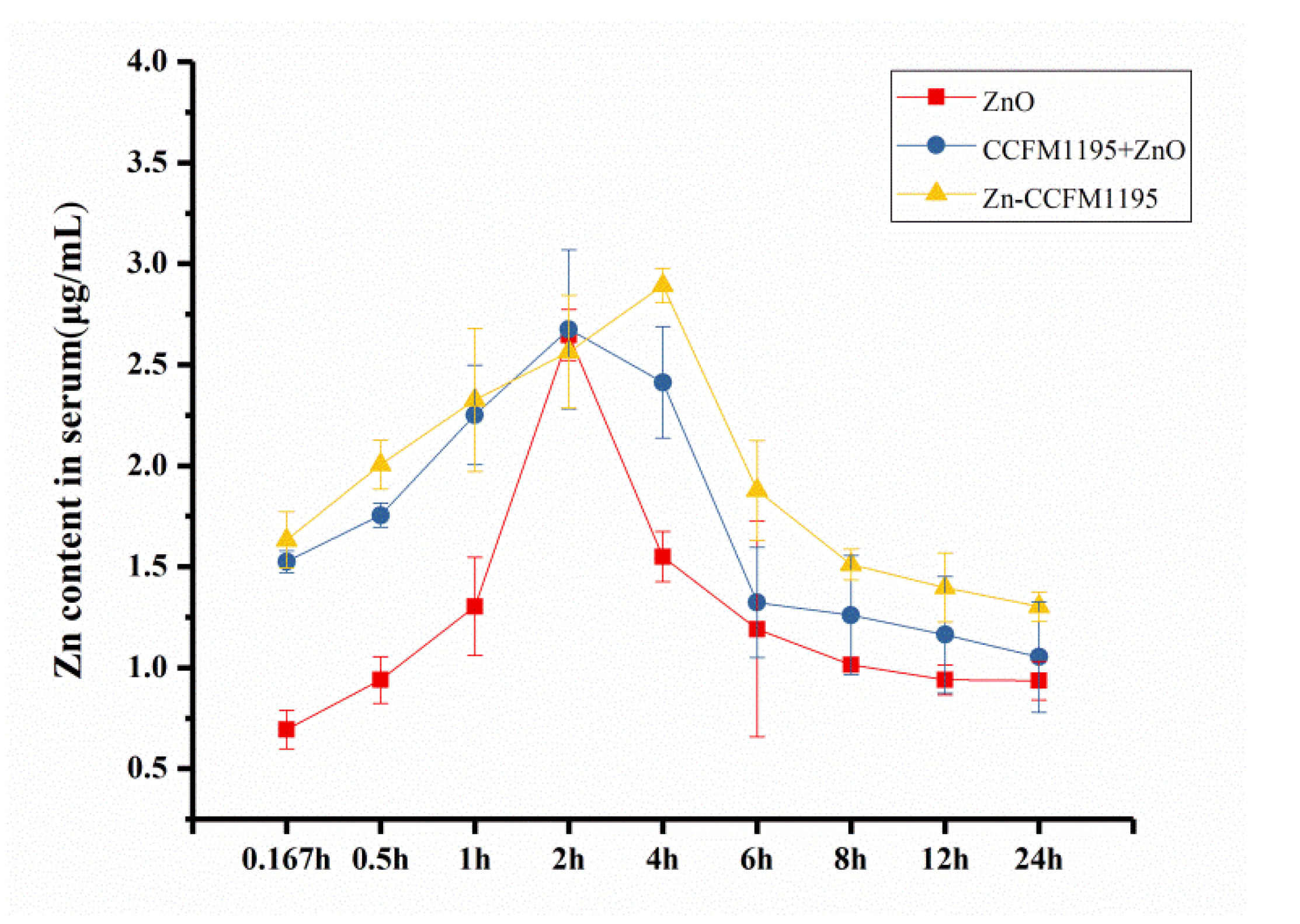
3.8. Pharmacokinetic Data Analysis
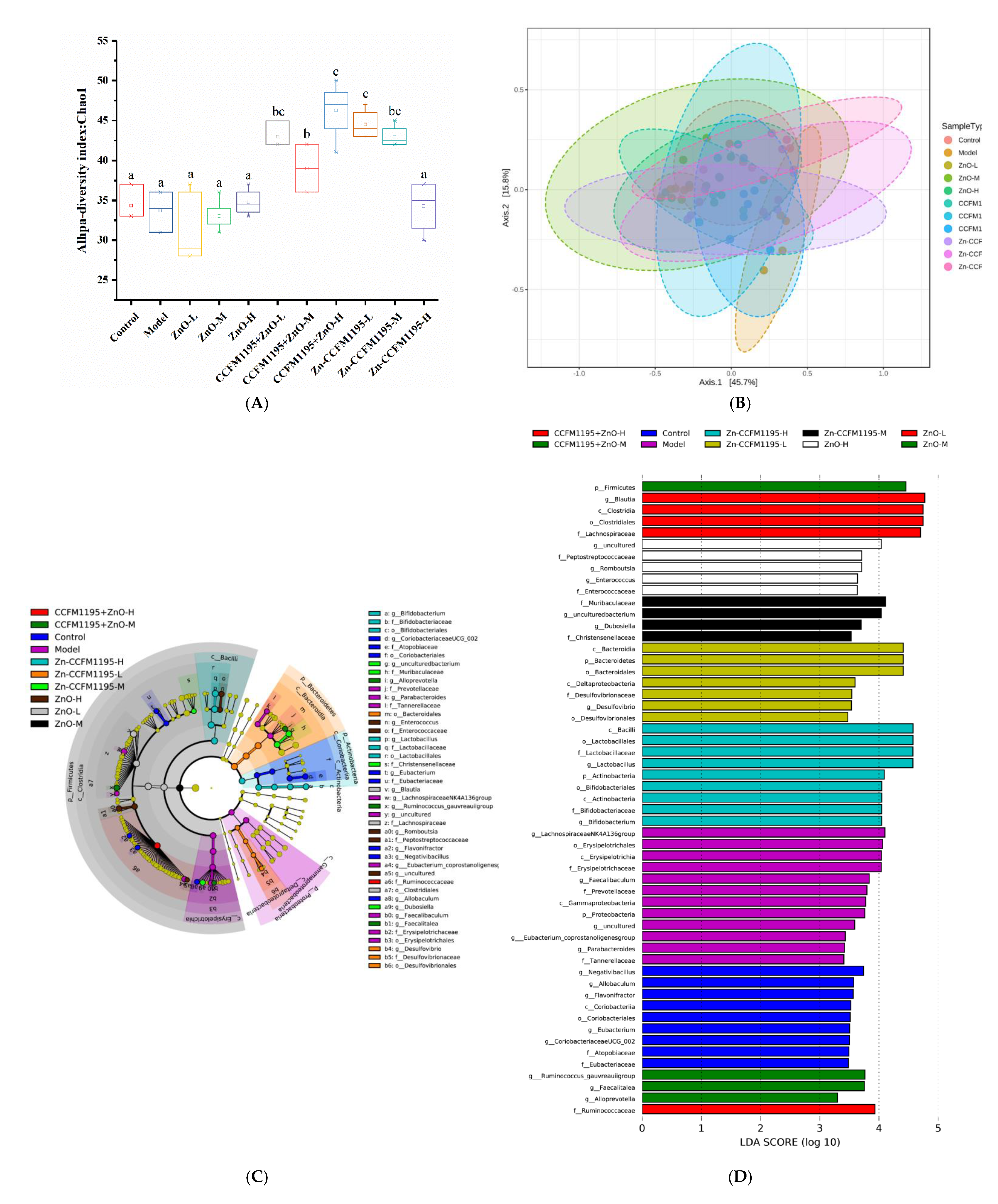
3.9. Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Strain Preservation Number | Latin Name |
|---|---|
| N9 | Lactobacillus plantarum |
| ST-III | L. plantarum |
| N13 | L. plantarum |
| CCFM8610 | L. plantarum |
| CCFM8661 | L. plantarum |
| X1 | L. plantarum |
| CCFM242 | L. plantarum |
| N8 | L. plantarum |
| CCFM259 | L. plantarum |
| NFM11 | L. plantarum |
| 138-1 | Lactobacillus reuteri |
| 9-5 | L. reuteri |
| 7-2 | L. reuteri |
| AA3-8 | L. reuteri |
| 5-1 | L. reuteri |
| CCFM339 | L. reuteri |
| CCFM325 | L. reuteri |
| P8-4 | L. reuteri |
| 9-2 | L. reuteri |
| CCFM311 | L. reuteri |
| CCFM1195 | Bifidobacterium longum |
| FHeNJZ44M8 | B. longum |
| CCFM688 | B. longum |
| CCFM 752 | B. longum |
| CCFM 1029 | B. longum |
| FZJHZD13M2 | B. longum |
| CCFM 751 | B. longum |
| L6 | B. longum |
| CCFM 760 | B. longum |
| CCFM 689 | B. longum |
| JS-WX-25-M8 | Bifidobacterium breve |
| AH-WH-9-M-5 | B. breve |
| FBJ-CP-1-M6 | B. breve |
| CCFM683 | B. breve |
| F-JS-ZJ-1-M5 | B. breve |
| FSHMX3M8 | B. breve |
| FBJHD5M2 | B. breve |
| JS-WX-23-M8 | B. breve |
| FSH-MX-3M1 | B. breve |
| CCFM1026 | B. breve |
| Components | Volume(1 L) |
|---|---|
| Trypyone | 10 g |
| Beef extract | 10 g |
| Yeast extract | 5 g |
| D(+)-Glucose anhydrous | 20 g |
| Sodium acetate | 2 g |
| MgSO4·7H2O | 0.1 g |
| MnSO4·H2O | 0.05 g |
| Diammonium hydrogen citrate | 2 g |
| K2HPO4 | 2 g |
| Tween 80 | 1 mL |
| L-Cysteine | 1 g |
| PCR System (50 μL) | |
|---|---|
| 2 × Taq MasterMix | 25 μL |
| ddH2O | 20 μL |
| 341F | 1.5 μL |
| 806R | 1.5 μL |
| DNA template | 2 μL |
References
- Fa Llah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Caulfield, L.E.; Black, R.E. Zinc Deficiency. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J., Eds.; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 257–279. [Google Scholar]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J. Zinc deficiency in human health. Encycl. Environ. Health 2011, 789–800. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Fons, C.; Brun, J.F.; Fussellier, M.; Cassanas, G.; Bardet, L.; Orsetti, A. Serum zinc and somatic growth in children with growth retardation. Biol. Trace Elem. Res. 1992, 32, 399–404. [Google Scholar] [CrossRef]
- Hamza, R.T.; Hamed, A.I.; Sallam, M.T. Effect of zinc supplementation on growth hormone-insulin growth factor axis in short Egyptian children with zinc deficiency. Ital. J. Pediatr. 2012, 38, 21. [Google Scholar] [CrossRef] [Green Version]
- Rider, S.A.; Davies, S.J.; Jha, A.N.; Clough, R.; Sweetman, J.W. Bioavailability of co-supplemented organic and inorganic zinc and selenium sources in a white fishmeal-based rainbow trout (Oncorhynchus mykiss) diet. J. Anim. Physiol. Anim. Nutr. 2010, 94, 99–110. [Google Scholar] [CrossRef]
- Dobrzanski, Z.; Jamroz, D.; Gorecka, H.; Opalinski, S. Bioavailability of selenium and zinc supplied to the feed for laying hens in organic and inorganic form. Electron. J. Pol. Agric. Univ. 2003, 6, #03. [Google Scholar]
- Li, S.L.; Center, T.; Co, Y.Y. Manufacturing technology of compound amino acid chelated zinc. Yunnan Chem. Technol. 2017, 44, 101–103. [Google Scholar]
- Leonardi, A.; Zanoni, S.; de Lucia, M.; Amaretti, A.; Raimondi, S.; Rossi, M. Zinc uptake by lactic acid bacteria. ISRN Biotechnol. 2013, 2013, 312917. [Google Scholar] [CrossRef] [Green Version]
- Mrvčić, J.; Prebeg, J.; Barišić, T.; Stanzer, L.; Bačun-Družina, D.; Stehlik-Tomas, V. Zinc binding by lactic acid bacteria. Food Technol. Biotechnol. 2009, 47, 381–388. [Google Scholar]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 240–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, P.; Pons, N.; Agrawal, A.; Oozeer, R.; Guyonnet, D.; Brazeilles, R.; Faurie, J.M.; van Hylckama Vlieg, J.E.; Houghton, L.A.; Whorwell, P.J.; et al. Changes of the human gut microbiome induced by a fermented milk product. Sci. Rep. 2014, 4, 6328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katouli, M.; Melin, L.; Jensen-Waern, M.; Wallgren, P.; Mollby, R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J. Appl. Microbiol. 2010, 87, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Crane, J.K.; Naeher, T.M.; Shulgina, I.; Zhu, C.; Boedeker, E.C. Effect of zinc in enteropathogenic Escherichia coli infection. Infect. Immun. 2007, 75, 5974–5984. [Google Scholar] [CrossRef] [Green Version]
- Marteau, P.; Guyonnet, D.; de Micheaux, P.L.; Gelu, S. A randomized, double-blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I-2494 in healthy women reporting minor digestive symptoms. Neurogastroenterol. Motil. 2013, 25, 331-e252. [Google Scholar] [CrossRef]
- Fudagawa, N.; Kawase, A. Determination of copper and zinc in tea by atomic absorption spectrometry. Bunseki Kagaku 1978, 27, 353–358. [Google Scholar] [CrossRef]
- Jia, Y. Morphological Analysis and Subcellular Distribution of Zinc in Zinc Enriched Yeast. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2009. [Google Scholar]
- Tomoko, G.; Michio, K.; Hitoshi, S. Long-term zinc deficiency decreases taste sensitivity in rats. J. Nutr. 2001, 131, 305–310. [Google Scholar]
- Zhang, S.Q.; Zhang, H.B.; Zhang, Y. Quantification of selenomethionine in plasma using UPLC–MS/MS after the oral administration of selenium-enriched yeast to rats. Food Chem. 2018, 241, 1–6. [Google Scholar] [CrossRef]
- Domellof, M.; Lonnerdal, B.; Dewey, K.G.; Cohen, R.J.; Hernell, O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am. J. Clin. Nutr. 2004, 79, 111–115. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.J. Trace elements in human and animal nutrition. Soil Sci. 1956, 82, 287. [Google Scholar] [CrossRef] [Green Version]
- Takeda, A.; Tamano, H.; Tochigi, M.; Oku, N. Zinc homeostasis in the hippocampus of zinc-deficient young adult rats. Neurochem. Int. 2005, 46, 221–225. [Google Scholar] [CrossRef]
- Toyoshima, K.; Shimamura, A. A histochemical study of the gustatory epithelium in the circumvallate papilla of zinc-deficient rats. J. Kyushu Dent. Soc. 1990, 44, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Lazarte, C.E.; Vargas, M.; Granfeldt, Y. Zinc bioavailability in rats fed a plant-based diet: A study of fermentation and zinc supplementation. Food Nutr. Res. 2015, 59, 27796. [Google Scholar] [CrossRef]
- Tesan, F.C.; Collia, N.; Arnoldi, S.; Fuda, J.; Torti, H.; Weill, R.; Salgueiro, M.J.; Boccio, J. Relative bioavailability of zinc in yogurt using body weight gain, femur weight and bone zinc content in rats as markers. Open Nutraceuticals J. 2009, 2, 16–19. [Google Scholar] [CrossRef]
- Scrimgeour, A.G.; Marchitelli, L.J.; Whicker, J.S.; Song, Y.; Ho, E.; Young, A.J. Phytase supplementation increases bone mineral density, lean body mass and voluntary physical activity in rats fed a low-zinc diet. J. Nutr. Biochem. 2010, 21, 653–658. [Google Scholar] [CrossRef]
- Ahmed, A.; Anjum, F.M.; Randhawa, M.A.; Farooq, U.; Akhtar, S.; Sultan, M.T. Effect of multiple fortification on the bioavailability of minerals in wheat meal bread. J. Food Sci. Technol. 2012, 49, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Sadingi, A.; Roshan, M.M.; Moradi, A.; Ostadrahimi, A. The effects of zinc supplementation on serum zinc, alkaline phosphatase activity and fracture healing of bones. Saudi Med. J. 2008, 29, 1276–1279. [Google Scholar]
- Godden, W. “Trace” elements in human and animal nutrition. J. Soc. Chem. Ind. 2007, 58, 791–796. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Halifeoglu, I. Effects of zinc deficiency and supplementation on plasma leptin levels in rats. Biol. Trace Elem. Res. 2005, 104, 41–46. [Google Scholar] [CrossRef]
- Sun, J.Y.; Wang, J.F.; Zi, N.T.; Jing, M.Y.; Weng, X.Y. Effects of zinc supplementation and deficiency on bone metabolism and related gene expression in rat. Biol. Trace Elem. Res. 2011, 143, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Pluhator, M.M.; Thomson, A.B.R.; Fedorak, R.N. Clinical aspects of trace elements: Zinc in human nutrition—Zinc metabolism. Can. J. Gastroenterol. 1995, 9, 327–332. [Google Scholar] [CrossRef]
- Fujimura, T.; Matsui, T.; Funaba, M. Regulatory responses to excess zinc ingestion in growing rats. Br. J. Nutr. 2012, 107, 1655–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semrad, C.E. Zinc and intestinal function. Curr. Gastroenterol. Rep. 1999, 1, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.T.; Failla, M.L.; Cousins, R.J. Identification of albumin as the plasma carrier for zinc absorption by perfused rat intestine. Biochem. J. 1979, 184, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int. J. Food Sci. Technol. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Hiroshi, H.; Ayako, K.; Takanori, K. Contribution of the cecum and colon to zinc absorption in rats. J. Nutr. 2000, 130, 83–89. [Google Scholar]
- Yu, Y.; Lu, L.; Wang, R.L.; Xi, L.; Luo, X.G.; Liu, B. Effects of zinc source and phytate on zinc absorption by in situ ligated intestinal loops of broilers. Poult. Sci. 2010, 89, 2157–2165. [Google Scholar] [CrossRef]
- Cummings, J.H. Short chain fatty acids in the human colon. Gut 1981, 22, 763–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Levrat, M.A.; Remesy, C.; Demigne, C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J. Nutr. 1991, 121, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Mogna, L.; Pane, M.; Nicola, S.; Raiteri, E. Screening of different probiotic strains for their in vitro ability to metabolise oxalates: Any prospective use in humans? J. Clin. Gastroenterol. 2014, 48 (Suppl. 1), S91–S95. [Google Scholar] [CrossRef]
- Abratt, V.R.; Reid, S.J. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv. Appl. Microbiol. 2010, 72, 63–87. [Google Scholar]
- Giardina, S.; Scilironi, C.; Michelotti, A.; Samuele, A.; Borella, F.; Daglia, M.; Marzatico, F. In vitro anti-inflammatory activity of selected oxalate-degrading probiotic bacteria: Potential applications in the prevention and treatment of hyperoxaluria. J. Food Sci. 2014, 79, M384–M390. [Google Scholar] [CrossRef]
- Al-Wahsh, I.; Wu, Y.; Liebman, M. Acute probiotic ingestion reduces gastrointestinal oxalate absorption in healthy subjects. Urol. Res. 2012, 40, 191–196. [Google Scholar] [CrossRef]
- Okombo, J.; Liebman, M. Probiotic-induced reduction of gastrointestinal oxalate absorption in healthy subjects. Urol. Res. 2010, 38, 169–178. [Google Scholar] [CrossRef]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc absorption in human small intestine. Am. J. Physiol. 1989, 256, 87–91. [Google Scholar] [CrossRef]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef] [Green Version]
- Butt, E.M. Trace elements in human and animal nutrition. Am. J. Clin. Pathol. 1958, 30, 360. [Google Scholar] [CrossRef] [Green Version]
- Solomons, N.W. Biological availability of zinc in humans. Am. J. Clin. Nutr. 1982, 35, 1048–1075. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrc, R.F. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef]

| Strain | Number of Viable Bacteria (cfu/g) | Zn Content (mg/g) |
|---|---|---|
| CCFM1195 | (3.8 ± 0.3) × 108 | — |
| Zn-enriched CCFM1195 | (1 ± 0.1) × 109 | 1.7 ± 0.22 |
| CCFM1195 + ZnO | H2O | EDTA | Digest | Sum |
|---|---|---|---|---|
| Zn content (μg/g) | 15.91 ± 0.18 | 11.99 ± 0.57 | 1.29 ± 0.05 | 29.19 ± 0.51 |
| Percent (%) | 54.51 | 41.08 | 4.40 | 100 |
| CCFM1195 | H2O | EDTA | Digest | Sum |
|---|---|---|---|---|
| Zn content (μg/g) | 119.94 ± 1.96 | 628.68 ± 3.37 | 2644.54 ± 4.83 | 3393.16 ± 10.03 |
| Percent (%) | 3.53 | 18.53 | 77.94 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Liu, F.; Zhang, Q.; Mao, B.; Tang, X.; Huang, J.; Guo, R.; Zhao, J.; Zhang, H.; Cui, S.; et al. Effects of Zn-Enriched Bifidobacterium longum on the Growth and Reproduction of Rats. Nutrients 2022, 14, 783. https://doi.org/10.3390/nu14040783
Han X, Liu F, Zhang Q, Mao B, Tang X, Huang J, Guo R, Zhao J, Zhang H, Cui S, et al. Effects of Zn-Enriched Bifidobacterium longum on the Growth and Reproduction of Rats. Nutrients. 2022; 14(4):783. https://doi.org/10.3390/nu14040783
Chicago/Turabian StyleHan, Xinran, Fei Liu, Qiuxiang Zhang, Bingyong Mao, Xin Tang, Jie Huang, Renmei Guo, Jianxin Zhao, Hao Zhang, Shumao Cui, and et al. 2022. "Effects of Zn-Enriched Bifidobacterium longum on the Growth and Reproduction of Rats" Nutrients 14, no. 4: 783. https://doi.org/10.3390/nu14040783
APA StyleHan, X., Liu, F., Zhang, Q., Mao, B., Tang, X., Huang, J., Guo, R., Zhao, J., Zhang, H., Cui, S., & Chen, W. (2022). Effects of Zn-Enriched Bifidobacterium longum on the Growth and Reproduction of Rats. Nutrients, 14(4), 783. https://doi.org/10.3390/nu14040783






